Introduction
Search strategy and study selection criteria
Terminology
Intracranial arteries compared to extracranial arteries
Anatomy
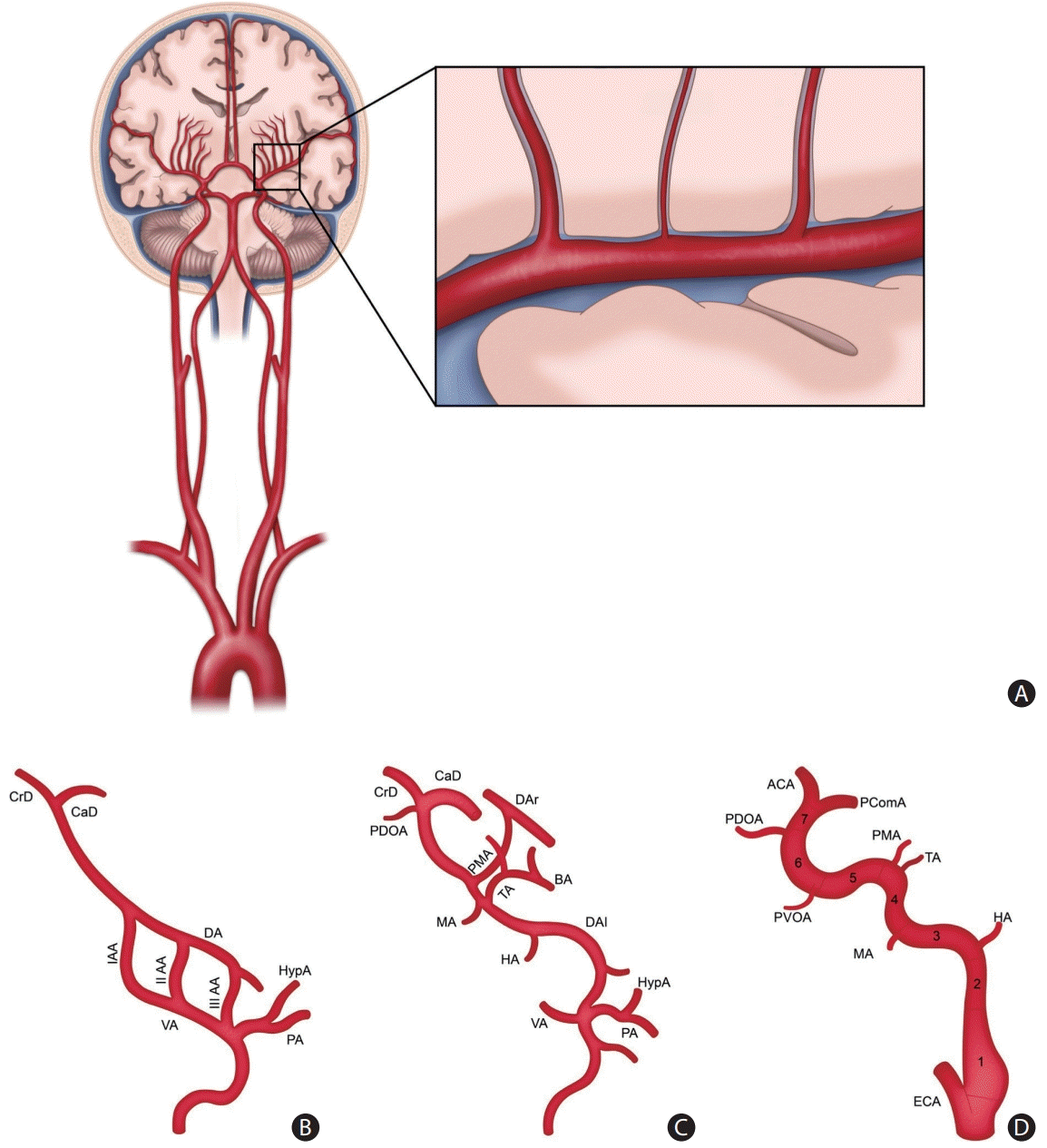 | Figure 1.Overview of cerebrovascular anatomy and embryology. (A) Schematic drawing of the anatomy of the intracranial and extracranial arteries. The middle cerebral artery traverses through the subarachnoid space and extends perforators into the cerebral parenchyma. (B) In the early embryo, the three aortic arches (AA) link the ventral (VA) and dorsal aorta (DA). (C) As the first (I AA) and second aortic arches (II AA) regress, the DA and the third aortic arch (III AA) remain as the primitive internal carotid artery (ICA). The remnant I AA corresponds to the mandibular artery (MA), and the remnant II AA corresponds to the hyoid artery (HA). Other embryonic arteries arise, such as the primitive maxillary artery (PMA), trigeminal artery (TA), and primitive dorsal ophthalmic artery (PDOA). (D) The embryonic arteries that regress divide the primitive ICA into seven segments. The first segment corresponds to the remnant of the III AA. The second segment is the remnant of the DA between the II AA and III AA. The third to seventh segments are divided into the MA, PMA, PDOA, primitive ventral OA (PVOA), and ICA bifurcation. CrD, cranial division; CaD, caudal division; HypA, hypoglossal artery; PA, proatlantal artery; DAr, dorsal aorta right; DAl, dorsal aorta left; BA, basilar artery; ACA, anterior cerebral artery; PComA, posterior communicating artery; ECA, external carotid artery. |
Embryology
Histology
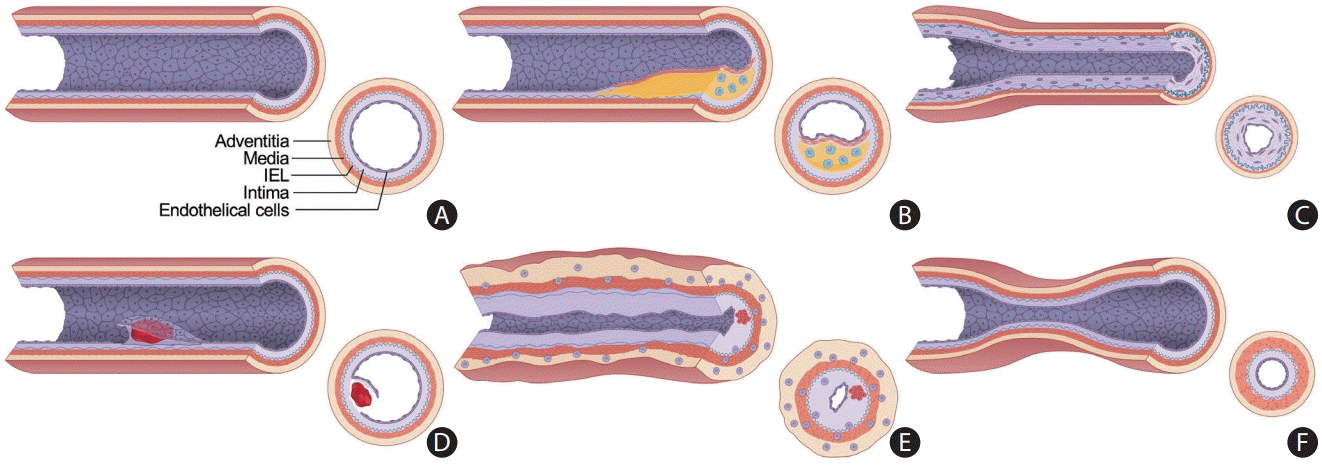 | Figure 2.Schematic diagram displaying the heterogeneity of intracranial arterial diseases. (A) The structure of a normal intracranial vessel wall consists of endothelial cells, intima, internal elastic lamina (IEL), media, and adventitia. There is no external elastic lamina in intracranial arteries. Intracranial arteries have fewer elastin fibers in the media, thinner adventitia, and thicker IEL compared to extracranial arteries. (B) Atherosclerosis is characterized by a lipid core covered by fibrous caps. Infiltrating macrophages are found within the lipid core. More advanced forms of atherosclerosis are infrequently seen in intracranial arteries. (C) Intracranial arteries in moyamoya disease undergo negative remodeling due to fibrosis and thickening of the intima and proliferation of smooth muscle cells. There is also infolding and chronic contraction of the IEL. (D) Intracranial arterial dissection. The intima is torn inward to form an intimal flap and a double lumen. Intramural hematoma is sometimes seen. (E) Mononuclear and granulomatous adventitial inflammation and focal fibrin thrombus formation in vasculitis. Concentric thickening of the vessel wall is seen. (F) In reversible cerebral vasoconstriction syndrome, the diameter of the entire vessel wall is reduced due to the contraction of smooth muscle in the media layer. |
ICAD: heterogeneity in their pathogeneses
ICAD: atherosclerosis
ICAD: RNF213 spectrum disorder or MMD spectrum
ICAD: intracranial dissection
ICAD: vasculitis
Role of high-resolution vessel wall MR imaging in the evaluation of ICAD
Clinical considerations on the image acquisition of HR-VWI
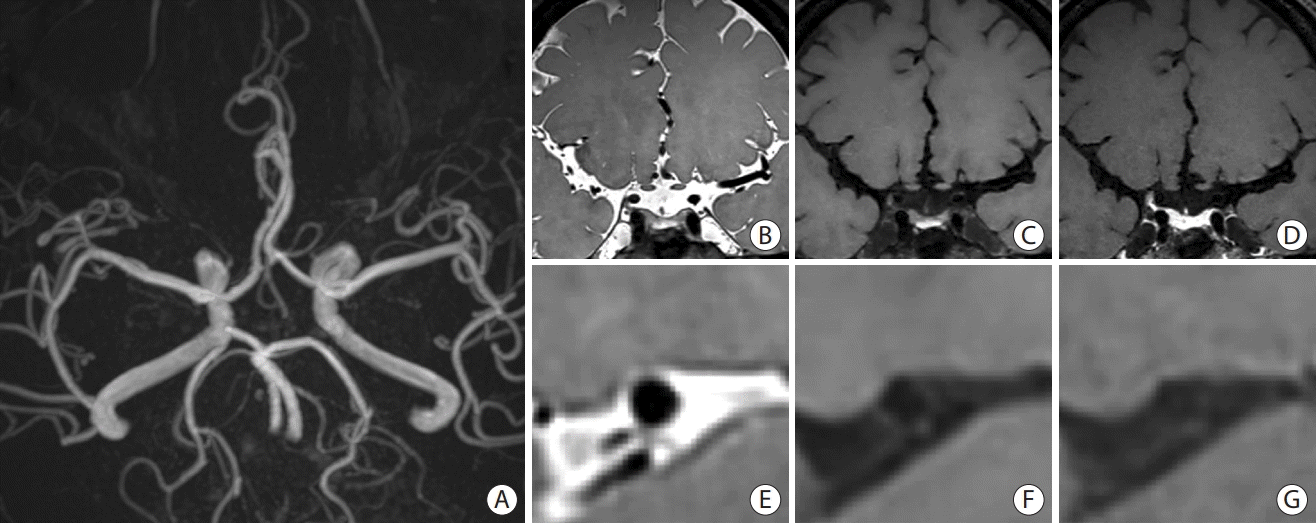 | Figure 3.A representative image of high-resolution vessel wall imaging of normal intracranial arteries. (A) Time-of-flight image. (B-D) Proton density (PD), T1-weighted, and T1-weighted with gadolinium (T1GD) coronal images showing the left middle cerebral artery (MCA). (E-G) PD, T1-weighted, and T1GD coronal image showing a cross-sectional view of the left MCA. |
Table 1.
Histological and pathological correlation of HR-VWI findings
Utilization of high-resolution vessel wall MR imaging in clinical practice
Clinical interpretation of HR-VWI findings in ICAD
ICAD: atherosclerosis
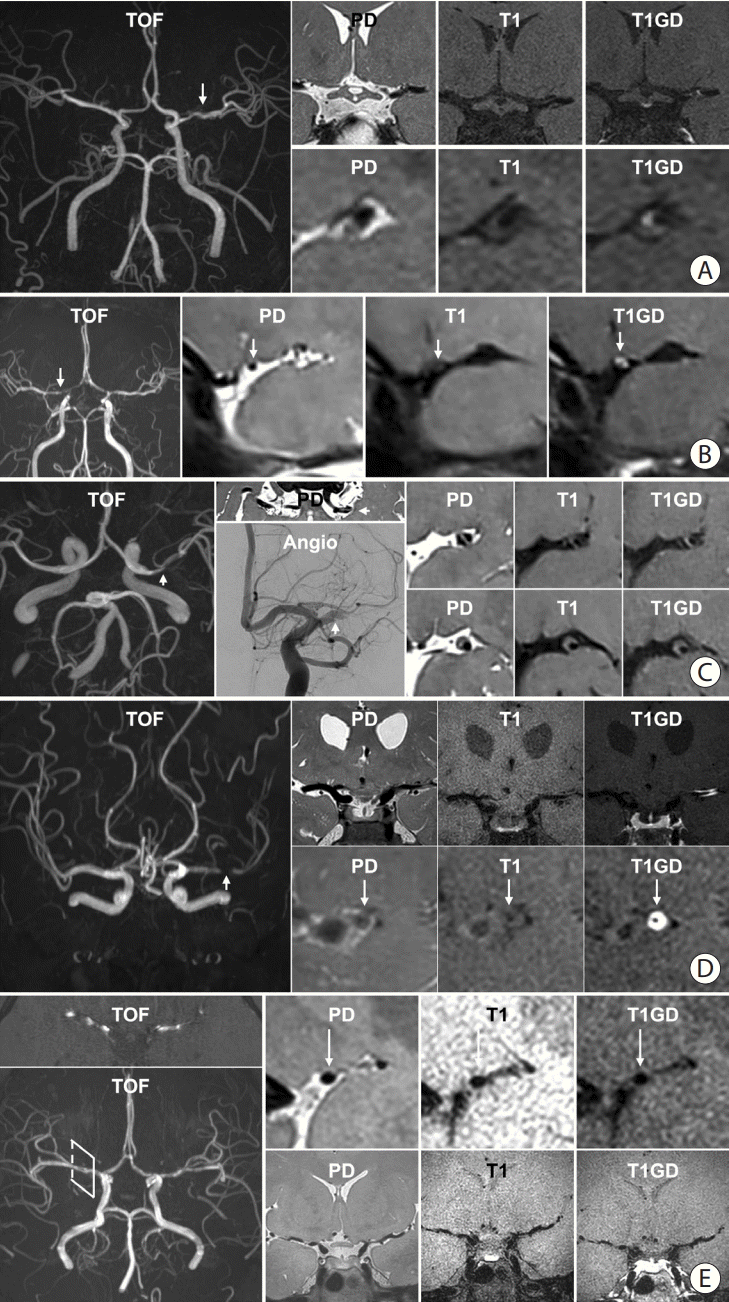 | Figure 4.Representative high-resolution vessel wall imaging (HR-VWI) images of various intracranial arterial diseases. (A) HR-VWI findings of atherosclerosis in a 45-year-old male with prehypertension and hypercholesterolemia. A stenotic portion (arrow) at the left middle cerebral artery (MCA) is identified on the time-of-flight (TOF) image. The proton density (PD) image shows no total vessel wall diameter stenosis. The T1 image shows eccentric vessel wall thickening with isointense signal suggesting a lipid core or fibrous cap. The area appears to be enhanced on the T1-weighted with gadolinium (T1GD) image. (B) HR-VWI in a 35-year-old female with moyamoya disease presenting with right MCA infarction. A heterozygous RNF213 c.14429G>A mutation was found. The TOF image shows stenosis at both distal internal carotid artery (ICA) to proximal A1 and M1. The arrow indicates the most stenotic part of the right MCA. Concentric wall thickening involving the right MCA with enhancement and negative remodeling was noted on PD, T1, and T1GD images. (C) HR-VWI of a left MCA dissection in a 48-year-old male who underwent endovascular treatment due to a left MCA infarction 4 months earlier. TOF-magnetic resonance angiography (MRA) shows a stenotic portion of the left MCA (arrow). The intimal flap is clearly visible on the PD axial image. Angiography at the time of endovascular treatment shows the intimal flap (arrow). PD, T1, and T1GD sagittal images show a double lumen and an intramural hematoma. A lesion with hyperintense PD and hyperintense T1 signal and no enhancement suggests a late subacute stage of the intramural hematoma. (D) HR-VWI in a 47-year-old female with vasculitis. TOF image shows a severely stenotic portion of the left MCA (arrow). PD, T1, and T1GD coronal images show wall thickening and prominent enhancement of the left M1. Sagittal images show concentric wall thickening with negative remodeling with strong enhancement. The stenosis later progressed despite steroid treatment. (E) HR-VWI in a 27-year-old female with reversible cerebral vasoconstriction syndrome presenting with severe headache. TOF-MRA axial image shows multifocal stenoses of both MCAs. Reconstructed TOF-MRA shows multifocal mild to moderate stenoses at the distal ICA, MCA, anterior cerebral artery, and basilar artery. Sagittal images of the right MCA show normal appearance of the vessel wall. Coronal images show stenotic segments without any abnormal vessel wall signal. Intracranial vessels were significantly improved on follow-up HR-VWI. |
ICAD: MMD or RNF213 spectrum disease
ICAD: intracranial dissection
ICAD: vasculitis
ICAD: reversible cerebral vasoconstriction syndrome
Clinical significance of follow-up HR-VWI
Table 2.
| Pathophysiology | Study | Patients | Number | Country | Follow-up | Temporal change in HR-VWI findings |
|---|---|---|---|---|---|---|
| Atherosclerosis | Chung et al. [62] | ≤7 d IS with atherosclerotic ICAD | 77 | Korea | 6 mo | After high dose statin: enhancement↓, wall area index↓, and stenosis degree↓ |
| Shi et al. [71] | <8 wk IS, stenosis >30% | 58 | China | 6.2 mo | Plaque burden progression in FU was associated with recurrent stroke | |
| Lee et al. [72] | Symptomatic atherosclerotic ICAD | 35 | Korea | 12–24 mo | Initial presence and enhancement of plaque was associated with stenosis aggravation | |
| Zhang et al. [73] | Symptomatic atherosclerotic ICAD+EVT | 45 | China | Pre- and post- procedure | Drug-coated balloon angioplasty reduced plaque enhancement (vs. bare balloon angioplasty) | |
| Kwee et al. [61] | Symptomatic atherosclerotic ICAD | 14 | USA | 3–6 mo (6) | Culprit lesion: enhancement not changed | |
| ≥6 mo (6) | Non-culprit lesion: enhancement↓ | |||||
| <3 mo (2) | ||||||
| Shen et al. [60] | Symptomatic atherosclerotic ICAD | 67 | China | 334 d | Recur (+) group: plaque features not changed | |
| Recur (-) group: stenosis ratio↓, plaque burden↓, enhancement ratio↓ | ||||||
| Meng et al. [64] | Symptomatic atherosclerotic ICAD+drug coated balloon angioplasty | 29 | China | <3 mo (4) | Balloon angioplasty: stenosis degree↓, plaque hyperintensity↓, wall enhancement↓ | |
| 3–6 mo (14) | ||||||
| >6 mo (11) | ||||||
| Lin et al. [63] | Symptomatic atherosclerotic ICAD | 87 | China | 8 mo | Culprit plaque: plaque length↓, maximum thickness↓, NWI↓, stenosis degree↓, plaque contrast ratio↓ | |
| Nonculprit plaque: NWI, stenosis degree↓ | ||||||
| Xiao et al. [74] | Symptomatic atherosclerotic ICAD | 29 | China, USA | 8 mo | Recurrent group: maximum wall thickness↑, plaque-wall contrast ratio↑, plaque enhancement ratio↑ | |
| Huang et al. [75] | Symptomatic atherosclerotic ICAD | 37 | China | 3, 6, 9, 12 mo | 12 mo of medical treatment: plaque burden↓, enhancement↓ | |
| 6 mo after balloon angioplasty: plaque burden↓ | ||||||
| Wu et al. [65] | Severe atherosclerotic ICAD+angioplasty and stenting | 24 | Taiwan | 24 h, 4.5 mo | HR-VWI ≤24 h can predict a 1-year outcome following intracranial stenting | |
| Dissection | Kwon et al. [76] | Dissection | 43 | Korea | 40 d | Extracranial dissection group: stenosis improved, residual wall enhancement↓, intramural hematoma↓ |
| Lee et al. [66] | <7 days IS | 17 | Korea | 3–12 mo | Hematoma disappears in 6 months. Most lesions healed in 12 months. | |
| MMD | Kim et al. [56] | Adult MMD | 66 | Korea | 30 mo | Negative remodeling occurred less in the cilostazol group (vs. other antiPLT vs. no antiPLT) |
| Lu et al. [67] | Adult MMD | 170 | China | 9.9 mo | Initial presence and progression of enhancement was associated with rapid stenosis progression. Vessel wall enhancement and rapid stenosis progression was associated with recurrent stroke. | |
| Vasculitis | Shimoyama et al. [38] | CNS vasculitis | 9 | Japan | 15.6 mo | Relapsed cases: enhancement↑ |
| Intensive immunosuppressive Tx: enhancement↓ | ||||||
| Cho et al. [36] | Varicella zoster vasculopathy | 1 | Korea | 2, 5 mo | After steroid and valacyclovir: enhancement↓ | |
| Song et al. [39] | Pediatric tuberculosis vasculitis | 1 | USA | 13, 21, 99, and 237 d | After anti-tuberculosis medication: leptomeningeal, wall, and parenchymal enhancement↓ | |
| Patzig et al. [37] | CNS vasculitis | 45 | Germany | 239.5 d | Enhancement (+): relapse↑ | |
| Kang et al. [77] | CNS vasculitis | 41 | China | 5.3 mo | Less enhancement: stenosis improved | |
| Yeo et al. [68] | Antiphospholipid syndrome | 8 | Korea | 110 d | Extremely high B2GPIs titer → diffuse narrowing↑ despite medical Tx | |
| RCVS | Choi et al. [69] | Atypical RCVS | 1 | Korea | 1 wk, 1 mo | After nimodipine: stenosis improved |
HR-VWI, high-resolution vessel wall imaging; ICAD, intracranial arterial disease; IS, ischemic stroke; FU, follow-up; EVT, endovascular treatment; NWI, normalized wall index; MMD, moyamoya disease; antiPLT, antiplatelet agent; CNS, central nervous system; RCVS, reversible cerebral vasoconstriction syndrome; B2GPIs, beta-2-glycoprotein I.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download