Abstract
Cochlear hair cells convert sound into electrical signals that are relayed via the spiral ganglion neurons to the central auditory pathway. Hair cells are vulnerable to damage caused by excessive noise, aging, and ototoxic agents. Non-mammals can regenerate lost hair cells by mitotic regeneration and direct transdifferentiation of surrounding supporting cells. However, in mature mammals, damaged hair cells are not replaced, resulting in permanent hearing loss. Recent studies have uncovered mechanisms by which sensory organs in non-mammals and the neonatal mammalian cochlea regenerate hair cells, and outlined possible mechanisms why this ability declines rapidly with age in mammals. Here, we review similarities and differences between avian, zebrafish, and mammalian hair cell regeneration. Moreover, we discuss advances and limitations of hair cell regeneration in the mature cochlea and their potential applications to human hearing loss.
Hearing loss is a devastating disability that impairs everyday communication. The World Health Organization estimates that it affects more than 1.5 billion people globally at present and predicts that it will affect 2.5 billion by 2050 [1]. Sensorineural hearing loss accounts for a considerable proportion of all hearing impairment and is primarily caused by the loss of cochlear hair cells [2,3]. Hair cells are specialized mechanoreceptors essential for hearing and are interspersed with non-sensory supporting cells in the mammalian and non-mammalian cochlea [4]. Hair cells are also present in the vestibular organs of both mammalian and nonmammalian species, as well as the lateral line system in aquatic vertebrates such as fish [5,6]. Cochlear hair cells do not regenerate in mature mammals, leading to irreversible hearing loss in mice and humans [7-9]. Neonatal mice and rats have a limited ability to regenerate lost hair cells, but this ability is lost after the first postnatal week [10-14]. Unlike mammals, mature avian hair cells can spontaneously regenerate after damage, almost completely restoring cochlear function [15-23]. Similarly, the zebrafish lateral line can fully regenerate lost hair cells and restore its ability to detect underwater currents [24-27]. In both birds and zebrafish, supporting cells have the capacity to regenerate hair cells [18,20,26,28-33]; thus, non-mammalian sensory organs serve as vital model systems for studying mechanisms of hair cell regeneration. In the avian cochlea, hair cells regenerate by two distinct mechanisms: mitotic regeneration and direct transdifferentiation (Fig. 1). In mitotic regeneration, supporting cells re-enter the cell cycle, divide, and produce daughter cells, which give rise to more supporting cells and/or new hair cells, whereas in direct transdifferentiation, the molecular signature of supporting cells is altered, resulting in direct transformation into new hair cells without intervening mitosis [18,20,28,30,34-36]. Over the past decade, studies have revealed novel mechanisms regulating hair cell differentiation and proliferation in sensory organs of mammals and non-mammals [37-39].
In this review, we first discuss the similarities and differences in hair cell regeneration in the neonatal mouse cochlea and mature sensory organs of non-mammalian vertebrates. In addition, we summarize recent studies on promising approaches to stimulate regeneration in the mature mammalian cochlea and their potential applications for treating human hearing loss.
The chicken cochlea (basilar papilla) does not undergo proliferation or hair cell turnover during homeostasis. However, it mounts a robust proliferative and regenerative response after damage [28]. Hair cells are replaced via both mitotic regeneration and direct transdifferentiation in spatially and temporally specific manners [40]. In the neural region, where tall hair cells reside, mitotic regeneration predominates, whereas direct transdifferentiation is the primary mode of regeneration in the abneural region where short hair cells are located (Fig. 1) [18,34,41]. Direct transdifferentiation occurs first (within 24 hours after hair cell damage), while mitotic regeneration occurs a few days later [34,35]. Interestingly, supporting cells are more densely packed in the abneural region than in the neural region, and the ratio of supporting cells to hair cells is significantly higher in the former [34]. Whether cell density contributes to the mechanisms of hair cell regeneration remains to be determined.
More recently, single-cell RNA sequencing (scRNA-seq) studies have further characterized the heterogeneity of supporting cells in the basilar papilla [41-43]. Using undamaged chicken basilar papilla, Janesick et al. [41] have identified two transcriptionally distinct subgroups of supporting cells: those in the neural region marked by lecithin-cholesterol acyltransferase (Lcat), glioma pathogenesis-related protein 1-like (Glipr1l), and Dickkopf WNT signaling pathway inhibitor 3 (Dkk3) and those in the abneural regions marked by netrin-4-like (Ntn4l), secreted protein acidic cysteine rich related modular calcium binding 2 (Smoc2), and tissue inhibitor of metalloproteinase 3 (Timp3) (Table 1). Using microarrays, researchers examined the transcriptome of chicken sensory epithelium during hair cell regeneration [44,45]. Signaling pathways such as transforming growth factor-β (Tgf-β), Notch, Wnt, nuclear factor kappa B (Nfκb), insulin-like growth factor 1 (Igf1), and activating protein 1 (Ap-1) were found to be upregulated during hair cell regeneration according to large-scale gene analysis [44]. Moreover, in vitro experiments using small interfering RNA (siRNA) showed that paired box (Pax), Wnt, and Ap-1 pathways are required for supporting cell proliferation [45]. Recently, Benkafadar et al. [42] used scRNA-seq to analyze the transcriptome of degenerating hair cells in the aminoglycoside-damaged basilar papilla. Both hair cells in the neural and abneural regions of the basilar papilla showed activated stress signaling and apoptosis pathways. However, only dying hair cells in the abneural region downregulated adenosine monophosphate-activated protein kinase (AMPK) signaling and upregulated potassium voltage-gated channel subfamily Q member 1 (Kcnq1) and potassium voltagegated channel subfamily E regulatory subunit 1 (Kcne1) expression, suggesting that they might play a role in maintaining potassium conductance [42]. This study suggests that dying hair cells in different regions of the basilar papilla respond differently to damage; however, the mechanisms regulating mitotic regeneration and direct transdifferentiation in the neural and abneural regions, respectively, remain to be determined. The authors also postulated that the initial events that trigger regeneration may originate from dying hair cells rather than supporting cells [42], an intriguing concept that warrants further investigation.
Compared to the undamaged avian basilar papilla, post-damage regeneration involves the upregulation of inflammation and immune responses such as interferons [39,41]. Transcriptomic analyses of supporting cells in the damaged basilar papilla have revealed the upregulation of immune genes [39]. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, which plays important roles in carcinogenesis and inflammation [46], is essential for the upregulation of damage-response immune genes such as interferon alpha inducible protein 6 (Ifi6), interferon induced protein with tetratricopeptide repeats 5 (Ifit5) and radical S-adenosyl methionine domain containing 2 (Rsad2) in supporting cells after hair cell loss, and the inhibition of JAK/STAT signaling using ruxolitinib, an inhibitor of both JAK1 and JAK2, has been shown to prevent the upregulation of immune-related genes in supporting cells [39]. These immune related genes are not expressed in newly regenerated hair cells after damage; instead, those cells highly express ubiquitin specific peptidase 18 (Usp18) and suppressor of cytokine signaling 3 (Socs3), which are negative regulators of JAK/STAT signaling, suggesting that newly regenerated hair cells may participate in a negative feedback loop with the JAK/STAT signaling response [39]. While immune-related genes linked to JAK/STAT signaling are robustly upregulated in supporting cells after hair cell damage, future studies are needed to determine if the increase of immune-related genes observed is a trigger for regeneration. At present, the role of immune response during hair cell regeneration is unclear.
Atonal basic helix-loop-helix transcription factor 1 (Atoh1), a transcription factor required for hair cell development, is normally absent in mature hair cells or supporting cells during homeostasis. After damage, supporting cells in the basilar papilla re-express Atoh1 during the process of transdifferentiation and mitotic regeneration [34], suggesting that Atoh1 plays a key role in directing the transformation of supporting cells into hair cells during regeneration. Single-cell transcriptomic analyses have found Atoh1 expression to be upregulated in supporting cells after damage in vitro and shown that regenerated hair cells express both early (growth factor independent 1 transcriptional repressor [Gfi1], LIM homeobox 3 [Lhx3], and RNA binding motif protein 24 [Rbm24]) and late markers (oncomodulin [Ocm], Myosin7a, and solute carrier family 34 member a2 [Slc34a2]) of hair cell differentiation [39,47]. However, some postmitotic cells from 7- to 10-day-old post-hatch chicks with high Atoh1 levels can differentiate into supporting cells in vitro [48], suggesting that Atoh1 expression alone may be insufficient to drive progenitor cells towards a hair cell fate.
Cell fate specification is a process that likely depends on the interplay of multiple signaling pathways, including Notch, fibroblast growth factor (FGF), Wnt, and bone morphogenetic protein (BMP) signaling [49-52]. For example, activating Wnt signaling in the basilar papilla in vitro increased the proliferation of supporting cells, while inhibition of Wnt signaling prevented the proliferation and differentiation of regenerated hair cells in vitro [52]. Meanwhile, fibroblast growth factor receptor 3 (Fgfr3) is expressed in the mature chicken basilar papilla and is downregulated after damage [49]. In the initial stages of hair cell regeneration, Fgfr3 mRNA is absent in supporting cells that have reentered the cell cycle. As hair cells are newly regenerated, Fgfr3 mRNA and protein levels are restored to baseline levels, suggesting that the downregulation of Fgfr3 may be important for supporting cell division [49]. Additionally, in the chicken basilar papilla, Fgf-2 inhibited supporting cell proliferation after hair cell damage [8].
Bmp4 is expressed in mature hair cells and is lost after damage, prior to an increase in Atoh1 mRNA expression [50]. Inhibiting Bmp4 signaling in vitro promotes the upregulation of Atoh1 in supporting cells and subsequent hair cell regeneration, while treatment with Bmp4 after hair cell damage prevents Atoh1 expression and division of supporting cells [50]. Taken together, this suggests that Bmp4 in hair cells acts as a negative regulator of hair cell fate and proliferation of supporting cells [50].
More recently, Gomez-Dorado et al. [37] found that the adenovirus early gene 2 binding factor (E2F) transcription factor activates Atoh1 expression in the basilar papilla, and that this upstream regulator is absent in mammals, which may contribute to the lack of regeneration in the mammalian cochlea. Gomez-Dorado et al. [37] found that chickens have three 3´ enhancers, two of which share similar homology with one of the Atoh1 enhancers in the developing mouse cochlea [53], whereas the third one is unique to chickens and zebrafish. It is possible that the regenerative ability of the avian basilar papilla is in part due to the activation of Atoh1 expression by E2f1.
The Notch pathway plays a crucial role during cochlear development by regulating hair cell fate through the process of lateral inhibition [54]. In the undamaged basilar papilla, supporting cells express the Notch1 receptor and Serrate1 ligand, while the expression of Delta1 (another Notch ligand) is not detected in either hair cells or supporting cells [19]. Notch activity in the undamaged basilar papilla most likely regulates cell fate (e.g., prevents supporting cells from converting into hair cells), since Notch signaling regulates lateral inhibition. Moreover, a low level of Notch pathway activity may help maintain mitotic quiescence in the sensory epithelium [19]. However, after ototoxic damage, Delta1 expression increases in supporting cells and expression remains high as they differentiate into hair cells [55]. In the undamaged epithelium, in vitro inhibition of Notch activity with a γ-secretase inhibitor (DAPT) had no effect on supporting cells. However, in damaged epithelia treated with DAPT, Atoh1 transcripts increased compared to vehicle-treated cultures and hair cells were regenerated by supporting cells via both mitotic and non-mitotic mechanisms [51]. Therefore, Notch signaling prevents supporting cells from regenerating excessive hair cells after damage, although this pathway appears to be expressed at low levels under homeostatic conditions and the degree of Atoh1 upregulation is correlated with increasing Notch inhibition via DAPT [51]. Thus, during hair cell regeneration, Notch signaling plays a pivotal role in maintaining supporting cells by acting as a negative regulator of Atoh1.
Vascular endothelial growth factor (VEGF), which promotes angiogenesis and endothelial cell proliferation, is expressed in hair cells, while supporting cells express the corresponding receptors VEGFR1 and VEGFR2 [56,57]. In organotypic cultures of the avian cochlea that were damaged by streptomycin, inhibiting VEGF receptors decreased both supporting cell proliferation and the number of regenerated hair cells [57]. Conversely, recombinant human VEGFA increased both supporting cell division and the regeneration of hair cells in vitro, indicating that VEGF signaling is both necessary and sufficient to increase avian hair cell regeneration. Further research is needed to determine how VEGF signaling affects hair cell regeneration in other species, such as zebrafish and mammals.
The zebrafish lateral line system runs along the body’s surface and detects water currents [15,58-60]. Because of the superficial location of the lateral line system, ease of live imaging, and available genetic tools, zebrafish are an excellent model system for studying the development and regeneration of hair cells [25,61-63]. The lateral line consists of individual neuromasts, which runs along the body’s surface [58,59]. Neuromasts are sensory patches made up of about 20 hair cells that are surrounded by supporting cells (Fig. 2) [64]. The structure of neuromast and neural connections resemble those found in the inner ear sensory organs [65]. Even though fish also have inner ear organs containing hair cells and supporting cells [66], most hair cell regeneration studies in fish to date have examined the lateral line system.
In the lateral line, hair cells undergo constant turnover, replenished by supporting cells, throughout the life of the animal [25,26]. After damage, hair cells are rapidly replaced via mitotic regeneration [67,68] and transdifferentiation of supporting cells [32,69-71], with the former playing the dominant role during regeneration [72]. However, the significance of the latter’s role remains a matter of debate [70,71,73]. Like the chicken basilar papilla, hair cell loss elicits differential responses from lateral line supporting cells, suggesting a degree of heterogeneity among them. Cruz et al. [24] used transgenic fish (myo6b: NLS-Eos) to track hair cell regeneration after damage, and found that supporting cells regenerate hair cells via three regionally and temporally distinct mechanisms—those in the anterior-posterior pole undergo slow cell division prior to regenerating hair cells, whereas those in the dorso-ventral pole undergo rapid cell division before regenerating hair cells, further contrasting with those in the center that directly transdifferentiate into new hair cells [24,74]. These findings suggest that supporting cells in the dorsoventral regions are the most proliferative and regenerative. In support of the existence of distinct supporting cell subtypes, time-lapse imaging and scRNA-seq revealed that transdifferentiation and mitotic regeneration take place in distinct spatial compartments in the lateral line [74]. Two recent studies similarly identified three spatially segregated groups of supporting cells that regenerate hair cells [38,73]. By contrast, a recent study showed that many supporting cells from the dorso-ventral poles can convert into hair cells without cell division [73]. Therefore, whether regionally distinct mechanisms of hair cell regeneration (i.e., mitotic regeneration in dorso-ventral vs. transdifferentiation in central regions) exist in the lateral line system remains unresolved. Supporting cells may decide their fate according to their location in the neuromast both during homeostasis and regeneration. Interestingly, supporting cells in the dorso-ventral poles regenerated after selective ablation, implying that proliferation is not solely triggered by hair cell damage [73].
In the lateral line of zebrafish, hair cell regeneration occurs rapidly after ototoxic damage, with regenerated hair cells detected as early as five hours after damage [38]. As mentioned above, there exist at least three distinct groups of supporting cells within the neuromast; thus, one may hypothesize that there are regionally distinct signaling events that govern their behavior [73]. As a well-known regulator of stem cell renewal and regeneration, Wnt signaling promotes supporting cell proliferation during both homeostasis and regeneration in the zebrafish lateral line. In contrast, inhibition of the Wnt pathway by hs:dkk2, which blocks the binding of Wnt ligands, prevents proliferation of supporting cells but does not affect regeneration [74,75]. It is unlikely, however, that the Wnt/β-catenin signaling pathway is the initiating signal for regeneration because it is not upregulated until hours after damage-induced proliferation begins [75,76]. In addition, the Wnt/β-catenin signaling pathway can interact with the FGF pathway, which is also known to promote proliferation and regeneration, possibly through its interaction with the Wnt/β-catenin signaling pathway [77,78]. Inconsistent evidence has been reported regarding the involvement of FGF signaling in regeneration. The expression of FGF genes and their receptors is downregulated immediately after hair cell death [76,78]. Additionally, fgf3-deficient mutant larvae (fgf3-/-) show more supporting cell proliferation during homeostasis and regeneration, and scRNA-seq has revealed the activation of Wnt pathway genes after hair cell damage in fgf3-/- larvae [78]. In contrast with these findings, Tang et al. showed that FGF upregulation (via bFGF treatment) partially restored the proliferation of supporting cells after proliferation was reduced by Wnt inhibition [77]. Neomycin-damaged larvae exposed to bFGF exhibited more proliferation of supporting cells in their neuromast compared to controls, which was followed by enhanced hair cell regeneration [77]. Further, pharmacological inhibition of the FGF receptor using SU5402 increased Wnt target gene expression and decreased proliferation and hair cell regeneration [77]. Collectively, these conflicting findings on the FGF pathway in hair cell regeneration of zebrafish suggest that its role remains unclear.
More recently, the yes-associated protein (yap)-lin-28 homolog a (lin28a) pathway has been shown to play a role in progenitor cell repopulation after severe damage [79]. Lin28a has been described to regulate progenitor proliferation in the developing mouse inner ear by inhibiting let7 microRNA processing, with the Wnt pathway acting downstream of lin28a [80]. Ye et al. [79] established a severe damage injury paradigm, where both hair cells and supporting cells were depleted in the zebrafish lateral line. In this damage model, the yap-lin28a signaling pathway promotes repopulation of the sex determining region Y-box 2 (sox2)+ progenitors by regulating the let7-Wnt pathway and increasing Lin28a expression, which are both necessary and sufficient for restoring the depleted sox2+ progenitors during regeneration [79]. Following damage, there is an initial upregulation of Atoh1a+ hair cell precursor cells, which also begin expressing sox2 [79]. Interestingly, the number of sox2+ cells decreased after yap inhibition (using verteporfin) and this reduction was rescued by lin28a overexpression [79]. Further, overexpression of let-7 inhibited lin28a-induced proliferation, suggesting that let7 acts downstream of lin28a [79]. Conversely, lin28 is downregulated by let-7, and the suppression of let-7 activity led to the upregulation of lin28 in neural stem cells in vitro, suggesting a feedback loop between lin28 and let-7 [80,81]. In the mammalian cochlea, inducing hair cell regeneration via Atoh1 overexpression in supporting cells alone may be ineffective because it can exhaust the supporting cell population. Based on the results of this zebrafish study, replacing the depleted sox2+ progenitors via the yap-lin28a pathway in tandem with Atoh1 overexpression may be a promising approach for sustainable regeneration in mammals.
Notch signaling is active in homeostatic neuromasts, and is downregulated after damage, suggesting that Notch downregulation is essential for initiating regeneration and its inhibition stimulates supporting cell proliferation after hair cell damage [73,74]. Notch signaling inhibits the conversion of supporting cells to hair cells in the center of the neuromast; however, in the dorso-ventral poles, Notch signaling decreases the proliferation of supporting cells by inhibiting Wnt signaling [74,78]. Furthermore, due to the role of Notch signaling in lateral inhibition, sustained loss of signaling results in hair cell overproduction [76]. Active Notch signaling during regeneration reverts supporting cells to mitotic quiescence and maintains the proper hair cell number [72,74,78]. In addition, Notch signaling is spatially restricted in regenerating neuromasts, such that the Notch ligand delta is expressed in the dorso-ventral poles of supporting cells, while the Notch ligand jagged2b is expressed in the center of neuromasts [74]. Additionally, Notch receptor expression is heterogeneous, with notch3 being highly expressed in anterior-posterior poles of supporting cells in the neuromast and is thought to maintain supporting cell quiescence [70,73,74]. Notch inhibition significantly increased hair cell regeneration after damage, particularly in the anterior-posterior poles relative to the dorsoventral poles, indicating that supporting cells in the anterior-posterior poles are regulated to a greater extent by Notch signaling [73]. Collectively, these findings highlight the complex nature and interplay of an array of pathways guiding hair cell regeneration. Thus, the precise modulation of multiple pathways, including Notch and Wnt/β-catenin, may serve as a promising strategy for promoting hair cell regeneration in other species.
Baek and colleagues reported that hair cell regeneration occurs in three sequential phases based on transcriptomic analyses: (1) an acute inflammatory response capable of initiating hair cell regeneration, (2) temporary activation of regeneration-specific genes (between 30 minutes and one hour after hair cell ablation), and (3) activation of hair cell specification genes (between 3 and 10 hours after hair cell ablation) [38]. While hair cell regeneration and development share some characteristics, the acute inflammatory response is specific to regeneration. In addition, BRS-28 is an anti-inflammatory agent that suppresses proinflammatory factors such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α and nitric oxide. Reducing macrophage migration impedes regeneration regeneration as well as functional recovery of the neuromasts, as assessed via rheotaxis behavior [82]. However, conflicting evidence has been reported on the role of macrophages in hair cell regeneration, and this topic warrants further investigation. While macrophages migrate rapidly to the neuromasts after hair cell damage, macrophage-depleted fish do not show defects in hair cell regeneration, suggesting that macrophages are not required for hair cell regeneration [83]. Another study showed that in response to hair cell damage, glucocorticoid activation is initially seen in macrophages, followed by IL-10 signaling and then oxidative phosphorylation by IL-4/polyamine signaling, with both IL-4 and IL-10 being necessary for synaptogenesis of regenerated hair cells [84]. Whether these inflammatory states are directly related to hair cell damage or regeneration in zebrafish remains unclear. In the mammalian cochlea, several studies have found an influx of macrophages after damage [85-89]. However, as hair cell regeneration does not occur in the mature mammalian cochlea, it is unknown what role these cells play after damage, whether it be to assist in repair and the phagocytosis of dying hair cells, or to inhibit regeneration. Future research comparing macrophages and inflammation after cochlear hair cell damage in mammals and non-mammals is therefore essential and may help us better understand their roles in hair cell regeneration.
Investigating the differences in hair cell regeneration between zebrafish and mammals could potentially provide insights into the differential ability to regenerate hair cells between these two taxa. Further, genetic conservation in hair cell development makes the zebrafish a valuable model for discovering novel genes specific to regeneration.
The mature mammalian cochlea lacks the ability to proliferate or regenerate hair cells after damage, resulting in permanent hearing loss (Fig. 3) [5,6]. Numerous supporting cell subtypes are found in the neonatal and mature cochlea, including inner border cells (IBCs), inner phalangeal cells (IPhCs), Hensen cells, Deiters’ cells (DCs), Claudius cells, and inner and outer pillar cells, each with unique gene expression profiles [90]. Independent studies have reported that modest spontaneous hair cell regeneration occurs through both mitotic division and direct transdifferentiation when hair cells are damaged in the neonatal mouse cochlea (Fig. 4) [13,91]. The regenerated hair cells can form synaptic connections, and they express terminal differentiation markers of both inner hair cells (vesicular glutamate transporter 3, VGlut3) and outer hair cells (Ocm and prestin) after damage in the neonatal mouse cochlea [92]. However, this ability is lost after the first week of age [13].
Leucine rich repeat containing G protein coupled receptor 5 (Lgr5), a Wnt target gene that marks stem cells in self-renewing organs [93], is expressed in several supporting cell subtypes in the neonatal mouse cochlea [94,95]. When isolated and cultured, Lgr5+ supporting cells can act as hair cell progenitors [94,95]. After hair cell damage in vivo and in vitro, a modest number of regenerated hair cells arise from Lgr5+ supporting cells, especially in the apical region [13,91], which can be augmented by inhibiting Notch signaling to increase the regenerative response [91]. Interestingly, supporting cell subtypes other then Lgr5+ supporting cells can also contribute to hair cell regeneration in the neonatal cochlea [96]. For example, one study reported that the Wnt receptor Frizzled9 marks supporting cells that can generate similar numbers of new hair cells as Lgr5+ progenitors both in vivo and in vitro [97]. As Frizzled9 is expressed in fewer supporting cell subtypes (IPhCs, IBCs, and the third row of DCs) than in those expressing Lgr5, Frizzled9-expressing cells may represent cells with higher regenerative potential [97]. Taken together, these studies suggest that neonatal cochlear supporting cells may retain some regenerative capacity.
Supporting cells in the mature cochlea do not proliferate or regenerate, indicating that these cells and/or their environment are rather different from the immature mammalian cochlea. However, supporting cells retain a level of plasticity and can be converted to hair cell-like cells [98-101]. The mechanisms underlying this regenerative capacity of supporting cells are beginning to be revealed [102]. Relative to organoids derived from older mouse cochleae (postnatal day 5), those from younger cochleae (postnatal day 2) formed significantly more hair cells [103,104]. The transgenic overexpression of RNA binding protein lin28b, which regulates metabolism, stemness and tissue repair, led to an increase in hair cells and upregulated the hair cell transcription factors Atoh1 and POU class 4 homeobox 3 (Pou4f3, also known as Brn3.1) in organoids derived from postnatal day 5 cochleae [103]. Conversely, suppressing lin28b by overexpressing let-7 reduced hair cell production and Atoh1 expression in organoids derived from postnatal day 2 cochleae [103]. In a follow up study, Li et al. [105] discovered that activation of lin28b upregulates follistatin (a TGF-β superfamily protein that functions as an activin antagonist) [106] and that co-activation of follistatin and lin28b allows supporting cells in cochlear organoids to proliferate and generate new hair cells in vitro and in neonatal mice in vivo [105]. Follistatin is required to counteract TGF-β signaling by lin28b. Follistatin knockdown diminishes the ability of lin28b to promote hair cell production, while knockdown of TGF-β enhances it [105]. Taken together, lin28b and follistatin can promote supporting cell plasticity in the neonatal mouse cochlea. As noted above, yap can induce lin28a expression through the Wnt signaling pathway after severe damage of neuromasts in zebrafish [79]. Future studies are required to identify whether Yap upregulation can induce the expression of lin28a/b in the mammalian cochlea.
Tao and colleagues similarly reported that the ability of supporting cells to transdifferentiate into hair cells was mostly lost by postnatal day 6 in mice [107]. In postnatal day 1 cochlear supporting cells, hair cell gene enhancers are epigenetically primed by monomethylation of histone H3 at lysine 4 (H3K4me1) but silenced with the presence of trimethylation of histone H3 at lysine 27 (H3K27me3) [107]. The inhibition of Notch signaling removes this epigenetic silencing, allowing Atoh1 activation and subsequent conversion into hair cells [107]. This primed but silenced state gradually disappears by postnatal day 6 in maturing support cells as a result of the loss of H3K4me1 [107]. Epigenetic barriers are gradually established in the neonatal cochlea, contributing to the inability of adult cochlear supporting cells to transdifferentiate into hair cells. Further comparative epigenomic analyses of cochlear supporting cells at distinct postnatal ages and between damaged and undamaged organs should help reveal these epigenetic barriers.
In neonatal mice, after ablation of the supporting cell subtypes IBCs and IPhCs in vivo using transgenic mice (Plp1Cre; Rosadiphtheria toxin fragment A), Mellado Lagarde et al. [108] found that they spontaneously regenerated, presumably by surrounding cells in the greater epithelial ridge (GER) via non-mitotic mechanisms. However, regeneration of supporting cells does not occur in the damaged mature cochlea. The GER (also known as the Kolliker’s organ) is a transient structure in the neonatal cochlea consisting of columnar epithelial cells medial to the organ of Corti. When isolated and cultured, GER cells can differentiate and expand into hair cell-bearing organoids [109]. By combining a different supporting cell ablation model and a lineage tracing approach, Udagawa et al. similarly found that IPhCs are regenerated by GER cells in vivo in neonatal mice [110]. In contrast to previous findings, they reported that GER cells undergo proliferation before regenerating IPhCs, with more robust proliferation seen with increasing degrees of damage [110]. These studies indicate that the neonatal cochlear supporting cells and surrounding cells can regenerate both lost hair cells and supporting cells. The findings warrant further investigation comparing supporting cells in the immature and mature mammalian cochlea.
Atoh1 overexpression alone is sufficient in inducing supporting cells to acquire a hair cell fate in the neonatal mouse cochlea [111]. The rate of hair cell conversion is different among supporting cell subtypes and is particularly high among IPhCs, IBCs, and cells in the GER [111-113]. These Atoh1-induced ectopic hair cells, however, appear immature, roundly shaped and display shorter bundles than native hair cells [113,114]. In the mature cochlea, Atoh1 overexpression alone can also promote supporting cells converting into hair cells [115], albeit to a very limited extent [101], indicating that additional factors are needed to increase efficiency. Several studies have explored other approaches that can increase Atoh1-responsiveness. One study found that transient co-activation of Myc and Notch genes induces the proliferation of supporting cells, which become more responsive to Atoh1 overexpression and in turn generate more hair cell-like cells in the mature cochlea in vitro and in vivo [99]. Walters et al. [100] reported that concomitant deletion of p27kip1 (a cell cycle inhibitor that maintains quiescence of cochlear supporting cells) and ectopic Atoh1 expression increases the rate of conversion from supporting cells to hair cells in a non-mitotic manner in the adult cochlea in vivo. Moreover, they found that Gata3 or Pou4f3 activation in conjunction with Atoh1 also results in more effective conversion of supporting cells into hair cell-like cells in the adult cochlea than Atoh1 alone [100].
Gfi1 is a zinc finger transcription factor that plays an important role in hair cell development, a transcriptional repressor of neuronal cell fate, and an off-DNA transcriptional coactivator of Atoh1, where Gfi1 can interact with Atoh1 without directly binding to DNA [116-118]. A recent study revealed that co-expression of Atoh1 and Gfi1 improved the efficiency of supporting cell to hair cell conversion relative to Atoh1 alone in the mature mouse cochlea in vivo [98]. Similarly, the ectopic expression of Atoh1 and Isl1 (LIM domain transcription factor) produces significantly more regenerated hair cells both in vivo and in vitro than Atoh1 overexpression alone in the mature cochlea [101]. While multiple transcription factors can convert supporting cells to hair cells and the co-expression of multiple of these factors enhances the degree of conversion, these hair cells remain immature. As such, further studies are needed to understand how, and which transcription factors are necessary to produce mature hair cells.
The transcription factor Pou4f3, a downstream target of Atoh1, is a key hair cell survival factor and is expressed by ectopic hair cells in vitro [119] and during development [120]. In addition to promoting hair cell survival, Pou4f3 increases access to the enhancer network of Atoh1 and thereby creates a feed-forward mechanism in the neonatal cochlea [121]. As the overexpression of Gfi1, Pou4f3, and Atoh1 has been shown to be than Atoh1 alone in generating hair cells from embryonic stem cells [122], several groups have taken this approach to increase the efficiency of hair cell regeneration in the mammalian cochlea. By co-expressing Gfi1, Pou4f3, and Atoh1 in the neonatal cochlea, two independent groups found robust generation of hair cells from both the organ of Corti supporting cells and the GER [113,123]. Compared to Atoh1 only, the co-expression of Gfi1, Pou4f3, and Atoh1 significantly increased the number of regenerated hair cells with gene expression similar to that of native hair cells (transmembrane channel like 1 [Tmc1], transmembrane inner ear [Tmie], cadherin related 23 [Cdh23], calcium and integrin binding family member 2 [Cib2], microsomal glutathione S-transferase 3 [Mgst3], acyl-CoA binding domain containing 7 [Acbd7], and calbindin 2 [Calb2]), with synaptic elements, hair bundles, and FM1-43 uptake, suggesting a degree of maturation [113,123]. However, the developmental process was slowed compared to native hair cell development [123]. Interestingly, there are conflicting reports on the loss of endogenous hair cells after this approach to induce regeneration [113,123].
The development of cochlear hair cells involves the orderly expression of several transcription factors. First, Atoh1 is required for hair cell specification and differentiation [124,125]. Next, Gfi1 and Pou4f3, both downstream targets of Atoh1, mediate hair cell differentiation and survival [118,126]. Cochlear hair cells are then further specified into inner and outer hair cell subtypes, with the former serving as the primary mechanoreceptors and the latter as amplifiers that enhance the sensitivity of the organ [4]. Ablation of insulinoma-associated 1 (Insm1), which is specifically expressed in outer hair cells, leads them to acquire an inner hair cell phenotype, suggesting that it functions to maintain an outer hair cell fate [127]. While deletion of IKAROS family zinc finger 2 (Ikzf2) reduces the expression of outer hair cell genes, such as Prestin, without converting them into inner hair cell-like, overexpression of Ikzf2 can coerce inner hair cells to become outer hair cell-like in adult mice [128]. Recently, Sun and colleagues examined the role of Ikzf2 in the mature mouse cochlea; after the selective ablation of outer hair cells, co-expression of Ikzf2 and Atoh1 in supporting cells leads to the formation of Prestin+ outer hair cells [102]. As expected, the number of regenerated hair cells is limited, consistent with previous reports [3,114]. Interestingly, induced Prestin+ outer hair cells also express other outer hair cell genes (Insm1 and Ikzf2), indicating a degree of differentiation surpassing previous studies. Unfortunately, regenerated hair cells display immature hair bundles and are not fully functional, indicating that additional factors may be needed to further enhance their maturation.
T-box transcription factor 2 (Tbx2) is another transcription factor recently shown to govern differentiation of inner hair cells [129]. Indeed, ablation of Tbx2 in inner hair cells during development leads them to become outer hair cell-like [129,130]. Similarly, the combination of overexpressing Tbx2 and transient overexpression of Atoh1 induces ectopic hair cells that are inner hair cell-like in the immature cochlea [130]. These ectopic hair cells express some inner hair cell genes, such as vGlut3 and otoferlin, yet only display immature hair bundles histologically [130]. Collectively, these studies suggest that co-manipulating transcription factors such as Ikzf2, Insm1, and Tbx2 can be a promising approach to guide maturation of regenerated/ectopic hair cells in the mammalian cochlea.
The Wnt/β-catenin signaling pathway regulates cell proliferation and hair cell differentiation, and is essential for cochlear development [74,131]. In the neonatal mouse cochlea, Wnt agonists and β-catenin (the central mediator of canonical Wnt signaling) overexpression promote the proliferation and subsequent formation of hair cells in Lgr5+ supporting cells in vitro, whereas Wnt antagonists inhibit their proliferation and hair cell-forming capacity [95,104,132-134]. Ectopic co-expression of β-catenin and Atoh1 in neonatal cochlear Lgr5+ cells significantly increases the number of ectopic hair cells [135]. In addition, Sox2 haploinsufficiency promotes hair cell regeneration and mitosis induced by the Wnt/β-catenin signaling pathway after damage in the neonatal cochlea in vivo [136]. Recently, Zhang et al. [137] showed that adeno-associated virus-neuroepithelia transforming gene 1 (Net1) can also activate the Wnt/β-catenin signaling pathway, promoting supporting cell proliferation and hair cell regeneration in neonatal cochleae in vivo. Net1 is a guanine nucleotide exchange factor that regulates RhoA activity and is implicated in cell proliferation, apoptosis, and differentiation [138,139]. Wnt activation alone has not been shown to be able to stimulate proliferation and hair cell regeneration in the adult mammalian cochlea [140]. However, recently, a combination of Atoh1 overexpression, valproic acid, siRNAs against Myc suppressors, the Wnt agonist lithium chloride, and the cAMP agonist forskolin was reported to stimulate significantly more regenerated hair cell-like cells in the mature mouse cochlea than Atoh1 overexpression alone [141].
The blockade of Notch signaling after damage results in the transdifferentiation of supporting cells into immature hair cells in the neonatal cochlea in vitro [142]. Apart from the damage model, research has also highlighted that the addition of DAPT causes supporting cells to take on a hair cell fate in undamaged cochlea both in vitro and in vivo [143]. Similarly, Notch inhibition leads to Atoh1 activation in neonatal mice in vivo, although the ability of Notch inhibition to promote hair cell formation declines precipitously with age [144]. Conversely, spontaneous hair cell regeneration is prevented by increasing Notch signaling in the neonatal mouse cochlea in vivo [145]. In adult mice deafened by acoustic trauma, Notch inhibition alone has been reported to promote supporting cell transdifferentiation to outer hair cells, and with slightly improved auditory brainstem response thresholds [146,147]. These findings indicate that Notch signaling inhibits the differentiation of supporting cells into hair cells and suggest that manipulating the cell fate of cochlear sensory cells through pharmacological inhibition of Notch signaling is a potential therapeutic approach to hair cell regeneration in the mammalian cochlea.
Significant advances have been made to reveal the molecular mechanisms of hair cell regeneration in sensory organs in chickens and zebrafish. Distinct subtypes of supporting cells acting as hair cell progenitors with their corresponding transcriptomes have been uncovered. These results have fueled new research directions and approaches to stimulate cochlear hair cell regeneration in mammals. In addition, several studies have revealed that supporting cells in the neonatal mouse cochlea can act as bona fide hair cell progenitors [13,91,143,148], and mechanisms limiting regeneration in the adult mammalian cochlea have begun to be elucidated [106,107].
Several approaches have shown efficacy in increasing hair cell formation in the neonatal mouse cochlea. However, their ability to induce hair cell formation in supporting cells in the mature mouse cochlea is rather limited. In the near future, it will be critical to further expand our knowledge on the transcriptome of supporting cells, with a particular focus on their chromatin accessibility in the damaged mature cochlea [149]. Given that hair cells can inhibit neighboring supporting cells from acquiring a hair cell fate, it is likely that the responsiveness of the damaged cochlea is dramatically different from that of the undamaged organ. At present, it is unknown how supporting cells early after damage differ from those long after damage, and whether they become a “flat epithelium.” A better understanding of the milieu of the damaged mature cochlea resulting from a variety of etiologies (e.g., noise, genetic mutations, and cisplatin) may help guide approaches to induce hair cell regeneration.
In the mature cochlea, the concurrent manipulation of genes/pathways has shown promise in inducing proliferation or transforming supporting cells into hair cells [99,100]. Remarkably, studies on hair cell development and maturation have informed approaches to induce the regeneration of outer and inner hair cell subtypes. However, regenerated hair cells still do not fully mature. Moreover, it remains to be determined whether regenerated hair cells are properly innervated by spiral ganglion neurons and how a lack of certain developmental cues (e.g., spontaneous activities in supporting cells and hair cells) will affect maturation of regenerated hair cells. Thus, these exciting discoveries have highlighted the challenges ahead and the need to further probe regenerated hair cells and the damaged mature cochlea.
In summary, numerous recent studies have demonstrated that genetic and epigenetic factors contribute to hair cell regeneration. In addition, by combining various genes and pathways, hair cell regeneration can be achieved in the mature cochlea. With advancements in delivery techniques for therapeutic agents, mammalian hair cell regeneration has the potential to be used as a biological therapy for hearing loss.
▪ The neonatal mouse cochlea is capable of regenerating hair cells and supporting cells, but this ability declines with organ maturation.
▪ Reprogramming approaches using multiple transcription factors can stimulate hair cell regeneration in the adult mouse cochlea.
▪ Regenerated hair cells in the mouse cochlea are inner and outer hair cell-like, but do not fully mature.
▪ Distinct subgroups of supporting cells in the non-mammalian and mammalian sensory organs have a greater regenerative capacity.
ACKNOWLEDGMENTS
We would like to thank P. Atkinson for excellent input on the manuscript, N. Benkafadar for sharing figures, and M. Jung for figure illustration.
This research was supported by Maternal & Child Health Research (MCHRI) and Natural Sciences and Engineering Research Council of Canada (NSERC) postdoctoral fellowships (JMA), a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1C1C1004183) (S.W.C), and NIH/NIDCD RO1DC021110 and RO1DC016919 (AGC).
REFERENCES
1. Chadha S, Kamenov K, Cieza A. The world report on hearing, 2021. Bull World Health Organ. 2021; Apr. 99(4):242–A.
2. Wu PZ, O’Malley JT, de Gruttola V, Liberman MC. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J Neurosci. 2020; Aug. 40(33):6357–66.
3. Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: similarities and differences. Development. 2015; May. 142(9):1561–71.
4. Hudspeth AJ, Choe Y, Mehta AD, Martin P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc Natl Acad Sci U S A. 2000; Oct. 97(22):11765–72.
5. Denans N, Baek S, Piotrowski T. Comparing sensory organs to define the path for hair cell regeneration. Annu Rev Cell Dev Biol. 2019; Oct. 35:567–89.
6. Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006; Nov. 7(11):837–49.
7. Bohne BA, Ward PH, Fernandez C. Irreversible inner ear damage from rock music. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1976; 82(1):ORL50–9.
8. Oesterle EC, Bhave SA, Coltrera MD. Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J Comp Neurol. 2000; Aug. 424(2):307–26.
9. Hawkins JE. Microcirculation in the labyrinth. Arch Otorhinolaryngol. 1976; Sep. 212(4):241–51.
10. Brigande JV, Heller S. Quo vadis, hair cell regeneration. Nat Neurosci. 2009; Jun. 12(6):679–85.
11. Malgrange B, Belachew S, Thiry M, Nguyen L, Rogister B, Alvarez ML, et al. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002; Mar. 112(1-2):79–88.
12. Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011; 1:26.
13. Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014; Feb. 141(4):816–29.
14. White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006; Jun. 441(7096):984–7.
15. Corwin JT, Oberholtzer JC. Fish n’ chicks: model recipes for hair-cell regeneration. Neuron. 1997; Nov. 19(5):951–4.
16. Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009; Jun. 1277:12–23.
17. Rubel EW, Furrer SA, Stone JS. A brief history of hair cell regeneration research and speculations on the future. Hear Res. 2013; Mar. 297:42–51.
18. Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988; Jun. 240(4860):1772–4.
19. Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999; Feb. 126(5):961–73.
20. Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988; Jun. 240(4860):1774–6.
21. Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987; Oct. 113(10):1058–62.
22. Froymovich O, Rebala V, Salvi RJ, Rassael H. Long-term effect of acoustic trauma on distortion product otoacoustic emissions in chickens. J Acoust Soc Am. 1995; May. 97(5 Pt 1):3021–9.
23. Poje CP, Sewell DA, Saunders JC. The effects of exposure to intense sound on the DC endocochlear potential in the chick. Hear Res. 1995; Feb. 82(2):197–204.
24. Cruz IA, Kappedal R, Mackenzie SM, Hailey DW, Hoffman TL, Schilling TF, et al. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev Biol. 2015; Jun. 402(2):229–38.
25. Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol. 2003; Jun. 4(2):219–34.
26. Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000; May. 143(1-2):171–81.
27. Hernandez PP, Moreno V, Olivari FA, Allende ML. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio). Hear Res. 2006; Mar. 213(1-2):1–10.
28. Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007; 51(6-7):633–47.
29. Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008; Feb. 28(9):2261–73.
30. Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996; Feb. 205(1):17–20.
31. Roberson DW, Kreig C, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit Neurosci. 1996; 2:195–205.
32. Hernandez PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML. Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker sox2 and proliferation-dependent and-independent mechanisms of hair cell renewal. Dev Neurobiol. 2007; Apr. 67(5):637–54.
33. Lopez-Schier H, Hudspeth AJ. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc Natl Acad Sci U S A. 2006; Dec. 103(49):18615–20.
34. Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007; Jan. 236(1):156–70.
35. Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004; Nov. 78(4):461–71.
36. Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996; Sep. 16(17):5466–77.
37. Gomez-Dorado M, Daudet N, Gale JE, Dawson SJ. Differential regulation of mammalian and avian ATOH1 by E2F1 and its implication for hair cell regeneration in the inner ear. Sci Rep. 2021; Sep. 11(1):19368.
38. Baek S, Tran NT, Diaz DC, Tsai YY, Acedo JN, Lush ME, et al. Single-cell transcriptome analysis reveals three sequential phases of gene expression during zebrafish sensory hair cell regeneration. Dev Cell. 2022; Mar. 57(6):799–819.
39. Janesick AS, Scheibinger M, Benkafadar N, Kirti S, Heller S. Avian auditory hair cell regeneration is accompanied by JAK/STAT-dependent expression of immune-related genes in supporting cells. Development. 2022; Apr. 149(8):dev200113.
40. Matsunaga M, Kita T, Yamamoto R, Yamamoto N, Okano T, Omori K, et al. Initiation of supporting cell activation for hair cell regeneration in the avian auditory epithelium: an explant culture model. Front Cell Neurosci. 2020; 14:583994.
41. Janesick A, Scheibinger M, Benkafadar N, Kirti S, Ellwanger DC, Heller S. Cell-type identity of the avian cochlea. Cell Rep. 2021; Mar. 34(12):108900.
42. Benkafadar N, Janesick A, Scheibinger M, Ling AH, Jan TA, Heller S. Transcriptomic characterization of dying hair cells in the avian cochlea. Cell Rep. 2021; Mar. 34(12):108902.
43. Ellwanger DC, Scheibinger M, Dumont RA, Barr-Gillespie PG, Heller S. Transcriptional dynamics of hair-bundle morphogenesis revealed with CellTrails. Cell Rep. 2018; Jun. 23(10):2901–14.
44. Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, et al. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One. 2007; Jun. 2(6):e525.
45. Alvarado DM, Hawkins RD, Bashiardes S, Veile RA, Ku YC, Powder KE, et al. An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J Neurosci. 2011; Mar. 31(12):4535–43.
46. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017; Apr. 77(5):521–46.
47. Matsunaga M, Yamamoto R, Kita T, Ohnishi H, Yamamoto N, Okano T, et al. Stepwise fate conversion of supporting cells to sensory hair cells in the chick auditory epithelium. iScience. 2023; Feb. 26(2):106046.
48. Lewis RM, Hume CR, Stone JS. Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear Res. 2012; Jul. 289(1-2):74–85.
49. Bermingham-McDonogh O, Stone JS, Reh TA, Rubel EW. FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev Biol. 2001; Oct. 238(2):247–59.
50. Lewis RM, Keller JJ, Wan L, Stone JS. Bone morphogenetic protein 4 antagonizes hair cell regeneration in the avian auditory epithelium. Hear Res. 2018; Jul. 364:1–11.
51. Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009; Feb. 326(1):86–100.
52. Jacques BE, Montgomery WH, Uribe PM, Yatteau A, Asuncion JD, Resendiz G, et al. The role of Wnt/β-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev Neurobiol. 2014; Apr. 74(4):438–56.
53. Luo Z, Du Y, Li S, Zhang H, Shu M, Zhang D, et al. Three distinct Atoh1 enhancers cooperate for sound receptor hair cell development. Proc Natl Acad Sci U S A. 2022; Aug. 119(32):e2119850119.
54. Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005; Feb. 132(3):541–51.
55. Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998; Dec. 125(23):4645–54.
56. Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995; May. 129(4):895–8.
57. Wan L, Lovett M, Warchol ME, Stone JS. Vascular endothelial growth factor is required for regeneration of auditory hair cells in the avian inner ear. Hear Res. 2020; Jan. 385:107839.
58. Baxendale S, Whitfield TT. Methods to study the development, anatomy, and function of the zebrafish inner ear across the life course. Methods Cell Biol. 2016; 134:165–209.
59. Pickett SB, Raible DW. Water waves to sound waves: using zebrafish to explore hair cell biology. J Assoc Res Otolaryngol. 2019; Feb. 20(1):1–19.
60. Matsui JI, Ryals BM. Hair cell regeneration: an exciting phenomenon... but will restoring hearing and balance be possible. J Rehabil Res Dev. 2005; 42(4 Suppl 2):187–98.
61. Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996; Dec. 123:37–46.
62. Teitz T, Fang J, Goktug AN, Bonga JD, Diao S, Hazlitt RA, et al. CDK2 inhibitors as candidate therapeutics for cisplatin- and noise-induced hearing loss. J Exp Med. 2018; Apr. 215(4):1187–203.
63. Kimmel CB. Genetics and early development of zebrafish. Trends Genet. 1989; Aug. 5(8):283–8.
64. Villegas R, Martin SM, O’Donnell KC, Carrillo SA, Sagasti A, Allende ML. Dynamics of degeneration and regeneration in developing zebrafish peripheral axons reveals a requirement for extrinsic cell types. Neural Dev. 2012; Jun. 7:19.
65. Haehnel M, Taguchi M, Liao JC. Heterogeneity and dynamics of lateral line afferent innervation during development in zebrafish (Danio rerio). J Comp Neurol. 2012; May. 520(7):1376–86.
66. Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Dev Dyn. 2002; Apr. 223(4):536–43.
67. Venuto A, Erickson T. Evaluating the death and recovery of lateral line hair cells following repeated neomycin treatments. Life (Basel). 2021; Nov. 11(11):1180.
68. Breitzler L, Lau IH, Fonseca PJ, Vasconcelos RO. Noise-induced hearing loss in zebrafish: investigating structural and functional inner ear damage and recovery. Hear Res. 2020; Jun. 391:107952.
69. Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010; Feb. 338(2):262–9.
70. Wibowo I, Pinto-Teixeira F, Satou C, Higashijima S, Lopez-Schier H. Compartmentalized Notch signaling sustains epithelial mirror symmetry. Development. 2011; Mar. 138(6):1143–52.
71. Mackenzie SM, Raible DW. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS One. 2012; 7(10):e47257.
72. Pinto-Teixeira F, Viader-Llargues O, Torres-Mejia E, Turan M, Gonzalez-Gualda E, Pola-Morell L, et al. Inexhaustible hair-cell regeneration in young and aged zebrafish. Biol Open. 2015; May. 4(7):903–9.
73. Thomas ED, Raible DW. Distinct progenitor populations mediate regeneration in the zebrafish lateral line. Elife. 2019; Mar. 8:e43736.
74. Romero-Carvajal A, Navajas Acedo J, Jiang L, Kozlovskaja-Gumbriene A, Alexander R, Li H, et al. Regeneration of sensory hair cells requires localized interactions between the Notch and Wnt pathways. Dev Cell. 2015; Aug. 34(3):267–82.
75. Mi XX, Yan J, Li Y, Shi JP. Wnt/β-catenin signaling was activated in supporting cells during exposure of the zebrafish lateral line to cisplatin. Ann Anat. 2019; Nov. 226:48–56.
76. Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014; Apr. 111(14):E1383–92.
77. Tang D, He Y, Li W, Li H. Wnt/β-catenin interacts with the FGF pathway to promote proliferation and regenerative cell proliferation in the zebrafish lateral line neuromast. Exp Mol Med. 2019; May. 51(5):1–16.
78. Lush ME, Diaz DC, Koenecke N, Baek S, Boldt H, St Peter MK, et al. scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. Elife. 2019; Jan. 8:e44431.
79. Ye Z, Su Z, Xie S, Liu Y, Wang Y, Xu X, et al. Yap-lin28a axis targets let7-Wnt pathway to restore progenitors for initiating regeneration. Elife. 2020; Apr. 9:e55771.
80. Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008; Aug. 10(8):987–93.
81. Golden EJ, Benito-Gonzalez A, Doetzlhofer A. The RNA-binding protein LIN28B regulates developmental timing in the mammalian cochlea. Proc Natl Acad Sci U S A. 2015; Jul. 112(29):E3864–73.
82. Zhang R, Liu X, Li Y, Wang M, Chen L, Hu B. Suppression of inflammation delays hair cell regeneration and functional recovery following lateral line damage in zebrafish larvae. Biomolecules. 2020; Oct. 10(10):1451.
83. Warchol ME, Schrader A, Sheets L. Macrophages respond rapidly to ototoxic injury of lateral line hair cells but are not required for hair cell regeneration. Front Cell Neurosci. 2020; Jan. 14:613246.
84. Denans N, Tran NT, Swall ME, Diaz DC, Blanck J, Piotrowski T. An anti-inflammatory activation sequence governs macrophage transcriptional dynamics during tissue injury in zebrafish. Nat Commun. 2022; Sep. 13(1):5356.
85. Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, et al. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J Neurosci. 2015; Nov. 35(45):15050–61.
86. Manickam V, Gawande DY, Stothert AR, Clayman AC, Batalkina L, Warchol ME, et al. Macrophages promote repair of inner hair cell ribbon synapses following noise-induced cochlear synaptopathy. J Neurosci. 2023; Mar. 43(12):2075–89.
87. Fredelius L, Rask-Andersen H. The role of macrophages in the disposal of degeneration products within the organ of corti after acoustic overstimulation. Acta Otolaryngol. 1990; 109(1-2):76–82.
88. Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005; Aug. 489(2):180–94.
89. Sato E, Shick HE, Ransohoff RM, Hirose K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol. 2010; Jun. 11(2):223–34.
90. Kolla L, Kelly MC, Mann ZF, Anaya-Rocha A, Ellis K, Lemons A, et al. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat Commun. 2020; May. 11(1):2389.
91. Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014; Mar. 2(3):311–22.
92. Heuermann ML, Matos S, Hamilton D, Cox BC. Regenerated hair cells in the neonatal cochlea are innervated and the majority coexpress markers of both inner and outer hair cells. Front Cell Neurosci. 2022; Sep. 16:841864.
93. Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996; Oct. 6(10):1290–301.
94. Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011; Aug. 12(4):455–69.
95. Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012; Jul. 32(28):9639–48.
96. McGovern MM, Randle MR, Cuppini CL, Graves KA, Cox BC. Multiple supporting cell subtypes are capable of spontaneous hair cell regeneration in the neonatal mouse cochlea. Development. 2019; Development. 146(4):–dev171009.
97. Zhang S, Liu D, Dong Y, Zhang Z, Zhang Y, Zhou H, et al. Frizzled-9+ supporting cells are progenitors for the generation of hair cells in the postnatal mouse cochlea. Front Mol Neurosci. 2019; 12:184.
98. Lee S, Song JJ, Beyer LA, Swiderski DL, Prieskorn DM, Acar M, et al. Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea. Sci Rep. 2020; Dec. 10(1):21397.
99. Shu Y, Li W, Huang M, Quan YZ, Scheffer D, Tian C, et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat Commun. 2019; Dec. 10(1):5530.
100. Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, et al. in vivo interplay between p27Kip1, GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice. Cell Rep. 2017; Apr. 19(2):307–20.
101. Yamashita T, Zheng F, Finkelstein D, Kellard Z, Carter R, Rosencrance CD, et al. High-resolution transcriptional dissection of in vivo Atoh1-mediated hair cell conversion in mature cochleae identifies Isl1 as a co-reprogramming factor. PLoS Genet. 2018; Jul. 14(7):e1007552.
102. Sun S, Li S, Luo Z, Ren M, He S, Wang G, et al. Dual expression of Atoh1 and Ikzf2 promotes transformation of adult cochlear supporting cells into outer hair cells. Elife. 2021; Sep. 10:e66547.
103. Li XJ, Doetzlhofer A. LIN28B/let-7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc Natl Acad Sci U S A. 2020; Sep. 117(36):22225–36.
104. McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, et al. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 2017; Feb. 18(8):1917–29.
105. Li XJ, Morgan C, Goff LA, Doetzlhofer A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci Adv. 2022; Feb. 8(6):eabj7651.
106. Pauklin S, Vallier L. Activin/Nodal signalling in stem cells. Development. 2015; Feb. 142(4):607–19.
107. Tao L, Yu HV, Llamas J, Trecek T, Wang X, Stojanova Z, et al. Enhancer decommissioning imposes an epigenetic barrier to sensory hair cell regeneration. Dev Cell. 2021; Sep. 56(17):2471–85.
108. Mellado Lagarde MM, Wan G, Zhang L, Gigliello AR, McInnis JJ, Zhang Y, et al. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc Natl Acad Sci U S A. 2014; Nov. 111(47):16919–24.
109. Kubota M, Scheibinger M, Jan TA, Heller S. Greater epithelial ridge cells are the principal organoid-forming progenitors of the mouse cochlea. Cell Rep. 2021; Jan. 34(3):108646.
110. Udagawa T, Atkinson PJ, Milon B, Abitbol JM, Song Y, Sperber M, et al. Lineage-tracing and translatomic analysis of damage-inducible mitotic cochlear progenitors identifies candidate genes regulating regeneration. PLoS Biol. 2021; Nov. 19(11):e3001445.
111. Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000; Jun. 3(6):580–6.
112. Liu Z, Fang J, Dearman J, Zhang L, Zuo J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS One. 2014; 9(2):e89377.
113. Iyer AA, Hosamani I, Nguyen JD, Cai T, Singh S, McGovern MM, et al. Cellular reprogramming with ATOH1, GFI1, and POU4F3 implicate epigenetic changes and cell-cell signaling as obstacles to hair cell regeneration in mature mammals. Elife. 2022; Nov. 11:e79712.
114. Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012; May. 32(19):6600–10.
115. Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005; Mar. 11(3):271–6.
116. Matern MS, Milon B, Lipford EL, McMurray M, Ogawa Y, Tkaczuk A, et al. GFI1 functions to repress neuronal gene expression in the developing inner ear hair cells. Development. 2020; Sep. 147(17):dev186015.
117. Jen HI, Singh S, Tao L, Maunsell HR, Segil N, Groves AK. GFI1 regulates hair cell differentiation by acting as an off-DNA transcriptional co-activator of ATOH1, and a DNA-binding repressor. Sci Rep. 2022; May. 12(1):7793.
118. Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003; Jan. 130(1):221–32.
119. Masuda M, Pak K, Chavez E, Ryan AF. TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa positive cells in nonsensory cochlear epithelium by ATOH1. Dev Biol. 2012; Dec. 372(1):68–80.
120. Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, et al. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997; Aug. 94(17):9445–50.
121. Yu HV, Tao L, Llamas J, Wang X, Nguyen JD, Trecek T, et al. POU4F3 pioneer activity enables ATOH1 to drive diverse mechanoreceptor differentiation through a feed-forward epigenetic mechanism. Proc Natl Acad Sci U S A. 2021; Jul. 118(29):e2105137118.
122. Costa A, Sanchez-Guardado L, Juniat S, Gale JE, Daudet N, Henrique D. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development. 2015; Jun. 142(11):1948–59.
123. Chen Y, Gu Y, Li Y, Li GL, Chai R, Li W, et al. Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea. Cell Rep. 2021; Apr. 35(3):109016.
124. Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999; Jun. 284(5421):1837–41.
125. Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J Neurosci. 2013; Jun. 33(24):10110–22.
126. Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, et al. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996; Jun. 381(6583):603–6.
127. Wiwatpanit T, Lorenzen SM, Cantu JA, Foo CZ, Hogan AK, Marquez F, et al. Trans-differentiation of outer hair cells into inner hair cells in the absence of INSM1. Nature. 2018; Nov. 563(7733):691–5.
128. Chessum L, Matern MS, Kelly MC, Johnson SL, Ogawa Y, Milon B, et al. Helios is a key transcriptional regulator of outer hair cell maturation. Nature. 2018; Nov. 563(7733):696–700.
129. Garcia-Anoveros J, Clancy JC, Foo CZ, Garcia-Gomez I, Zhou Y, Homma K, et al. Tbx2 is a master regulator of inner versus outer hair cell differentiation. Nature. 2022; May. 605(7909):298–303.
130. Bi Z, Li L, Ren M, Gu Y, Zhu T, Li S, et al. Development and trans-differentiation into inner hair cells require Tbx2. Natl Sci Rev. 2022; Dec. 9(12):nwac156.
131. Jansson L, Kim GS, Cheng AG. Making sense of Wnt signaling-linking hair cell regeneration to development. Front Cell Neurosci. 2015; 9:66.
132. Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012; May. 109(21):8167–72.
133. Hu L, Lu J, Chiang H, Wu H, Edge AS, Shi F. Diphtheria toxin-induced cell death triggers Wnt-dependent hair cell regeneration in neonatal mice. J Neurosci. 2016; Sep. 36(36):9479–89.
134. Ni W, Zeng S, Li W, Chen Y, Zhang S, Tang M, et al. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget. 2016; Oct. 7(41):66754–68.
135. Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. in vivo cochlear hair cell generation and survival by coactivation of β-catenin and Atoh1. J Neurosci. 2015; Jul. 35(30):10786–98.
136. Atkinson PJ, Dong Y, Gu S, Liu W, Najarro EH, Udagawa T, et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J Clin Invest. 2018; Apr. 128(4):1641–56.
137. Zhang L, Fang Y, Tan F, Guo F, Zhang Z, Li N, et al. AAV-Net1 facilitates the trans-differentiation of supporting cells into hair cells in the murine cochlea. Cell Mol Life Sci. 2023; Mar. 80(4):86.
138. Murray D, Horgan G, Macmathuna P, Doran P. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br J Cancer. 2008; Oct. 99(8):1322–9.
139. Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005; Feb. 6(2):167–80.
140. Samarajeewa A, Lenz DR, Xie L, Chiang H, Kirchner R, Mulvaney JF, et al. Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea. Development. 2018; Nov. 145(23):dev166579.
141. Quan YZ, Wei W, Ergin V, Rameshbabu AP, Huang M, Tian C, et al. Reprogramming by drug-like molecules leads to regeneration of cochlear hair cell-like cells in adult mice. Proc Natl Acad Sci U S A. 2023; Apr. 120(17):e2215253120.
142. Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS One. 2013; 8(8):e73276.
143. Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY, et al. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A. 2015; Jan. 112(1):166–71.
144. Du X, Cai Q, West MB, Youm I, Huang X, Li W, et al. Regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs. Mol Ther. 2018; May. 26(5):1313–26.
145. McGovern MM, Zhou L, Randle MR, Cox BC. Spontaneous hair cell regeneration is prevented by increased notch signaling in supporting cells. Front Cell Neurosci. 2018; 12:120.
146. Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013; Jan. 77(1):58–69.
147. Tona Y, Hamaguchi K, Ishikawa M, Miyoshi T, Yamamoto N, Yamahara K, et al. Therapeutic potential of a gamma-secretase inhibitor for hearing restoration in a guinea pig model with noise-induced hearing loss. BMC Neurosci. 2014; May. 15:66.
148. Xia M, Wu M, Zhao L, Ma J, Li W, Li H. Selective ablation of inner hair cells and subsequent in-situ hair cell regeneration in the neonatal mouse cochlea. Hear Res. 2021; Aug. 407:108275.
149. Milon B, Shulman ED, So KS, Cederroth CR, Lipford EL, Sperber M, et al. A cell-type-specific atlas of the inner ear transcriptional response to acoustic trauma. Cell Rep. 2021; Sep. 36(13):109758.
Fig. 1.
Hair cell regeneration in the chicken basilar papilla. (A) Microscopic image of a whole-mount preparation of the basilar papilla. Image courtesy of Nesrine Benkafadar. (B) Schematic image of the cross-sectional anatomy of the basilar papilla. (C) Mechanisms of hair cell regeneration in chicken basilar papilla. VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; JAK/STAT, Janus kinase/signal transducer and activator of transcription.
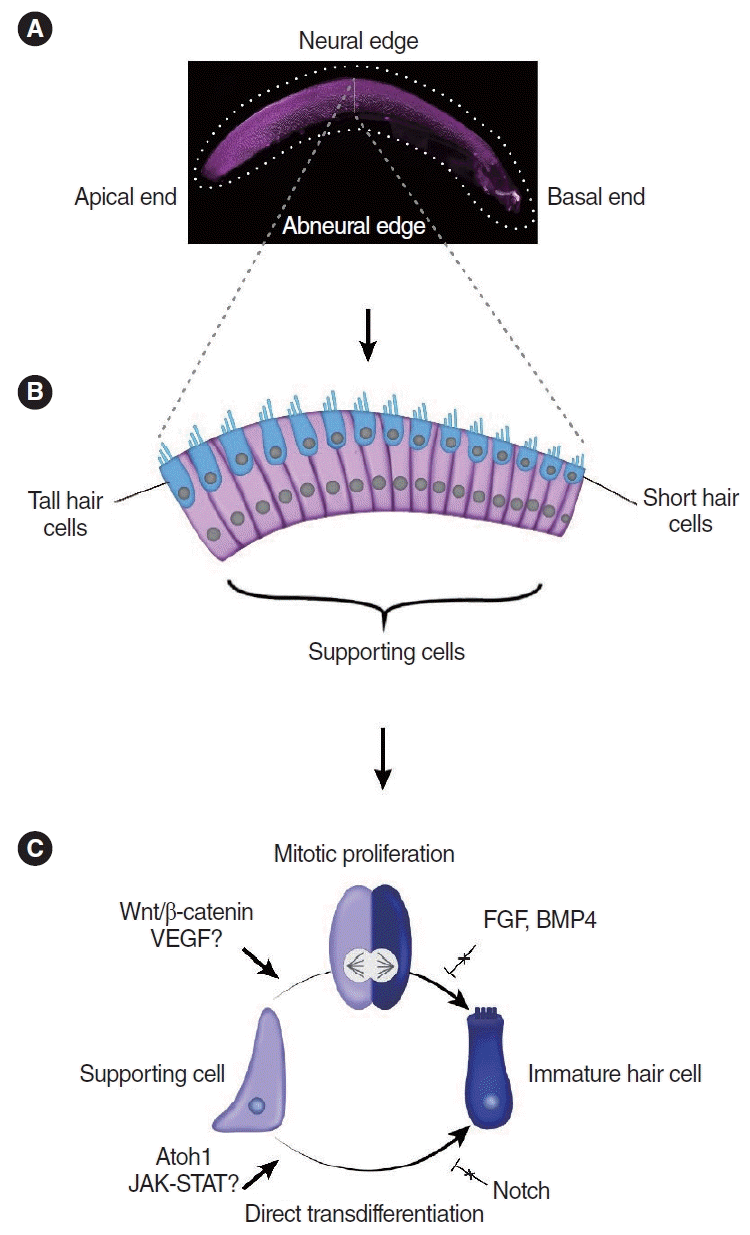
Fig. 2.
Hair cell regeneration in the zebrafish lateral line neuromast. (A) Schematic image of the zebrafish lateral line neuromast. (B) Mechanisms of hair cell regeneration in the zebrafish lateral line neuromast. Fgf, fibroblast growth factor.
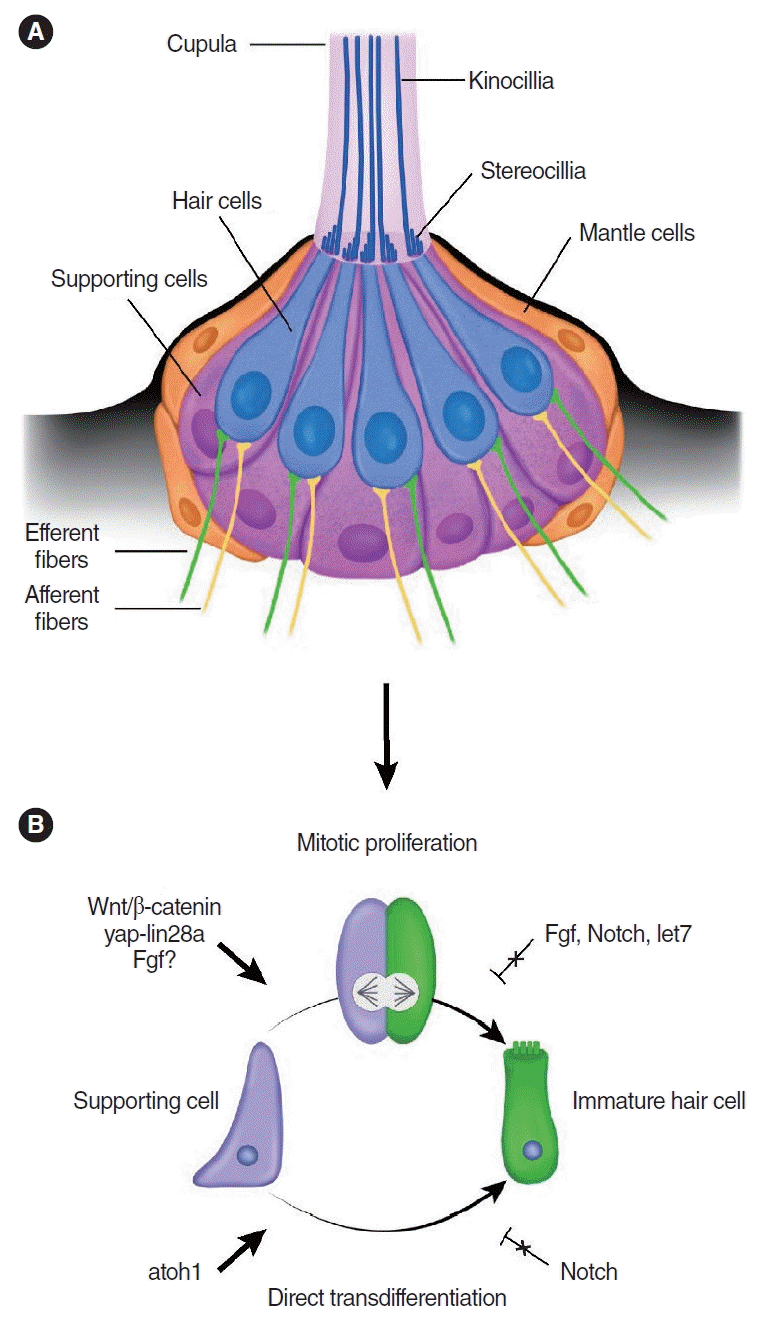
Fig. 3.
Schematic image of the mature organ of Corti. IBC, inner border cell; IPhC, inner phalangeal cell; IPC, inner pillar cell; OPC, outer pillar cell; DC, Deiters’ cell.
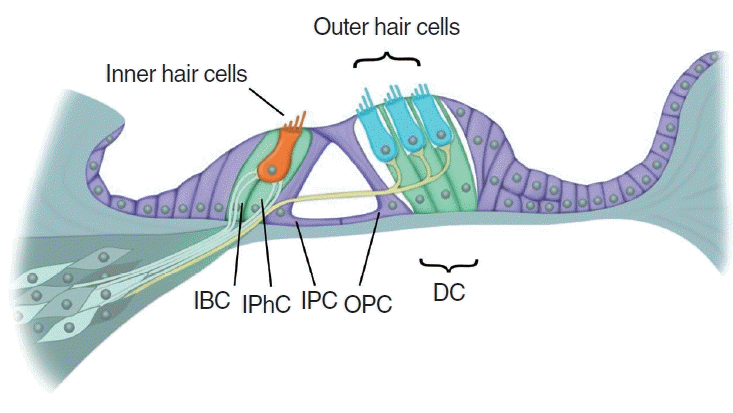
Fig. 4.
Hair cell regeneration in neonatal mammals’ organ of Corti. (A) Schematic image of the neonatal mammalian organ of Corti. (B) Mechanisms of hair cell regeneration in the neonatal mammalian organ of Corti. GER, greater epithelial ridge; IBC, inner border cell; IPhC, inner phalangeal cell; PC, pillar cell; DC, Deiters’ cell; HeC, Hensen cell; LER, lesser epithelial ridge.
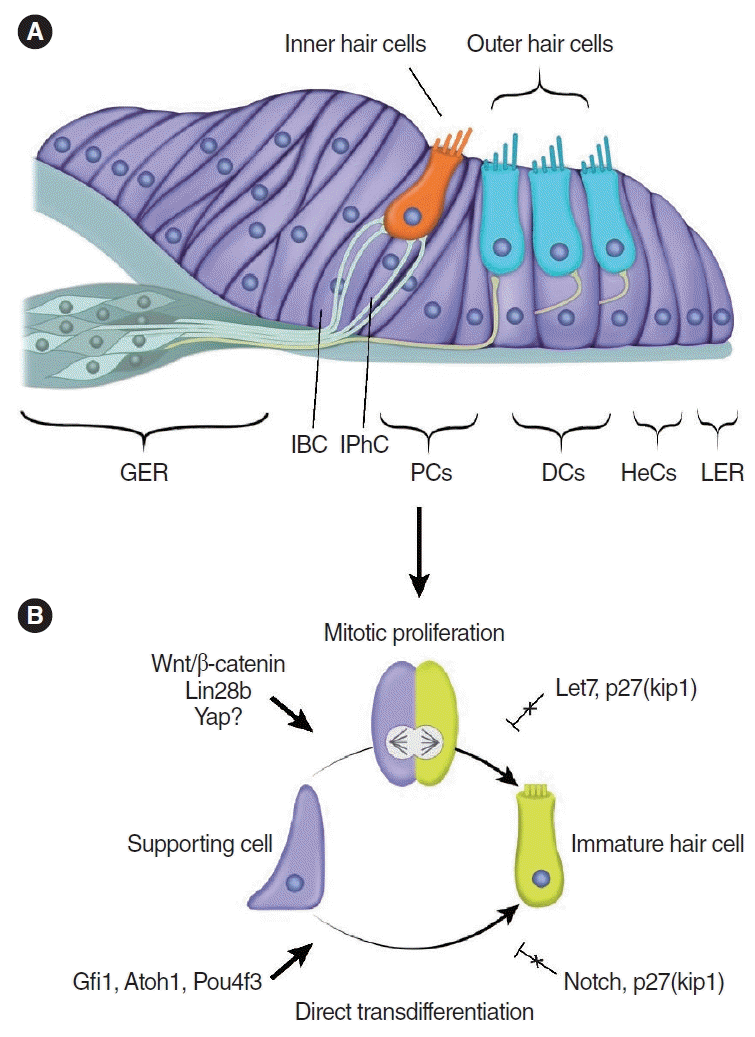
Table 1.
List of abbreviations




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download