Abstract
Objectives.
As cochlear implantation (CI) experiences rapid innovations and its indications expand, the characteristics of revision CI (RCI) are evolving. This study investigated changes in the RCI profile and explored their clinical implications.
Methods.
A retrospective chart review was conducted of all CIs performed at a tertiary medical institution between October 2001 and January 2023. The rates of and reasons for RCI were evaluated in relation to the manufacturer and device model. Kaplan-Meier analysis was employed to examine cumulative and device survival curves. Cumulative and device survival rates were additionally analyzed based on age group, period of primary CI, and manufacturer. A Cox proportional hazards model was employed to evaluate the association between RCI and the device manufacturer.
Results.
Among 1,430 CIs, 73 (5.1%) required RCI. The predominant reason for RCI was device failure (40 of 73 RCIs [54.8%]), with an overall device failure rate of 2.8%. This was followed by flap-associated problems and migration (nine of 73 RCIs each [12.3%]). Flap retention issues emerged as a new cause in three cases (two involving the CI 632 and one involving the SYNCHRONY 2 implant), and six instances of electrode tip fold-over arose (four for the CI 600 series and two for the CI 500 series). The overall 10-year cumulative and device survival rates were 93.4% and 95.8%, respectively. After excluding models with recall issues, significant differences in cumulative (P =0.010) and device (P =0.001) survival rates were observed across manufacturers.
Conclusion.
While the overall CI survival rate is stable, device failure persists as the predominant reason for RCI. Moreover, the types of complications leading to revision (including issues with flap retention and electrode tip fold-over) have shifted, particularly for newer implant models. Given the clinical importance of device failure and subsequent reoperation, clinicians should remain informed about and responsive to these trends.
Cochlear implantation (CI) is the most effective method for auditory rehabilitation in individuals with severe-to-profound sensorineural hearing loss. Over recent years, CI has demonstrated benefits across a wider range of age groups, enlarging the pool of potential recipients. A growing body of research supports the notion that early implantation leads to improved speech outcomes [1,2]. In 2020, the U.S. Food and Drug Administration reduced the minimum age for bilateral profound hearing loss candidates to 9 months. Similarly, the use of CIs in older populations is on the rise [3]. Recent studies have documented significant auditory benefits in patients over the age of 80 years [4,5]. Given these findings and current trends, it is anticipated that the rate of implantation will continue to climb. Despite the general consensus that CI is a safe intervention, the incidence of complications and the need for revision surgery are also expected to increase.
Revision CI (RCI) due to medically intractable complications presents a challenging scenario for both neuro-otologists and patients, as CI is generally regarded as a permanent solution. The rate of RCI varies in the literature, with figures ranging from 2.6% to 11.7% [6-13]. Common reasons for RCI include device failure, flap-associated problems, device migration, and electrode misinsertion [6,14]. Of these, device failure is acknowledged as a leading reason for RCI, although reported rates vary across studies, from 1.9% to 8.1% [6-13]. Recent investigations indicate that 10-year device survival rates have improved to over 95.0%, a testament to the technological advancements made in the last two decades [6,13]. Additionally, variations in device failure rates based on factors such as the manufacturer, model, patient age, or period of implantation have been examined in several studies [8,10,12,13].
Due to the rapid innovations and expanding indications of CI, ongoing evaluation and understanding of RCI are essential. A comparison with our prior investigation, which included CI cases up to March 2019 [6], revealed notable changes in the characteristics of RCI over the past 4 years. These trends were particularly evident with respect to differences among device manufacturers and models. Nonetheless, few studies have been conducted to examine the latest models from various manufacturers. Consequently, this study was designed to examine recent trends in RCI and device failure, with the goal of sharing our findings and emphasizing the clinical importance of these issues.
A retrospective chart review was conducted for all CIs performed at our institution between October 2001 and January 2023. The Institutional Review Board of Samsung Medical Center approved this study, and informed consent was waived (No. 2023-05-117). After a detailed counseling in which models from each manufacturer were shown and the benefits and drawbacks of each were explained, the patient chose the manufacturer and device model on the advice of the surgeon in charge. Compared to our previous study, three models from Cochlear (CI 612, CI 622, and CI 632), one model from MED-EL (SYNCHRONY 2), and one model from Advanced Bionics (HiRes Ultra3D) were newly implanted.
Demographic data, the date and age of primary CI, follow-up duration from implantation, as well as inner device models and their manufacturer were collected for 1,430 CIs. In addition, data on the cause of hearing loss, inner ear anomalies, and intraoperative findings such as the approach route for electrode insertion, electrode type, impedance test results, and electrically evoked compound action potential (ECAP) results, were collected for 73 RCIs. The interval between primary CI and RCI was calculated and the reasons for RCIs were based on the following classification used in our previous study: device failure, flap problems, migration, hematoma, cerebrospinal fluid (CSF) leakage, and misinsertion [6].
The overall cumulative survival and device survival curves depicted with a Kaplan-Meier curve. To analyze survival curves and rates, the date of the primary CI was used as an initial point, the RCI was used as a primary event, and the end point of observation was July 1, 2023. In addition, overall survival curves were drawn again after excluding devices with recall issues (CI 512, Clarion CII, HiRes 90K, and HiRes Ultra3D) to provide more practical representation of device survival. Cumulative and device survival rates were further analyzed based on age group (children <19 years and adults), the period of primary CI (2001–2012 and 2013–2023), and manufacturer (Cochlear, Advanced Bionics, and MED-EL). The log-rank test was used to examine the difference in survival curves between subgroups and the hazard ratio (HR) and 95% confidence interval were calculated using the Cox proportional hazards model to evaluate the association between RCI and manufacturers. Statistical analyses were performed using IBM SPSS ver. 27.0 (IBM Corp.), and a two-sided P <0.05 was considered statistically significant.
Of the 1,430 CIs analyzed, 992 devices (69.4%) were implanted in children, while 438 (30.6%) were implanted in adults. This cohort included 265 cases of bilateral implantation. The mean age at implantation was 18.8 years (range, 0–90 years). Regarding the distribution by manufacturer, Cochlear accounted for 816 of the implants (57.1%), MED-EL for 440 (30.8%), Advanced Bionics for 170 (11.9%), and Oticon for four (0.3%).
The characteristics of patients who underwent RCI are summarized in Table 1. A total of 73 RCIs were performed, constituting a 5.1% revision rate. Of these cases, 48 (65.8%) were in children and 25 (34.2%) in adults. These figures correspond to 4.8% and 5.7% of all CIs in their respective age groups. The right side was involved in 35 cases (47.9%), while the left side was affected in 38 (52.1%). The mean time to RCI was 739.3 days (range, 1–5,826 days). Congenital hearing loss was identified as the most common cause of hearing loss (60.3%), while inner ear anomalies were noted in 19 cases (26.0%). A round window approach had been adopted for primary CI in 63 cases (86.3%), while cochleostomy had been applied in 10 cases (13.7%). Regarding the type of electrode used, 38 cases (52.1%) employed a lateral wall (LW) electrode, while 35 (47.9%) involved a perimodiolar (PM) electrode. Intraoperative impedance test results were normal for all patients, and completely normal ECAPs were observed in 54 cases (74.0%). Only two cases (2.7%) exhibited no response.
Table 2 presents the reasons for RCI and the associated survival rates by manufacturer. Device failure was the predominant cause of RCI, accounting for 40 cases (54.8%), with an overall device failure rate of 2.8%. Flap-associated complications and migration each occurred in nine instances (12.3%), followed by misinsertion (seven [9.6%]), CSF leakage (five [6.8%]), and hematoma (three [4.1%]). Specifically, the device failures consisted of 36 hard and four soft failures. The flap-associated issues comprised six infections and three retention problems, with two involving the CI 632 model and one involving the SYNCHRONY 2. In one migration case, only the magnet was dislodged from the device, a CI 24RE implant. Electrode misinsertion was primarily due to tip fold-over (TF), affecting two CI 622, two CI 632, one CI 512, and one CI 532 device, along with a single case of direct misinsertion into the apical turn of a CI 24RE implant. Twelve patients required multiple RCIs for various reasons. Again, device failure was the most frequent cause (six cases [50.0%]), followed by CSF leakage (two cases [16.7%]), migration (one case [8.3%]), flap infection (one case [8.3%]), a flap retention issue (one case [8.3%]), and TF (one case [8.3%]).
Regarding the manufacturer, MED-EL exhibited the highest revision rate (26 of 440 implants [5.9%]), followed by Advanced Bionics (nine of 170 implants [5.3%]) and Cochlear (38 of 816 implants [4.7%]). The highest device failure rate was observed for Advanced Bionics (seven of 170 [4.1%]), followed by MED-EL (16 of 440 [3.6%]) and Cochlear (17 of 816 [2.1%]). Regarding device models, the CI 512 displayed the highest rates for both revision (13 of 66 [19.7%]) and device failure (eight of 66 [12.1%]). The SYNCHRONY (seven of 132 [5.3%]) and HiRes 90K (seven of 142 [4.9%]) devices had the highest failure rates among the models from their respective manufacturers. None of the Oticon implants required revision.
The results of the cumulative and device survival analyses are detailed in Table 3. The mean duration of follow-up was 5.8 years, with a standard deviation of 4.7 years. Fig. 1 displays the overall cumulative and device survival curves, both including and excluding recalled devices. The overall 5-, 10-, and 15-year cumulative survival rates were 94.7%, 93.4%, and 91.5%, respectively. When recalled devices were excluded, these rates became 95.6%, 94.8%, and 91.5%, respectively. The overall device survival rates at 5, 10, and 15 years were 97.0%, 95.8%, and 94.9%, respectively. Without the recalled devices, these respective rates were 97.6%, 97.1%, and 95.5%.
Table 3 and Fig. 2 summarize the cumulative and device survival rates based on various factors. Regarding age group, children showed a higher 5-year cumulative survival rate than adults, whereas adults exhibited a greater 5-year device survival rate. With respect to the timing of primary CI surgery, the 5-year cumulative survival rate was more favorable for CIs performed between 2001 and 2012, while the 5-year device survival rate was better for CIs performed between 2013 and 2023. Among the three manufacturers—Cochlear, Advanced Bionics, and MED-EL—Cochlear had the highest 5-year cumulative and device survival rates, while MED-EL had the lowest. However, these differences were not statistically significant. On the other hand, when models subject to recall were excluded, significant differences emerged in both cumulative survival (P=0.010) and evice survival (P=0.001) among manufacturers. Cox proportional hazards analysis revealed no significant differences in cumulative survival for MED-EL (HR, 1.44; 95% confidence interval, 0.87–2.39; P=0.155) and Advanced Bionics (HR, 0.89; 95% confidence interval, 0.43–1.85; P=0.751) compared to Cochlear. In terms of device survival, relative to Cochlear, MED-EL was associated with a greater risk (HR, 2.14; 95% confidence interval, 1.06–4.29; P=0.033), while Advanced Bionics displayed no significant difference (HR, 1.40; 95% confidence interval, 0.58–3.42; P=0.458).
Our institution serves as a representative national referral center for CI and has implanted devices from major manufacturers in a relatively balanced manner. This balance provides an advantage when analyzing the causes of RCI and device failure across manufacturers. Following our prior investigation, in which we assessed the reasons for revision and device failure in approximately 1,000 CIs over a 19-year period [6], the number of CIs placed has grown by nearly 500 in about 4 years. In the present series, the rates of RCI and device failure were 5.1% and 2.8%, respectively. These figures represent a slight increase in the RCI rate and a decrease in the device failure rate compared to our previous study, which reported rates of 4.6% and 3.0%, respectively. Regarding the specific causes of RCI, the proportion attributed to device failure decreased from 65.1% to 54.8%. In contrast, flap issues and migration both increased from 9.6% to 12.3%, and the rate of electrode misinsertion rose from 4.7% to 9.6%. The 10-year cumulative and device survival rates in this study were 93.4% and 95.8%, respectively.
The most salient finding of this study was the variation in cumulative and device survival rates across manufacturers. In our prior report, MED-EL exhibited the lowest rates of RCI (2.6%) and device failure (1.5%), followed by Cochlear and Advanced Bionics. A report by Lane et al. corroborated these results, indicating the highest RCI and device failure rates for Advanced Bionics and the lowest for MED-EL [8]. Chen et al. [13] observed comparable trends, with Advanced Bionics displaying the highest rates of RCI and device failure, while MED-EL exhibited the third-highest RCI rate and the second-highest device failure rate. However, a key limitation of these studies is that they predate 2020 and therefore do not account for the most recent device models. To our knowledge, the present investigation is the first to evaluate changes in RCI profile, while specifically including the latest models released by various manufacturers. Compared to our previous investigation, the rates for MED-EL have increased for both RCI (rising from 2.6% to 5.9%) and device failure (increasing from 1.5% to 3.6%). These changes may be attributable to the SYNCHRONY model, which experienced a rise in device failure from 3.2% in the previous study to 5.3%. These findings suggest that failure can occur years after implantation, and the number of events has presumably increased as the follow-up duration of SYNCHRONY, which has seen more widespread use in recent years, has lengthened. While the latest models from each manufacturer seem to demonstrate exceptional safety, with only a single device failure reported, the long-term prognosis of these newest models warrants more comprehensive evaluation in the years to come.
Due to the clinical importance of device failure and the need for subsequent reoperation, many clinicians have sought to identify the factors associated with RCI. Recent systematic reviews have reported an overall RCI rate of 5.5%–6.0% and a device failure rate of 2.2%–3.6% [8,10], findings that align with our own series. However, no consensus has been reached on the factors that contribute to RCI or device failure, and several studies have reported conflicting results. For instance, a study by Chen et al. [13] found that younger patients and those who received CIs during the earlier years of the investigation (1996 to 2005) were relatively likely to require revision surgery. Additionally, cumulative and device survival rates were higher among older patients. Another study indicated that devices manufactured after 2000 exhibited improved cumulative survival rates compared to earlier models, while device failure rates varied significantly among manufacturers, with Advanced Bionics exhibiting the lowest rate [8]. In contrast, Layfield et al. [10] reported no significant distinctions in revision rates among manufacturers. Similarly, our previous study did not reveal significant differences in cumulative or device survival rates by manufacturer. In the present study, although no significant differences in cumulative or device survival rates were initially found among manufacturers, significant differences did emerge when models with recall issues were excluded. Further analysis using the Cox proportional hazards model revealed an HR of 2.14 for device failure in MED-EL compared to Cochlear, which may reflect the recent shift in device failure patterns mentioned earlier. However, evidence regarding this issue is still lacking, and additional research is required to better understand the potential factors influencing survival rates.
The present study also uncovered several findings that reflect recent changes in RCI. For instance, technological advancements have led to a decline in the incidence of delayed device failure for CIs performed in recent periods, as depicted in Fig. 2B. Notably, no device failures have been reported after 5 years of CI in patients who underwent the procedure after 2013, suggesting that recently introduced devices demonstrate superior long-term durability compared to those from earlier periods. However, alongside the reduction in device failure, new challenges have arisen; these include issues with flap retention, which emerged as a novel issue during the study period. In recent years, the advent of diametric magnets has enabled safe magnetic resonance imaging up to 3 T without the need for additional measures such as compression bandages or magnet removal, which were necessary with axial magnets [15]. However, diametric magnets exert comparatively weak magnetic forces, potentially resulting in suboptimal signal transmission or detachment of the sound processor [16]. As a result, the recommended skin flap thickness for securing the sound processor has been reduced. For example, Cochlear now advises a flap thickness of less than 6 mm for the off-the-ear sound processor in the CI 600 series, a reduction of 4 mm from the CI 500 series. This change often necessitates procedures such as soft-tissue debulking to thin the flap or positioning of the internal device above the temporalis muscle layer [17]. Notably, all three instances of flap retention issues in this study occurred with recent models equipped with diametric magnets. Another concern is that flap reduction surgery may lead to complications, including infection or skin necrosis [18,19]. Additionally, superficial placement of the internal device increases the risk of trauma or magnet exposure [16]. While factors like sex, body mass index, hair type, or sound processor model may also influence retention [16,20,21], special attention should be paid to flap retention and the potential risk associated with additional flap reduction procedures for newer devices.
With the development of slimmer and more flexible electrodes, the risk of incorrect placement within the cochlea has increased, even when the electrodes are successfully inserted into the scala tympani. In the present study, four of six TFs occurred with the use of later models equipped with PM electrodes. The positive association between TF and this type of electrode has been well established [22-25], with reported TF rates ranging from 1.7% to 5.3%. In contrast, LW electrodes have been associated with TF rates of approximately 0.2% to 1.0% [26,27]. A recent systematic review noted a TF rate of 5.9% for the CI 532 model, suggesting a heightened risk of TF with the use of slim PM electrodes with removable external sheaths [23]. Although research on the TF rate for the CI 600 series is limited [28], electrode-related complications are expected to follow a pattern similar to that of the CI 500 series, as the CI 600 series employs the same electrodes. The results of this study indicate a TF rate of 3.5% for slim PM electrodes (such as those present in the CI 532 and CI 632), while the rate for slim LW electrodes (as in the CI 522 and CI 622) was only 0.7%. Clinically, TF negatively impacts auditory outcomes and may necessitate revision surgery, underscoring the need for prevention and early detection of TF through intraoperative measures [25,29]. However, the role of intraoperative electrophysiological testing in predicting TF is currently limited [25,30,31]. In a recent study by Mittmann et al. [30], none of the four TF cases in their series exhibited significant abnormal ECAP findings. Similarly, in the present study, all six TF cases presented with normal impedance and ECAP results. Alternative approaches, such as intraoperative computed tomography or the assessment of the spread of excitation to evaluate neural selectivity near each electrode, have been proposed [31-33]. Further research is necessary to determine the practical roles of these techniques in predicting TF, which may help reduce the occurrence of reoperation due to TF in the future.
While the present study offers valuable insights into clinically significant issues regarding recent changes in RCI and device failure, it also had several limitations. The retrospective study design, which included cases from decades ago, meant that certain information was unavailable that may have aided in determining the reasons for RCI. Consequently, possible contributing factors such as body mass index or sex in relation to flap-related complications, as well as cochlear lumen diameter for instances of electrode misinsertion, were not accounted for in this analysis. Moreover, to corroborate our experiences and findings regarding the latest models, a longer observation period is required, particularly for device failure. Factors pertaining to the patient, such as head trauma, may also influence device failure and should be considered when interpreting our results. Additionally, since the data were sourced from a single tertiary center, generalizing the findings may prove challenging. Future studies involving multiple centers could therefore be instrumental in validating the findings of this investigation. Despite these limitations, this study included more than 1,400 CIs performed at a single center over the past 22 years and incorporated devices from all three leading manufacturers. The results provide updated details and insights into the patterns of RCI. The findings are poised to substantially aid in minimizing surgical failures and enhancing the outcomes of CIs in clinical practice.
In conclusion, as the number of CIs has risen and technological advancements have been made, the characteristics of RCIs have changed. While the overall cumulative and device survival rates have remained comparable to historical data, a discernible variation is now present in device failure rates across manufacturers, particularly for their most recent models. Moreover, issues related to flaps and electrodes have increased slightly due to the introduction of new technologies. Continuous monitoring and prompt action in response to these developments are crucial for improving the success rate of CI.
▪ Over 23 years of cochlear implantation (CI), a total of 73 revision CIs (RCIs) were performed, meaning that 5.1% of CIs required revision.
▪ Device failure was the predominant reason for RCI, comprising 40 cases (54.8%), and the overall rate of device failure was 2.8%.
▪ Flap retention problems emerged as a new issue, stemming from the incorporation of diametric magnets in recent implant models.
▪ Most instances of tip fold-over were observed in newer models equipped with slim perimodiolar electrodes.
▪ Given the rapid evolution of the RCI profile, continuous monitoring and prompt responses to these changes are crucial for improving the success rate of CI.
REFERENCES
1. Dettman SJ, Dowell RC, Choo D, Arnott W, Abrahams Y, Davis A, et al. Long-term communication outcomes for children receiving cochlear implants younger than 12 months: a multicenter study. Otol Neurotol. 2016; Feb. 37(2):e82–95.
2. Naik AN, Varadarajan VV, Malhotra PS. Early pediatric cochlear implantation: an update. Laryngoscope Investig Otolaryngol. 2021; Jun. 6(3):512–21.
3. Fakurnejad S, Vail D, Song Y, Alyono J, Blevins NH. Trends in age of cochlear implant recipients, and the impact on perioperative complication rates. Otol Neurotol. 2020; Apr. 41(4):438–43.
4. Bourn SS, Goldstein MR, Morris SA, Jacob A. Cochlear implant outcomes in the very elderly. Am J Otolaryngol. 2022; 43(1):103200.
5. Wichova H, Mills D, Beatty S, Peng K, Miller M. Cochlear implantation performance outcomes in patients over 80 years old. Laryngoscope Investig Otolaryngol. 2022; Jun. 7(3):847–53.
6. Kim SY, Kim MB, Chung WH, Cho YS, Hong SH, Moon IJ. Evaluating reasons for revision surgery and device failure rates in patients who underwent cochlear implantation surgery. JAMA Otolaryngol Head Neck Surg. 2020; May. 146(5):414–20.
7. Kimura KS, O’Connell BP, Nassiri AM, Dedmon MM, Haynes DS, Bennett ML. Outcomes of revision cochlear implantation. Otol Neurotol. 2020; Jul. 41(6):e705–11.
8. Lane C, Zimmerman K, Agrawal S, Parnes L. Cochlear implant failures and reimplantation: a 30-year analysis and literature review. Laryngoscope. 2020; Mar. 130(3):782–9.
9. Gumus B, Incesulu AS, Kaya E, Kezban Gurbuz M, Ozgur Pinarbasli M. Analysis of cochlear implant revision surgeries. Eur Arch Otorhinolaryngol. 2021; Mar. 278(3):675–82.
10. Layfield E, Hwa TP, Naples J, Maina I, Brant JA, Eliades SJ, et al. Failure and revision surgery after cochlear implantation in the adult population: a 10-year single-institution retrospective and systematic review of the literature. Otol Neurotol. 2021; Mar. 42(3):408–13.
11. Rayamajhi P, Kurkure R, Castellino A, Kumar S, Ha M, Nandhan R, et al. A clinical profile of revision cochlear implant surgery: MERF experience. Cochlear Implants Int. 2021; Mar. 22(2):61–7.
12. Sagiv D, Yaar-Soffer Y, Yakir Z, Henkin Y, Shapira Y. Rates, indications, and speech perception outcomes of revision cochlear implantations. J Clin Med. 2021; Jul. 10(15):3215.
13. Chen J, Chen B, Shi Y, Li Y. A retrospective review of cochlear implant revision surgery: a 24-year experience in China. Eur Arch Otorhinolaryngol. 2022; Mar. 279(3):1211–20.
14. Karamert R, Duzlu M, Tutar H, Eravci FC, Turkcan AK, Zorlu ME, et al. Assessment of cochlear implant revision surgeries in a cohort of 802 patients. Otol Neurotol. 2019; Apr. 40(4):464–70.
15. Rupp R, Balk M, Sievert M, Leibl V, Schleder S, Allner M, et al. Risk of magnetic resonance imaging-induced magnet dislocation for different types of cochlear implants: a single-center retrospective study. J Otolaryngol Head Neck Surg. 2023; Apr. 52(1):28.
16. Posner D, Scott A, Polite C, Lustig LR. External magnet displacement in cochlear implants: causes and management. Otol Neurotol. 2010; Jan. 31(1):88–93.
17. Ozturan O, Yenigun A, Senturk E, Calim OF, Aksoy F, Eren SB. Temporal scalp thickness, body mass index, and suprafascial placement of receiver coil of the cochlear implant. J Craniofac Surg. 2017; Nov. 28(8):e781–5.
18. Kim YH, Cho SI. Skin flap necrosis by bone marking with methylene blue in cochlear implantation. J Audiol Otol. 2015; Sep. 19(2):108–10.
19. Qin F, Li W, Qiu J, Zhang L, Zhong M. After cochlear implantation: complications related to flap around implants. J Otol. 2016; Dec. 11(4):198–201.
20. Searle T, Marshall E, Craddock L, Monksfield P. Skin flap thickness and magnet strength in cochlear implants. Cochlear Implants Int. 2021; Mar. 22(2):80–4.
21. Wagner L, Honig E, Frohlich L, Plontke S, Rahne T. Optimal retention force of audio processor magnets. Otol Neurotol. 2019; Jun. 40(5):e482–7.
22. Jwair S, Prins A, Wegner I, Stokroos RJ, Versnel H, Thomeer HG. Scalar translocation comparison between lateral wall and perimodiolar cochlear implant arrays: a meta-analysis. Laryngoscope. 2021; Jun. 131(6):1358–68.
23. Ishiyama A, Risi F, Boyd P. Potential insertion complications with cochlear implant electrodes. Cochlear Implants Int. 2020; Jul. 21(4):206–19.
24. Ramos-Macias A, R De Miguel A, Falcon-Gonzalez JC. Mechanisms of electrode fold-over in cochlear implant surgery when using a flexible and slim perimodiolar electrode array. Acta Otolaryngol. 2017; Nov. 137(11):1129–35.
25. Zuniga MG, Rivas A, Hedley-Williams A, Gifford RH, Dwyer R, Dawant BM, et al. Tip fold-over in cochlear implantation: case series. Otol Neurotol. 2017; Feb. 38(2):199–206.
26. Hogerle C, Englhard A, Simon F, Gruninger I, Mlynski R, Hempel JM, et al. Cochlear implant electrode tip fold-over: our experience with long and flexible electrode. Otol Neurotol. 2022; Jan. 43(1):64–71.
27. Gabrielpillai J, Burck I, Baumann U, Stover T, Helbig S. Incidence for tip foldover during cochlear implantation. Otol Neurotol. 2018; Oct. 39(9):1115–21.
28. Kay-Rivest E, McMenomey SO, Jethanamest D, Shapiro WH, Friedmann DR, Waltzman SB, et al. Predictive value of transimpedance matrix measurements to detect electrode tip foldover. Otol Neurotol. 2022; Oct. 43(9):1027–32.
29. Gomez Serrano M, Patel S, Harris R, Selvadurai D. Initial surgical and clinical experience with the Nucleus CI532 slim modiolar electrode in the UK. Cochlear Implants Int. 2019; Jul. 20(4):207–16.
30. Mittmann P, Lauer G, Ernst A, Mutze S, Hassepass F, Arndt S, et al. Electrophysiological detection of electrode fold-over in perimodiolar cochlear implant electrode arrays: a multi-center study case series. Eur Arch Otorhinolaryngol. 2020; Jan. 277(1):31–5.
31. Grolman W, Maat A, Verdam F, Simis Y, Carelsen B, Freling N, et al. Spread of excitation measurements for the detection of electrode array foldovers: a prospective study comparing 3-dimensional rotational x-ray and intraoperative spread of excitation measurements. Otol Neurotol. 2009; Jan. 30(1):27–33.
32. Cosetti MK, Troob SH, Latzman JM, Shapiro WH, Roland JT, Waltzman SB. An evidence-based algorithm for intraoperative monitoring during cochlear implantation. Otol Neurotol. 2012; Feb. 33(2):169–76.
33. Trakimas DR, Kozin ED, Ghanad I, Barber SR, Curtin H, Remenschneider AK. Precurved cochlear implants and tip foldover: a cadaveric imaging study. Otolaryngol Head Neck Surg. 2018; Feb. 158(2):343–9.
Fig. 1.
Overall cumulative and device survival. (A) Before exclusion of models subject to recall. The excluded models were the CI 512, Clarion CII, HiRes 90K, and HiRes Ultra3D. (B) After exclusion. CI, cochlear implantation.
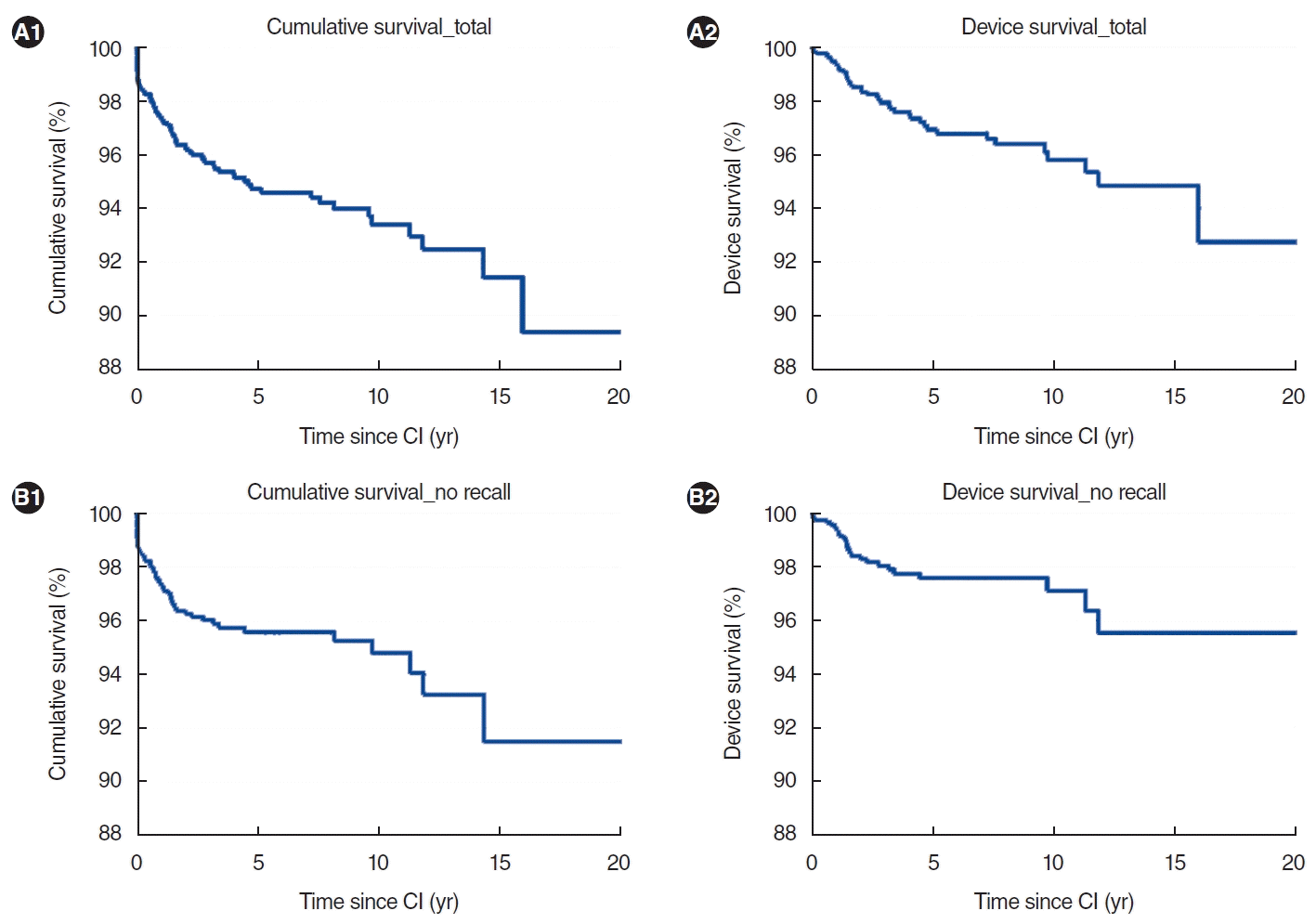
Fig. 2.
Cumulative survival and device survival. (A) By age group. (B) By period of primary implantation. (C) By manufacturer. CI, cochlear implantation.
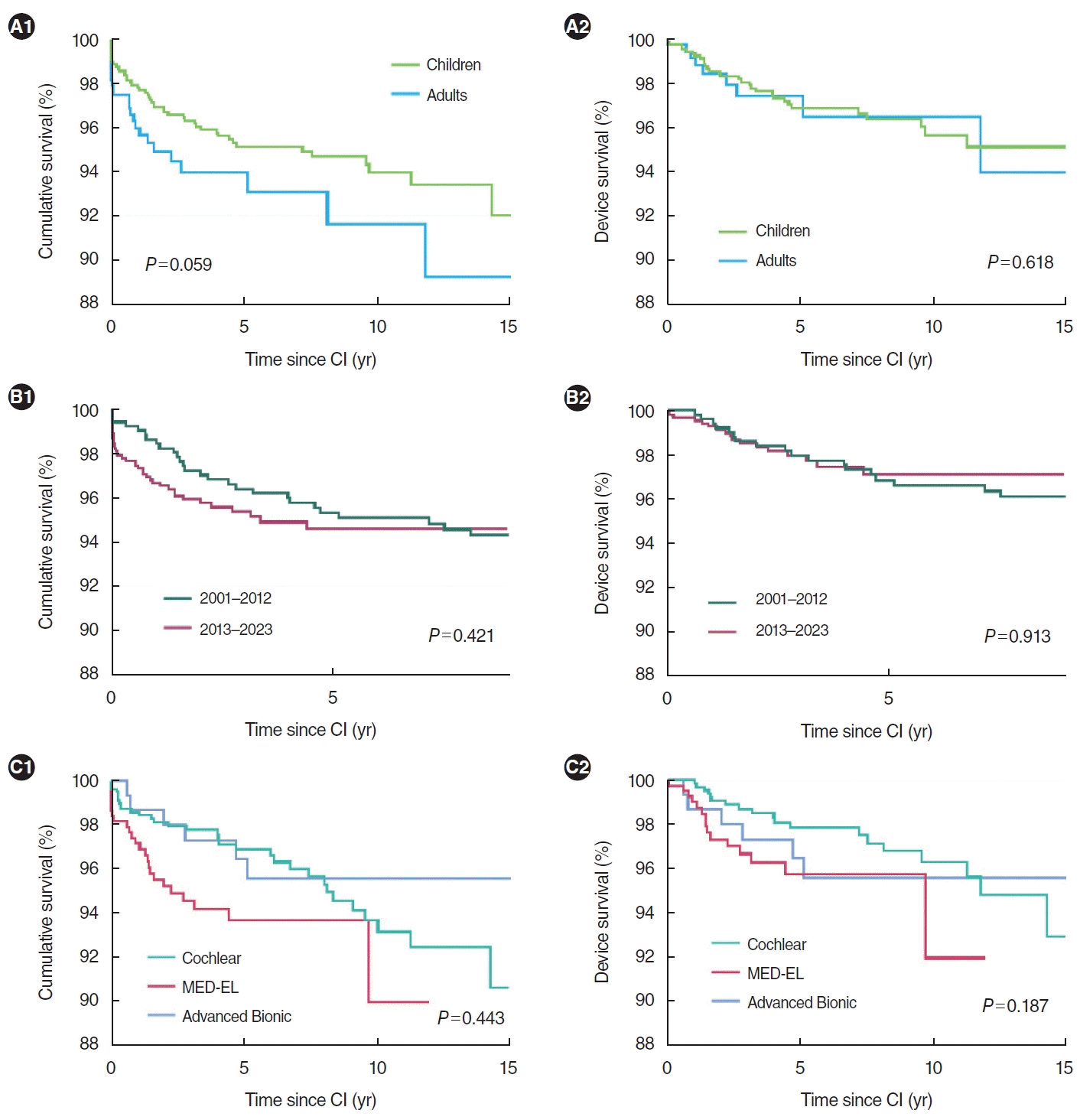
Table 1.
Summary of characteristics in revision cases
Table 2.
Reasons for and rates of revision by manufacturer
Table 3.
Cumulative and device survival rates by age group, period of primary implantation, and device manufacturer
|
Cumulative survival rate (%) |
Device survival rate (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 5 yr | 10 yr | 15 yr | P-valuea) | 5 yr | 10 yr | 15 yr | P-valuea) | |
| Total | 94.7 | 93.4 | 91.5 | 97.0 | 95.8 | 94.9 | ||
| Age group | 0.059 | 0.618 | ||||||
| Children | 95.2 | 94.0 | 92.1 | 96.9 | 95.7 | 95.1 | ||
| Adults | 94.0 | 91.8 | 89.4 | 97.4 | 96.5 | 94.0 | ||
| Period of primary CI | 0.421 | 0.913 | ||||||
| 2001–2012 | 95.3 | 94.0 | NA | 96.9 | 95.8 | NA | ||
| 2013–2023 | 94.6 | NA | NA | 97.2 | NA | NA | ||
| Manufacturer | 0.443 | 0.187 | ||||||
| Cochlear | 95.4 | 94.0 | 90.7 | 97.9 | 96.7 | 95.2 | ||
| Advanced Bionics | 95.3 | 94.5 | 94.5 | 96.4 | 95.6 | 95.6 | ||
| MED-EL | 93.3 | 89.5 | NA | 95.5 | 91.7 | NA | ||
| After excluding models with recall issueb) | 95.6 | 94.8 | 91.5 | 0.010 | 97.6 | 97.1 | 95.5 | 0.001 |
| Cochlear | 96.9 | 96.5 | 93.1 | 98.8 | 98.8 | 97.2 | ||
| Advanced Bionics | NA | NA | NA | NA | NA | NA | ||
| MED-EL | 93.3 | 89.5 | NA | 95.5 | 91.7 | NA | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download