1. Shin JH, Baek JH, Ha EJ, Lee JH. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012; 2012:919650.
2. Morelli F, Sacrini A, Pompili G, Borelli A, Panella S, Masu A, et al. Microwave ablation for thyroid nodules: a new string to the bow for percutaneous treatments? Gland Surg. 2016; 5:553–8.
3. Ahn HS, Kim SJ, Park SH, Seo M. Radiofrequency ablation of benign thyroid nodules: evaluation of the treatment efficacy using ultrasonography. Ultrasonography. 2016; 35:244–52.
4. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009; 33:1971–7.
5. Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an exvivo bovine liver tissue model. Korean J Radiol. 2014; 15:836–43.
6. Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008; 18:1244–50.
7. Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017; 18:615–23.
8. Kuo CY, Liu CL, Tsai CH, Cheng SP. Learning curve analysis of radiofrequency ablation for benign thyroid nodules. Int J Hyperthermia. 2021; 38:1536–40.
9. Russ G, Ben Hamou A, Poiree S, Ghander C, Menegaux F, Leenhardt L, et al. Learning curve for radiofrequency ablation of benign thyroid nodules. Int J Hyperthermia. 2021; 38:55–64.
10. Sinclair CF, Baek JH, Hands KE, Hodak SP, Huber TC, Hussain I, et al. General principles for the safe performance, training, and adoption of ablation techniques for benign thyroid nodules: an American Thyroid Association Statement. Thyroid. 2023; 33:1150–70.
11. Li YR, Chou WY, Chan WK, Cheng KL, Sun JH, Liu FH, et al. Successful applications of food-assisted and -simulated training model of thyroid radiofrequency ablation. Front Endocrinol (Lausanne). 2022; 13:809835.
12. Ha EJ, Baek JH, Che Y, Chou YH, Fukunari N, Kim JH, et al. Radiofrequency ablation of benign thyroid nodules: recommendations from the Asian Conference on Tumor Ablation Task Force. Ultrasonography. 2021; 40:75–82.
13. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018; 19:632–55.
14. Xiaoyin T, Ping L, Dan C, Min D, Jiachang C, Tao W, et al. Risk assessment and hydrodissection technique for radiofrequency ablation of thyroid benign nodules. J Cancer. 2018; 9:3058–66.
15. Chung SR, Baek JH, Choi YJ, Lee JH. Thermal ablation for the management of papillary thyroid microcarcinoma in the era of active surveillance and hemithyroidectomy. Curr Oncol Rep. 2022; 24:1045–52.
16. Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010; 194:1137–42.
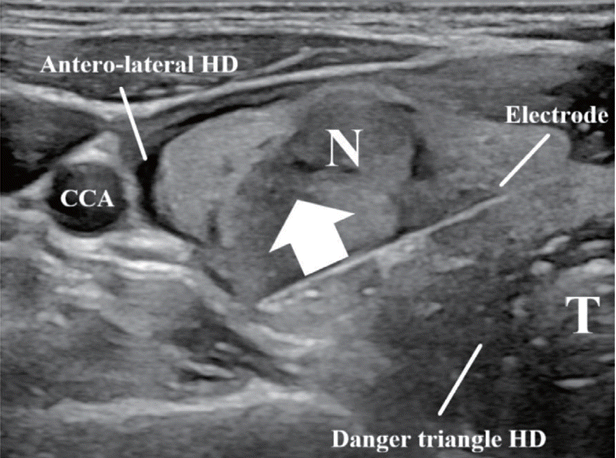
17. Jeong SY, Baek JH, Chung SR, Choi YJ, Chung KW, Kim TY, et al. Radiofrequency ablation of benign thyroid nodules: the value of anterolateral hydrodissection. Ultrasonography. 2023; 42:432–9.
18. Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017; 33:920–30.
19. Cho SJ, Baek JH, Chung SR, Choi YJ, Lee JH. Long-term results of thermal ablation of benign thyroid nodules: a systematic review and meta-analysis. Endocrinol Metab (Seoul). 2020; 35:339–50.
20. Ma Y, Wu T, Yao Z, Zheng B, Tan L, Tong G, et al. Continuous, large-volume hydrodissection to protect delicate structures around the thyroid throughout the radiofrequency ablation procedure. Eur Thyroid J. 2021; 10:495–503.
21. Lee M, Baek JH, Suh CH, Chung SR, Choi YJ, Lee JH, et al. Clinical practice guidelines for radiofrequency ablation of benign thyroid nodules: a systematic review. Ultrasonography. 2021; 40:256–64.
22. Pillai K, Akhter J, Chua TC, Shehata M, Alzahrani N, AlAlem I, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore). 2015; 94:e580.
23. Wang B, Han ZY, Yu J, Cheng Z, Liu F, Yu XL, et al. Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int J Hyperthermia. 2017; 33:459–64.
24. Zhao CK, Xu HX, Lu F, Sun LP, He YP, Guo LH, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. Clin Hemorheol Microcirc. 2017; 65:393–405.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download