Abstract
Antenatally diagnosed urinary tract dilatation (UTD), previously referred to as antenatal hydronephrosis, is the most commonly detected abnormality by prenatal ultrasonography. Several grading systems have been developed for the classification of antenatal UTD using prenatal and postnatal ultrasonography. UTD comprises a wide variety of congenital abnormalities of the kidney and urinary tract ranging from transient UTD to more significant abnormalities such as vesicoureteral reflux, ureteropelvic junction obstruction, ureterocele, ureterovesical junction obstruction, posterior urethral valves, and non-refluxing megaureter. Optimizing the evaluation of antenatally detected UTD is essential to recognize children with important disorders while avoiding excessive investigations. Conservative approach with close follow-up is increasingly accepted as an appropriate treatment option for patients with asymptomatic vesicoureteral reflux and ureteropelvic junction obstruction in recent years. However, predicting permanent kidney damage in an unselected group of children with antenatal UTD is still challenging. The management and follow-up of children with UTD should be individualized based on recommendations from a pediatric nephrologist, a pediatric urologist, or both. Future research directed at predicting long-term outcomes of children diagnosed with UTD from mild findings to severe disease is needed to refine management for those at higher risk of kidney disease progression.
Antenatal urinary tract dilatation (UTD) is commonly found on routine fetal ultrasound [1,2]. UTD is a preferred term while various terminology such as congenital, fetal, antenatal, or prenatal hydronephrosis has been used for years [3,4]. Given that it is associated with a broad spectrum of conditions ranging from a transient finding to congenital abnormalities of the kidney and urinary tract (CAKUT) leading to chronic kidney disease (CKD), it is important to avoid unnecessary testing and identify cases of significant urinary tract anomaly. Nguyen et al. [4] have recently updated the UTD classification system. A new guideline was subsequently developed for the evaluation of antenatal and postnatal UTD [4]. This review aimed to discuss a clinically significant question evaluating and managing antenatal UTD from a pediatric nephrologist’s view and to assist pediatricians in their decision-making about the antenatal UTD.
Different grading systems for UTD have been developed for a long time. The Society for Fetal Urology (SFU) system and renal pelvis anterior-posterior diameter (APD) measurements for grading UTD are commonly used [5]. However, the recently updated UTD classification [4] seems to be appropriate for predicting long-term renal outcome and need for surgery in addition to identifying postnatal CAKUT [6-8]. According to the UTD classification system [4], antenatal UTD is defined as a renal pelvis APD of ≥4 mm in the second trimester (<28 weeks) and/or ≥7 mm in the third trimester (≥28 weeks). The new system focuses not only on the kidney, but also on the entire urinary tract system, classifying UTD into two antenatal categories (UTD A1, UTD A2-3) and three postnatal categories (UTD P1, P2, and P3). UTD A1 is considered at low risk for postnatal CAKUT based on APD of 4 to <7 mm at <28 weeks and APD of 7 to <10 mm at ≥28 weeks. Abnormal kidney parenchyma (cortical thinning, hyperechogenicity, cystic dysplasia, or indistinct corticomedullary differentiation), calyces, ureters, bladder (wall thickening, ureterocele, or dilated posterior urethra), or amniotic fluid accompanied with a renal APD of ≥7 mm at <28 weeks or ≥10 mm at ≥28 weeks corresponds to antenatal UTD A2-3, which is considered to be at an increased risk for postnatal CAKUT. At least 48 hours after birth, the presence of a renal pelvis APD of < 10 mm without other abnormalities (no calyceal or ureteral dilation, no abnormalities of renal parenchyma or bladder) is defined as normal in the UTD classification system. In this system, an APD of 10 to 15 mm or central calyx dilatation is defined as UTD P1 (low risk) and an APD of ≥15 mm or peripheral calyceal dilatation or dilated ureter of >4 mm with an APD of ≥10 mm or calyceal dilatation is defined as UTD P2 (intermediate risk). The presence of renal parenchymal abnormality, bladder abnormality, or oligohydramnios combined with an APD of ≥10 mm or any calyceal dilatation is classified as UTD P3 (high risk) [3,4]. UTD P1, UTD P2, and UTD P3 are comparable to SFU grade I-II, SFU grade III, and SFU grade IV, respectively (Fig. 1) [9].
The main etiologies of antenatally diagnosed antenatal UTD can be grouped into three broad categories: (1) physiologic or transient dilation; (2) vesicoureteral reflux (VUR); and (3) obstructive uropathy. As the degree of antenatal and postnatal UTD increases, there is an increased risk of CAKUT except VUR [10]. A recent paper reported that one-third of children with antenatal UTD had the UTD before birth and that the UTD was resolved or stabilized by the end of 2 to 3 years for another third of children while UTD persisted or CAKUT was diagnosed for the remaining cases [3]. Transient or physiologic UTD might be associated with hydration status, bladder filling, transient narrowing of the ureteropelvic junction (UPJ), or delayed maturation of ureteral peristalsis [11]. UPJ obstruction and VUR are the two most common CAKUT conditions, both of which can be diagnosed in 10% to 12% of cases [1,12]. Other CAKUT conditions causing UTD include ureterovesical junction obstruction, primary non-refluxing megaureter, bladder outlet obstruction including posterior urethral valve (PUV) or ureterocele, duplex collecting system, multicystic dysplastic kidney, and so on [3,11].
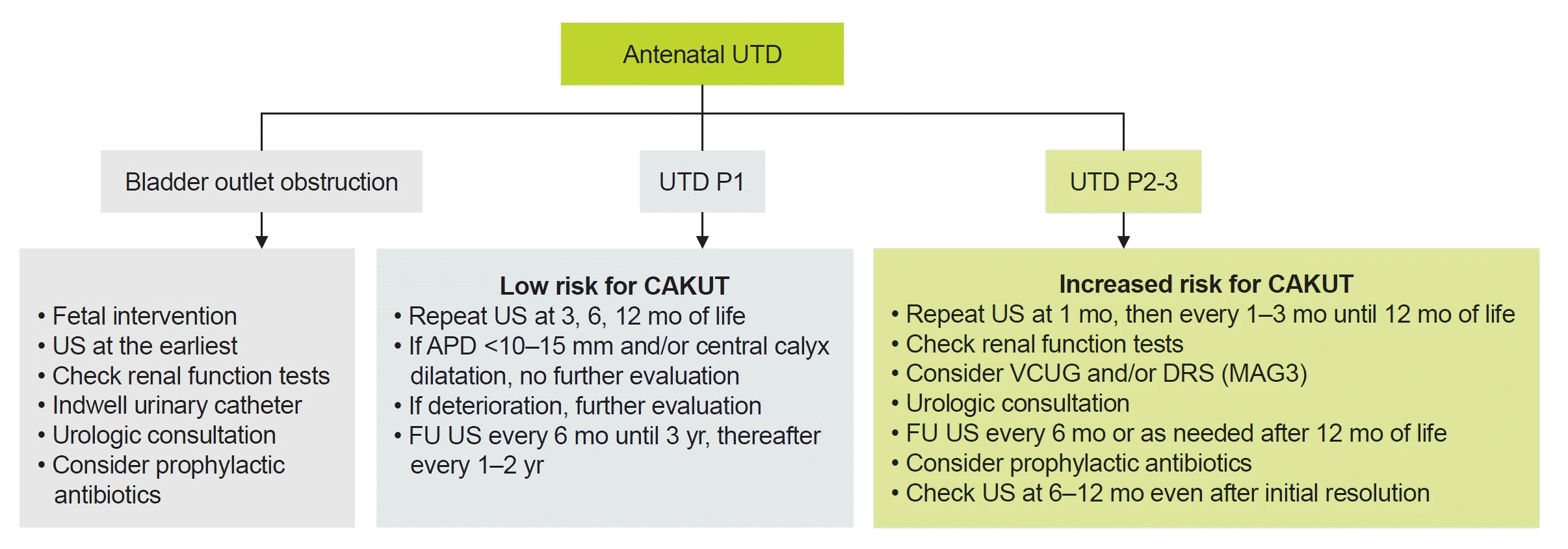
Extensive investigation for UTD is being proposed for cases with moderate or severe dilatation (UTD A2-3, P2, and P3). Nonetheless, it is recommended that all antenatal UTDs (UTD A1, A2-3) should be validated by two serial postnatal ultrasonography (US) at >48 hours after birth and a few weeks or months later [3,13]. For cases with suspected bladder outlet obstruction, kidney and bladder US should be checked as soon as possible postnatally and renal function should be checked together with indwelling urinary catheter. With urologic consultation, a follow-up US should be performed sooner [3,13]. For UTD P1, a follow-up US is recommended at 3, 6, and 12 months of life [3]. If there are only renal pelvis APD of <10 to 15 mm and/or central calyceal dilatation (UTD P1), further evaluation is not recommended [3]. However, peripheral calyceal dilation has been reported to increase the risk of a diagnosis of CAKUT [3,4]. If there are worsening findings on serial postnatal US, further work-up including renal function tests, voiding cystourethrogram (VCUG) or mercaptoacetyltriglycine (MAG3) scintigraphy (or diuretic renal scan) and urologic consultation should be considered. For UTD P2-3, a follow-up US in 1 to 3 months is suggested. Evaluation with renal function tests (especially serum creatinine, electrolytes, and blood gas analysis), VCUG, and MAG3 scan are considered [3,14]. Diuretic renal scan is usually performed from 6 to 8 weeks of age [3]. Measurement of serum cystatin C instead of creatinine may offer significant advantages in neonates and young infants given that serum cystatin C levels are less affected by age, sex, dietary protein intake, and muscle mass compared to creatinine [15]. Consultation to a pediatric urologist and the use of prophylactic antibiotics can be considered based on the severity of clinical conditions. Supplementary comments for the antibiotic prophylaxis will be mentioned in the following subject (Q5). A follow-up US at 6–12 months even after initial resolution could be recommended for some cases with UTD P2-3 since a recurrence of significant UTD has been reported in patients with spontaneous improvement (Fig. 2) [14,16].
The exact indications and suitable time for surgical intervention remain controversial. About 50% of postnatal UTDs will resolve and the remaining 40% to 45% will show improvement or stabilization of UTD within the first 3 years of life [3,14,17]. General indications for surgery include bladder outlet obstruction with oligohydramnios and recurrent urinary tract infections (UTIs) with VUR or UPJ obstruction. Increasing dilatation and/or decreasing split function (<40% with impaired renal drainage or >10% of renal function deterioration on a follow-up renal scan) are also indicative of the need for surgery [3,13,14]. The cumulative incidence of needing surgery was about 20% to 30% children with antenatal UTD in long-term studies [17-19], while another study reported a far smaller proportion of patients undergoing any surgical procedure [20]. Yang et al. [18] have found that the ipsilateral differential renal function is preserved only in the early pyeloplasty group, implying that early surgical treatment is important to preserve renal function in patients with persistent UTD P2-3 (SFU grade III or IV). In addition, several predictors for surgical intervention have been suggested, including initial postnatal APD, renal pyramidal thickness, delayed cortical tissue transit time on diuretic renal scan, and renal parenchyma-to-hydronephrosis area ratio [21-24].
In general, children with antenatally diagnosed UTD are at an increased risk of UTI. Infants with antenatal UTD are more likely to have acute pyelonephritis within the first year of life than those without UTD [25]. Several studies have shown a cumulative incidence of 7% to 14% for UTI during infancy [26-28]. While some studies reported higher incidence of 14% to 40% for UTI especially in cases with moderate or severe UTD [29,30], others revealed lower occurrence of UTI (3.3% to 6.83%) in children with antenatal diagnosis of UTD [31,32]. Among underlying uropathies, VUR has been shown to be the most important risk factor for UTI within the UTD population [28]. UTI rates were 3- to 6-fold higher in patients with hydroureteronephrosis than in those with isolated hydronephrosis according to a systematic review by Braga et al. [33]. For a long time, the use of continuous antibiotic prophylaxis (CAP) has been a challenging issue. A systematic review [34] has shown that uncircumcised boys and children with ureteral dilatation and/or high-grade UTD are more prone to develop UTI and that CAP is recommended for these subgroups of patients. However, benefits of CAP are limited in infants with mild to moderate UTD since the protective effect of CAP against UTI has not been revealed yet [34,35]. The use of CAP for UTI prevention in infants with prenatal UTD has been acknowledged as a low level of evidence by the American Urological Association, the SFU, and the Canadian Urological Association [33].
While permanent kidney damage is known to occur in about 40% of children with moderate or severe UTD [3], only a few studies have provided long-term outcomes of antenatally detected UTD [18,20,36,37]. Costa et al. [19] have reported the development of a composite event of hypertension, proteinuria, and/or reduced estimated glomerular filtration rate (eGFR) in 5% of a cohort of 447 children with isolated antenatal APD ≥5 mm at a median follow-up of 6.4 years. However, children with mild UTD did not have any chronic kidney damage during the follow-up period. Another study by Herthelius et al. [20] has shown that none of the children with antenatally detected UTD has proteinuria or reduced eGFR during 12 to 15 years of follow-up. Among confirmed cases with postnatal renal APD >7 mm and/or kidney parenchyma, calyces, ureters, or bladder pathology, persistent UTD occurred in 15% and persistent kidney damage assessed by renal DMSA (technetium 99m dimercaptosuccinic acid) scan or US was developed in 32% to 39%. They have concluded that it is unnecessary to perform long-term follow-up or use CAP in children with postnatal APD ≤7 mm and normal renal parenchyma, calyces, ureters, and bladder. According to a recent report by Herthelius [3], CAKUT is less likely to be diagnosed afterward in children older than 1 year who have a renal APD <15 mm without other abnormal findings on repeated exams. In contrast, there is a different story for a much longer follow-up of children with CAKUT [38,39]. In a study performed by Sanna-Cherchi et al. [38], renal deterioration was not evident until late adolescence apart from PUVs and bilateral hypodysplasia. However, 58 (18.6%) of 312 patients with CAKUT had started dialysis by 30 years of age. Patients with single kidney and those with renal hypodysplasia combined with PUVs were at increased risks for dialysis (hazard ratios: 2.43 and 5.1, respectively) compared to those with renal hypodysplasia, multicystic kidney, or horseshoe kidney [38]. Another study has also revealed that end-stage kidney disease caused by CAKUT is developed more often in adult age than in pediatric age [39]. Using data on the incidence and prevalence of renal replacement therapy (RRT) in a total of 212,930 patients, the median age at RRT start was found to be 31 years for patients with CAKUT and 61 years for those with non-CAKUT [39]. Patients with renal dysplasia required RRT at a very young age (median, 16 years) compared with those in other CAKUT categories. The incidence of RRT due to reflux-associated pyelonephritis increased sharply during the first two decades, reaching its peak in the early 20s. However, 50% of patients with CAKUT did not start RRT before turning 40s [39]. These studies suggest that ongoing loss of remnant nephrons can lead to CKD progression across the entire age range. Mild forms of CAKUT, including low nephron endowment at birth, seem to be more frequent than expected and be revealed in later adulthood [40]. Effective transition strategies from pediatric to adult nephrology service would be essential to achieve disease-specific good-quality care for this group of patients [39]. Individualized follow-up and management plans for children with UTD and/or CAKUT should be applied based on recommendations from a pediatric nephrologist or urologist, or both.
Since 2014, many studies have been performed to validate the correlation between the UTD classification system and clinical outcomes [6-9,20]. For predicting various outcomes such as surgical intervention, UTI risk, and chronic kidney damage, further extensive evaluation regarding the grading system would be necessary to assess its utility. Meanwhile, over the years, numerous urinary and serum biomarkers for UPJ obstruction and VUR have been studied, including neutrophil gelatinase-associated lipocalin [41-43], cystatin C [41], kidney injury molecule-1 [44], monocyte chemoattractant protein-1 [44], β2-microglobulin [43], and so on. Further studies are also needed to confirm the efficacy of these biomarkers to predict the development and progression of CKD as well as CAKUT itself. In addition, in line with the artificial intelligence era, investigations for grading UTD using machine learning algorithms (automated convolutional neural network model) have been reported. It was reported that a machine learning model classified 94% to 97.6% of patients correctly or within one grade of the diagnosis of radiologists of UTD [45,46]. Deep learning model could also predict renal complications in children with antenatal UTD concerning UPJ obstruction [47]. These models may offer great promise in their ability to affect clinical decision-making with a large amount of supplemental analytical data. Since genetic and environmental contributions for CAKUT have been identified, long-term prospective studies of patients with CAKUT coupled with comprehensive genomic analysis, functional validation of genetic variants, and an in-depth assessment of the in utero or perinatal environment are needed [40,48]. Recent advances in genetics, epigenetics, and molecular medicine might also offer an opportunity to expand our knowledge on the development of CAKUT and the proper management of patients with this condition.
Optimal evaluation of antenatal and/or postnatal UTD is essential as children with clinically significant abnormalities need to be identified while avoiding unnecessary testing. While most children with antenatal UTD have a favorable long-term outcome with a low risk of kidney disease progression, a greater portion of children with CAKUT need RRT during adulthood than during childhood. There is no definite answer to the question of at what time point we can stop the follow-up safely in a child with persistent UTD. In children with persistent moderate or severe UTD (UTD P2-P3, SFU III-IV), a non-negligible risk of permanent kidney damage exists. To improve the evaluation and management of these patients, future research studies should perform additional risk stratification and develop evidence-based interventions.
Notes
Conflicts of interest
Hyung Eun Yim is an editorial-in-chief of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
References
1. Ismaili K, Hall M, Donner C, Thomas D, Vermeylen D, Avni FE, et al. Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol. 2003; 188:242–6.

2. Feldman DM, DeCambre M, Kong E, Borgida A, Jamil M, McKenna P, et al. Evaluation and follow-up of fetal hydronephrosis. J Ultrasound Med. 2001; 20:1065–9.

3. Herthelius M. Antenatally detected urinary tract dilatation: long-term outcome. Pediatr Nephrol. 2023; 38:3221–7.

4. Nguyen HT, Phelps A, Coley B, Darge K, Rhee A, Chow JS. 2021 update on the urinary tract dilation (UTD) classification system: clarifications, review of the literature, and practical suggestions. Pediatr Radiol. 2022; 52:740–51.

5. Kim SY, Kim MJ, Yoon CS, Lee MS, Han KH, Lee MJ. Comparison of the reliability of two hydronephrosis grading systems: the Society for Foetal Urology grading system vs. the Onen grading system. Clin Radiol. 2013; 68:e484–90.

6. Melo FF, Mak RH, Simoes E Silva AC, Vasconcelos MA, Dias CS, Rosa LC, et al. Evaluation of urinary tract dilation classification system for prediction of long-term outcomes in isolated antenatal hydronephrosis: a cohort study. J Urol. 2021; 206:1022–30.

7. Melo FF, Vasconcelos MA, Mak RH, Silva AC, Dias CS, Colosimo EA, et al. Postnatal urinary tract dilatation classification: improvement of the accuracy in predicting kidney injury. Pediatr Nephrol. 2022; 37:613–23.

8. Hwang J, Kim PH, Yoon HM, Song SH, Jung AY, Lee JS, et al. Application of the postnatal urinary tract dilation classification system to predict the need for surgical intervention among neonates and young infants. Ultrasonography. 2023; 42:136–46.

9. Nguyen HT, Benson CB, Bromley B, Campbell JB, Chow J, Coleman B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatr Urol. 2014; 10:982–98.

10. Lee RS, Cendron M, Kinnamon DD, Nguyen HT. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics. 2006; 118:586–93.

11. Jain S, Chen F. Developmental pathology of congenital kidney and urinary tract anomalies. Clin Kidney J. 2018; 12:382–99.

12. Mallik M, Watson AR. Antenatally detected urinary tract abnormalities: more detection but less action. Pediatr Nephrol. 2008; 23:897–904.

13. Aksu N, Yavascan O, Kangin M, Kara OD, Aydin Y, Erdogan H, et al. Postnatal management of infants with antenatally detected hydronephrosis. Pediatr Nephrol. 2005; 20:1253–9.

14. Deshpande AV. Conversations for the future in the follow-up of antenatally diagnosed renal pelvicalyceal dilatation. Pediatr Nephrol. 2021; 36:5–8.

15. Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children: a meta-analysis. Clin Biochem. 2007; 40:383–91.
16. Matsui F, Shimada K, Matsumoto F, Takano S. Late recurrence of symptomatic hydronephrosis in patients with prenatally detected hydronephrosis and spontaneous improvement. J Urol. 2008; 180:322–5.

17. Zee RS, Herndon CD, Cooper CS, Kim C, McKenna PH, Khoury A, et al. Time to resolution: a prospective evaluation from the Society for Fetal Urology hydronephrosis registry. J Pediatr Urol. 2017; 13:316.

18. Yang Y, Hou Y, Niu ZB, Wang CL. Long-term follow-up and management of prenatally detected, isolated hydronephrosis. J Pediatr Surg. 2010; 45:1701–6.

19. Costa FP, Simoes E Silva AC, Mak RH, Ix JH, Vasconcelos MA, Dias CS, et al. A clinical predictive model of renal injury in children with isolated antenatal hydronephrosis. Clin Kidney J. 2019; 13:834–41.

20. Herthelius M, Axelsson R, Lidefelt KJ. Antenatally detected urinary tract dilatation: a 12-15-year follow-up. Pediatr Nephrol. 2020; 35:2129–35.

21. Dias CS, Silva JM, Pereira AK, Marino VS, Silva LA, Coelho AM, et al. Diagnostic accuracy of renal pelvic dilatation for detecting surgically managed ureteropelvic junction obstruction. J Urol. 2013; 190:661–6.

22. Song SH, Park S, Chae SY, Moon DH, Park S, Kim KS. Predictors of renal functional improvement after pyeloplasty in ureteropelvic junction obstruction: clinical value of visually assessed renal tissue tracer transit in 99mTc-mercaptoacetyltriglycine renography. Urology. 2017; 108:149–54.

23. Hodhod A, Capolicchio JP, Jednak R, Eid H, El-Doray AE, El-Sherbiny M. Is the renal pyramidal thickness a good predictor for pyeloplasty in postnatal hydronephrosis? J Pediatr Urol. 2018; 14:277.

24. Rickard M, Lorenzo AJ, Braga LH. Renal parenchyma to hydronephrosis area ratio (PHAR) as a predictor of future surgical intervention for infants with high-grade prenatal hydronephrosis. Urology. 2017; 101:85–9.

25. Walsh TJ, Hsieh S, Grady R, Mueller BA. Antenatal hydronephrosis and the risk of pyelonephritis hospitalization during the first year of life. Urology. 2007; 69:970–4.

26. Zareba P, Lorenzo AJ, Braga LH. Risk factors for febrile urinary tract infection in infants with prenatal hydronephrosis: comprehensive single center analysis. J Urol. 2014; 191:1614–8.

27. Zee RS, Herbst KW, Kim C, McKenna PH, Bentley T, Cooper CS, et al. Urinary tract infections in children with prenatal hydronephrosis: a risk assessment from the Society for Fetal Urology Hydronephrosis Registry. J Pediatr Urol. 2016; 12:261.

28. Visuri S, Jahnukainen T, Taskinen S. Incidence of urinary tract infections in infants with antenatally diagnosed hydronephrosis: a retrospective single center study. J Pediatr Surg. 2017; 52:1503–6.
29. Lee JH, Choi HS, Kim JK, Won HS, Kim KS, Moon DH, et al. Nonrefluxing neonatal hydronephrosis and the risk of urinary tract infection. J Urol. 2008; 179:1524–8.

30. Coelho GM, Bouzada MC, Pereira AK, Figueiredo BF, Leite MR, Oliveira DS, et al. Outcome of isolated antenatal hydronephrosis: a prospective cohort study. Pediatr Nephrol. 2007; 22:1727–34.

31. Sencan A, Carvas F, Hekimoglu IC, Caf N, Sencan A, Chow J, et al. Urinary tract infection and vesicoureteral reflux in children with mild antenatal hydronephrosis. J Pediatr Urol. 2014; 10:1008–13.

32. Pennesi M, Amoroso S, Bassanese G, Pintaldi S, Giacomini G, Barbi E. Frequency of urinary tract infection in children with antenatal diagnosis of urinary tract dilatation. Arch Dis Child. 2020; 105:260–3.

33. Braga LH, Easterbrook B, Jegatheeswaran K, Lorenzo AJ. From research question to conducting a randomized controlled trial on continuous antibiotic prophylaxis in prenatal hydronephrosis: a rational stepwise process. Front Pediatr. 2016; 4:27.

34. Silay MS, Undre S, Nambiar AK, Dogan HS, Kocvara R, Nijman RJ, et al. Role of antibiotic prophylaxis in antenatal hydronephrosis: a systematic review from the European Association of Urology/European Society for Paediatric Urology Guidelines Panel. J Pediatr Urol. 2017; 13:306–15.

35. Rianthavorn P, Phithaklimnuwong S. The role of antibiotic prophylaxis in mild to moderate isolated hydronephrosis detected in antenatal screening. Investig Clin Urol. 2020; 61:200–6.

36. Nef S, Neuhaus TJ, Sparta G, Weitz M, Buder K, Wisser J, et al. Outcome after prenatal diagnosis of congenital anomalies of the kidney and urinary tract. Eur J Pediatr. 2016; 175:667–76.

37. Sarhan OM, Helaly AE, Al Otay A, Ghanbar MA, Nakshabandi Z. Isolated low grade prenatally detected unilateral hydronephrosis: do we need long term follow-up? Int Braz J Urol. 2018; 44:812–8.

38. Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009; 76:528–33.

39. Wuhl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013; 8:67–74.

40. Murugapoopathy V, Gupta IR. A primer on congenital anomalies of the kidneys and urinary tracts (CAKUT). Clin J Am Soc Nephrol. 2020; 15:723–31.

41. Pavlaki A, Printza N, Farmaki E, Stabouli S, Taparkou A, Sterpi M, et al. The role of urinary NGAL and serum cystatin C in assessing the severity of ureteropelvic junction obstruction in infants. Pediatr Nephrol. 2020; 35:163–70.

42. Gavrilovici C, Dusa CP, Iliescu Halitchi C, Lupu VV, Spoiala EL, Bogos RA, et al. The role of urinary NGAL in the management of primary vesicoureteral reflux in children. Int J Mol Sci. 2023; 24:7904.

43. Kazlauskas V, Bilius V, Jakutis V, Komiagiene R, Burnyte B, Verkauskas G. Urine biomarkers combined with ultrasound for the diagnosis of obstruction in pediatric hydronephrosis. Front Pediatr. 2022; 9:762417.

44. Karakus S, Oktar T, Kucukgergin C, Kalelioglu I, Seckin S, Atar A, et al. Urinary IP-10, MCP-1, NGAL, Cystatin-C, and KIM-1 levels in prenatally diagnosed unilateral hydronephrosis: the search for an ideal biomarker. Urology. 2016; 87:185–92.

45. Smail LC, Dhindsa K, Braga LH, Becker S, Sonnadara RR. Using deep learning algorithms to grade hydronephrosis severity: toward a clinical adjunct. Front Pediatr. 2020; 8:1.

46. Ostrowski DA, Logan JR, Antony M, Broms R, Weiss DA, Van Batavia J, et al. Automated Society of Fetal Urology (SFU) grading of hydronephrosis on ultrasound imaging using a convolutional neural network. J Pediatr Urol. 2023; 19:566.

47. Weaver JK, Logan J, Broms R, Antony M, Rickard M, Erdman L, et al. Deep learning of renal scans in children with antenatal hydronephrosis. J Pediatr Urol. 2023; 19:514.

48. Son MH, Park E, Yim HE, Nam YJ, Lee YS, Choi EK, et al. Maternal exposure to airborne particulate matter during pregnancy and lactation induces kidney injury in rat dams and their male offspring: the role of vitamin D in pregnancy and beyond. Kidney Res Clin Pract. 2024 Jan 2 [Epub]. https://doi.org/10.23876/j.krcp.23.106.

Fig. 1.
UTD classification system. UTD, urinary tract dilatation; SFU, Society for Fetal Urology; APD, anterior-posterior diameter. a)With renal pelvic APD ≥4 mm or calyceal dilatation. b)Renal pelvic APD ≥10 mm or calyceal dilatation. Parenchymal abnormality includes cortical thinning, increased echogenicity, indistinct corticomedullary differentiation, or cystic dysplasia. Bladder abnormality includes bladder wall thickening, ureterocele, or dilated posterior urethra. For a more complete understanding of UTD classification system, please refer to the images in the article of Nguyen et al. [4].
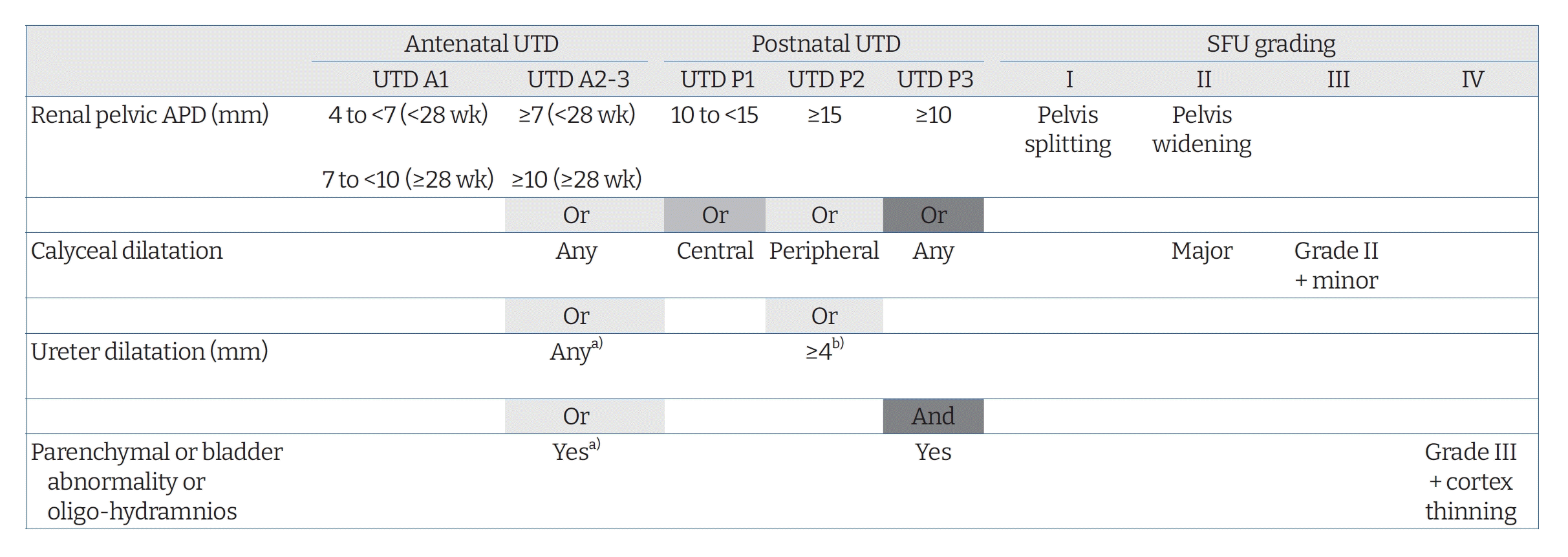
Fig. 2.
Evaluation, management, and follow-up for antenatal and postnatal UTD. UTD, urinary tract dilatation; US, ultrasonography; CAKUT, congenital abnormalities of the kidney and urinary tract; APD, anterior-posterior diameter; FU, follow-up; VCUG, voiding cystourethrogram; DRS, diuretic renal scan; MAG3, mercaptoacetyltriglycine. Adapted from Herthelius. Pediatr Nephrol 2023;38:3221-7 [3].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download