Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that affects multiple organs. It frequently involves various systems such as renal, joints, skin, cardiovascular, nervous, and hematologic systems [
1].
Childhood-onset SLE (cSLE) and adult-onset SLE (aSLE) have different phenotypes [
2]. Neuropsychiatric, renal, and hematological manifestations are more common in cSLE than in adult-onset disease [
2]. Patients with cSLE have common hematological manifestations such as leukopenia, lymphopenia, thrombocytopenia, and hemolytic anemia [
3]. In addition, kidney involvement is more frequent and shows more severe manifestations in cSLE patients than in aSLE [
1]. About 50% to 75% of cSLE patients have renal involvement, and >90% develop lupus nephritis within 2 years of diagnosis [
4]. Moreover, 10% to 30% of cSLE patients with lupus nephritis progress to end-stage renal disease (ESRD) within 15 years of diagnosis [
5].
Central nervous system (CNS) involvement in SLE occurs in 21% to 95% of patients [
6]. Neuropsychiatric manifestations include psychosis, anxiety, mood disorders, seizures, and hemorrhage [
6]. These manifestations are caused by the disease or secondary CNS sequelae of SLE induced by treatment with steroids and immunosuppressants [
6]. However, neurological complications such as cerebral infections, brain abscesses, and hemorrhage are seldom reported in lupus nephritis, even in adults. These are extremely rare in cSLE compared to aSLE, and the result is usually fatal. Here, we report a rare case of cSLE with ESRD, brain abscess, and cerebral hemorrhage.
Case report
A 9-year-old girl, previously diagnosed with idiopathic thrombocytopenic purpura (ITP) at another hospital, was referred to our hospital because of severe acute kidney injury (AKI) in June 2021. She was admitted to the pediatric intensive care unit (PICU) for continuous renal replacement therapy. She showed volume overload (uncontrolled hypertension, both pleural effusion, ascites) and decreased kidney function (blood urea nitrogen [BUN] 57.6 mg/dL, serum creatinine 0.76 mg/dL) with anuria, massive hematuria, and proteinuria, decreased platelet counts, a malar rash, joint pain, and immunologic findings of reduced complement levels, including complement components 3 and 4, and positive antinuclear antibody and anti-double-stranded DNA (
Tables 1,
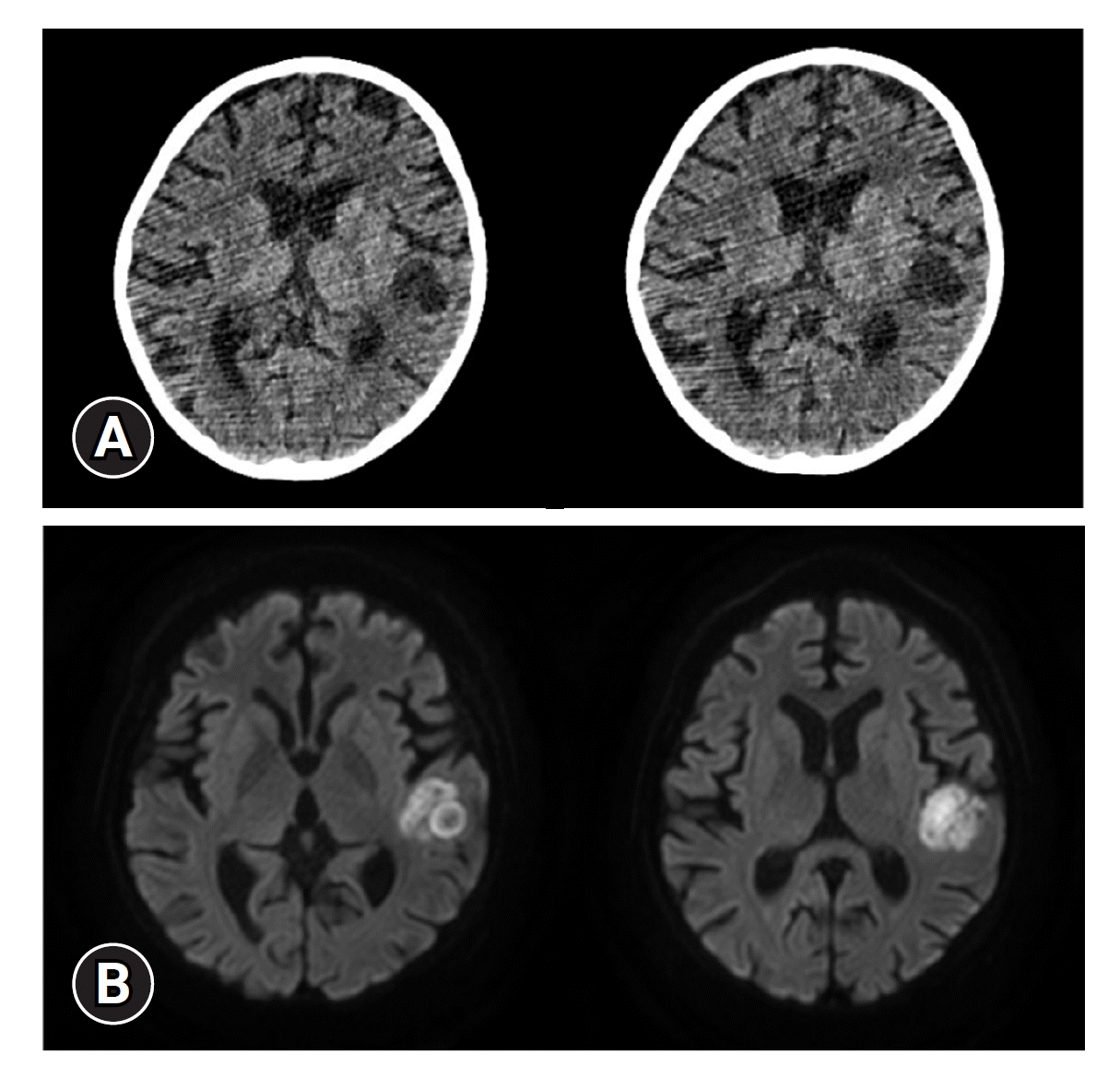
2). We finally diagnosed the patient with lupus nephritis, which was cSLE with kidney involvement. To treat the AKI caused by lupus nephritis, high-dose steroids (methylprednisolone 0.50–0.75 g/day for two doses) and immunosuppressant therapy (cyclophosphamide 500 mg/body surface area once in September 2021; mycophenolate mofetil 1,500 mg/day from August 2021 to January 2022) was administered. The AKI deteriorated, however, and after 3 months, she reached ESRD status. Given this situation, decreasing lupus activity became more important than preserving kidney function. So we had to add tacrolimus while using other immunosuppressants after 1 month (tacrolimus 2 mg/day from September 2021 to January 2022). Peritoneal dialysis (PD) alone was ineffective in resolving fluid retention, such as pericardial effusion, pleural effusions, hypertension, and elevation of BUN and serum creatinine levels. Therefore, we performed hemodialysis (HD) three times a week with daily PD. In December 2021, 6 months after her first admission, she was discharged with all dialysis plans. Unfortunately, she was hospitalized in the PICU again because of seizures January 2022. Brain magnetic resonance imaging was performed, and the patient was diagnosed with a brain abscess (
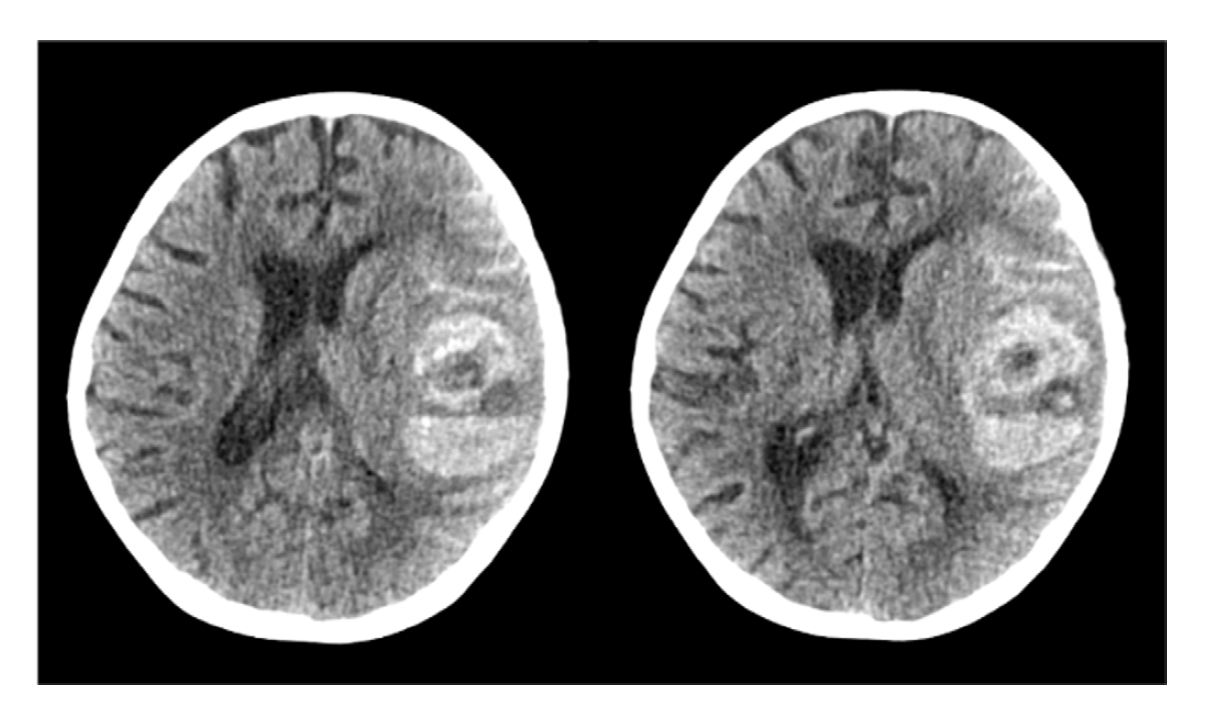
Fig. 1). During surgery, spontaneous cerebral hemorrhage was observed (
Fig. 2). Even though we performed a burr-hole operation immediately, her vital signs became unstable, and then shortly after, she died because of disseminated intravascular coagulation (DIC) (
Table 3,
Fig. 3).
Discussion
cSLE accounts for 15% to 20% of all SLE cases [
2]. Although both cSLE and aSLE involve multiple organs, cSLE has several different clinical manifestations, with hematological, renal, and neuropsychiatric manifestations being more common [
2].
Although lymphopenia is more prevalent with aSLE, both thrombocytopenia and hemolytic anemia are significantly more prevalent in the cSLE group [
3]. If only hematologic symptoms are present initially, it may be mistaken for a hematologic disease, and SLE may be missed in both age groups. Although there are limited studies in children, some studies reported that some patients developed SLE after ITP [
7]. The prevalence of these patients ranges from 3% to 12% [
7]. Patients initially have thrombocytopenia as an isolated presentation. Therefore, they could be diagnosed with ITP since they do not have symptoms of SLE other than thrombocytopenia [
7]. Another study reported that 2.96% of childhood ITP patients subsequently developed SLE [
8]. In our case, thrombocytopenia was an early symptom of SLE, and the patient was diagnosed and treated for ITP for 2 years. The patient was finally diagnosed with SLE when she was re-examined for symptoms and signs such as malar rash and renal manifestations such as pitting edema, oliguria, BUN, and serum creatinine elevation after transfer to our hospital. Even hematological problems alone may necessitate immunochemical tests for SLE. However, it is unclear whether our patient developed SLE after ITP or had an isolated presentation of thrombocytopenia as the initial symptom of SLE.
Kidney involvement is the most common manifestation of SLE and a predictor of poor survival outcomes [
7]. Lupus nephritis is more common and severe in cSLE than in aSLE [
9]. Although the 5-year renal survival rates in children with cSLE have markedly improved in recent decades, the most recent rate ranges from 52% to 91%, which is still low [
9]. Also, the mortality rate of cSLE with lupus nephritis is 19 times greater than that in the same age groups. It is higher than aSLE, which has an eight times higher mortality than the same age group [
9]. cSLE with lupus nephritis with frequent and severe symptoms of kidney involvement leads to high-dose corticosteroids and immunosuppressive treatment [
10]. Inevitable life-threatening side effects could occur when these drugs are used for remission of the disease. In addition, they could increase the risk of serious infections. A study reported that the poor prognosis of cSLE with ESRD is primarily associated with infectious disease [
11]. Infectious diseases such as septicemia, peritonitis, pulmonary infection, and other infections are secondary common causes of mortality during the 5 years from initiation of renal replacement therapy in cSLE with ESRD [
11].
cSLE shows more common psychiatric manifestations than aSLE [
12]. In our case, the patient was treated with systemic corticosteroids and immunosuppressants for lupus nephritis for 7 months. She had intractable fluid retention even though she was treated with simultaneous PD and HD, and concentrated administration of immunosuppressants was ineffective. There was a risk of infection while undergoing both dialyzes; however, immunosuppressive drugs had to be used to control the SLE activity. Immunosuppressants are not the only cause of the increased risk of infection. Several factors could contribute to the increased risk of infections in patients with SLE [
13]. Doria et al. [
13] insisted that there are factors that increase the risk of infections, such as organ injury, immunosuppressant use, and T- and B-cell exhaustion. In our study, our patient underwent HD and PD simultaneously. HD and PD catheters could be risk factors for infections, which could lead to prolonged hospitalization, poor morbidity, and mortality [
14]. Besides the aforementioned causes, infections can arise from several other factors. Uremia-induced immune dysfunction in ESRD patients increases their vulnerability to infection. Uremia is associated with impaired immune responses from neutrophils and lymphocytes, complement system dysfunction, and reduced antibody production, which leads to immunosuppression [
15]. Diabetes is also a major risk factor for nosocomial infections in HD patients [
15].
The patient had several factors which made her vulnerable to infection; renal failure due to SLE activity, long-term use of systemic corticosteroids, simultaneous and longer use of HD and PD catheters, longer hospital stays, and hyperglycemia due to long-term steroid use. Concerned about those risk of infection, we began immunosuppressant after 3 months. Nevertheless, she developed seizures and brain abscesses. She also experienced prolonged thrombocytopenia combined with a high bleeding tendency due to ESRD and uncontrolled DIC, which led to cerebral hemorrhage In short, she had multiple risk factors for severe infections and more potent treatment-related side effects, such as hematological and neurological complications, than other cSLE patients.
In conclusion, it is essential to recognize that even patients with isolated symptoms, such as ITP, may be exhibiting early manifestations of SLE. When Lupus nephritis is diagnosed, prompt treatment is essential to prevent the progression to ESRD. If resolved as soon as possible, it could prevent treatment-related complications and allow for discontinuation of medications. During treatment, it is important to consider the possibility of complications such as brain abscesses and hemorrhage. Although rare, these complications must always be considered in clinical practice because they increase mortality rates.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download