Abstract
Upper extremity anomalies are the second most common type of congenital malformations. Approximately 1% to 3% of newborns are born with congenital anomalies, and among them, roughly 10% have upper extremity anomalies. Congenital hand anomalies are often isolated phenomena but may also coexist with other congenital anomalies or syndromes. These anomalies cause not only aesthetic concerns, but also significant functional deficits and psychological issues for children and their families. Surgeons should conduct a thorough examination to make an accurate diagnosis and provide appropriate treatment or refer the patient to a specialized clinic if necessary. Operative procedures should aim to restore both function and aesthetics. This article reviews the embryology of the hand, the classification of congenital hand anomalies, and the clinical features and treatment of common major congenital hand anomalies.
Approximately 1% to 3% of newborns are born with congenital anomalies, and among them, approximately 10% have upper extremity anomalies. Congenital limb anomalies are the second most common congenital malformations after congenital heart disease [1]. Anomalies in limb development result from spontaneous mutation of genetic material, inheritance of abnormal genes, or subtle or gross insults to the limb bud. The anomaly may be isolated or syndromic [2].
Recent advancements in the understanding of limb anomalies are directly related to our increased understanding of embryogenesis, and research on gene misexpression and loss of function has enhanced our understanding of limb formation [3,4]. The hand surgeon must possess a basic comprehension of embryogenesis and limb formation to comprehend congenital limb anomalies to communicate relevant knowledge to the family of an affected child, and to convey reliable information is the responsibility of the physician caring for the family [1].
After approximately 4 weeks of gestation, the upper limb bud emerges, and by 8 weeks, finger separation occurs, leading to the formation of most limb structures. After 8 weeks, the existing structures undergo growth, maturation, and differentiation. Approximately 12 weeks later, a fully formed upper limb and hand, including nails, develops. The specific timings of hand formation are described in Table 1. Most upper extremity congenital anomalies occur during the 4- to 8-week period of rapid and fragile limb development [1,2,5].
The development of the upper limb occurs through growth along three spatial axes: proximal-distal, anterior-posterior, radial-ulnar, and dorsal-ventral. The signaling centers controlling these three spatial axes are the apical ectodermal ridge (AER), zone of polarizing activity (ZPA), and dorsal ectoderm [1,2,5].
The AER is a thickened layer of the ectoderm that induces the differentiation of the underlying mesenchyme-appropriate structures (Fig. 1 [6]). Actively differentiating mesenchymal tissue under AER is referred to as the progressive zone (PZ). Cells within the PZ ultimately differentiate and acquire specific cell types and locations. Wnt3 and fibroblast growth factor (Fgf) 10 secreted from the AER contribute to proximal-distal axis growth. Problems in this process can lead to abnormalities such as transverse deficiency [2,5].
The ZPA, located in the mesenchyme of the limb bud on the ulnar side, is a signaling center that regulates the growth of the radioulnar axis, allowing for distinct characteristics of the radial and ulnar sides. The ZPA secretes a morphogen called sonic hedgehog (Shh), which controls the patterning process of the radial-ulnar axis. In particular, it plays a role in the formation of the four digits on the ulnar side. In experimental models in which Shh was removed, the ulna and ulnar digits were missing, whereas the addition of Shh led to the occurrence of a mirror hand [2,5].
The dorsal ectoderm acts as a crucial signaling center for dorsal-ventral differentiation. Wnt7a from the dorsal ectoderm induces differentiation of the underlying mesenchyme, creating distinctive characteristics of the volar and dorsal sides of the hand [2,5].
Growth along these three axes is not independent, but interconnected; therefore, if there are abnormalities in one signaling system, it can inhibit the growth of the other axes (Table 2). The AER and ZPA are interconnected through reciprocal feedback that regulates the expression of Shh at the ulnar border of the limb bud adjacent to the AER during progressive growth [7-9]. When AER is removed Shh expression regresses, and Fgf signaling is lost if ZPA is ablated [8]. Wnt7a is secreted by the dorsal ectoderm and induces the expression of the homeodomain transcription factor Lmx1b in the underlying mesoderm. This asymmetrically dorsalizes the developing limbs [10,11]. Additionally, Wnt7a is involved in sustaining the secretion of Shh from the ZPA, connecting the dorsal-ventral and radial-ulnar axes [12]. If the dorsal ectoderm is removed, Shh expression decreases, leading to disturbances in posterior, ulnar patterning [13]. Overall, Shh plays a crucial role in limb development by integrating the proximal-distal, radial-ulnar, and dorsal-ventral axes [13].
At the end of the 5th week of development, the hand plates became visible. Current research indicates that the establishment of digit number is controlled by an intrinsic patterning mechanism within the hand plate, which utilizes reciprocal interactions between activators (bone morphogenetic protein, BMP) and inhibitors (Wnt) to define the alternating pattern of digits and interdigits [14,15].
Fig. 2 [16] shows the diffusion-driven instability model proposed by Turing. During limb development, BMP (green) is suggested to act as an activator, while Wnt (brown) serves as an inhibitor of diffusion-driven instability. Both these factors regulate the expression of Sox9 (represented in purple), which is involved in cartilage formation. The Fgfs and Hox/Gli pathways influence the balance between activators and inhibitors. Fgfs originating from the AER determine digit length and control the number of digits in conjunction with the distal Hox/Gli pathway. The presence of Fmn1 limits BMP expression, and mutations in Fmn1 can lead to elevated BMP levels, resulting in abnormal forearms such as radioulnar synostosis, and hand patterning such as oligosyndactyly [2].
Fig. 2 also shows a series of hand plates (note that hand plate development remains static in this illustration for clarity), illustrating the progression from fluctuating interactions between the activator and inhibitor (representing noise) to a stabilized pattern of five digits. On the right side, because of the progressive loss of digit-suppressing Hox/Gli transcription factors (represented by the red bar), the autonomous patterning mechanism generated an increased number of digits [2].
Dysmorphological terminology provides the fundamental concepts for understanding the causes of congenital limb malformations. Malformation refers to the abnormal tissue formation caused by abnormal cell development. On the other hand, deformation refers to the alteration of tissue shape into an abnormal form as a result of insults or disruptions affecting previously developed normal cells. Dysplasia is a condition in which tissue formation occurs with abnormal morphology owing to the failure of normal organization during the assembly of cells into tissues. This is exemplified by conditions, such as achondroplasia, in which abnormal skeletal features are observed. Disruption refers to the destruction of already-formed normal tissues, as observed in conditions such as constriction band syndrome [2,5].
The classification of congenital limb malformations was first established in 1832 by Isidore St. Hilaire. In this classification, malformations are divided into simple categories, such as phocomelia (showing limb-like fins), hemimelia (partial absence of limbs), and ectromelia (complete absence of limbs). To date, the most widely used classification is the Swanson/International Federation of Societies for Surgery of the Hand (IFSSH), which was initially proposed by Swanson et al. [17] in 1968 and subsequently revised (Table 3). However, this system was developed based on embryological and morphological knowledge from the 1960s and the 1970s, which raises inconsistencies when compared to current molecular-level knowledge. For example, the concepts of “differentiation” and “formation” occur simultaneously, making it challenging to separate them. Modifications to the classification have been proposed by the Japan Society for Surgery of the Hand, such as adding the category of “abnormal induction of digital rays” to the Swanson/IFSSH classification. In 2010, Oberg et al. [18] proposed a new classification called the OMT (Oberg, Manske, and Tonkin) classification, which divides congenital limb malformations into malformations, deformations, and dysplasia based on the underlying mechanisms and further subdivides them according to the affected region (Table 4). Ultimately, as knowledge of genes accumulates, the most accurate framework for classifying malformations is expected to become available. For example, syndactyly is associated with HoxD13, which is located on chromosome 2q31-32. This allows the estimation of specific genes that cause problems at particular stages of development and their potential to result in specific malformations [2,5].
Polydactyly is characterized by the presence of more than five digits in at least one extremity. It is one of the most common congenital anomalies that occurs in the upper extremities, along with syndactyly. Polydactyly is a common disorder of duplication. It can cause not only aesthetic issues but also functional impairment, depending on the level of the affected digit. The prevalence of polydactyly varies with race and sex. According to Finley et al. [19], the occurrence rate per 1,000 births was 2.3 for white males and 0.6 for white females, whereas it was 13.5 for black males and 11.1 for black females. Data from Kim et al. in 2003 [20], based on a Korean population, showed a prevalence rate of 93 per 100,000 births, which is similar to that observed in white populations.
Polydactyly is classified based on its location of occurrence. When it occurs in the thumb, it is referred to as thumb or preaxial polydactyly. When it occurs in the index, middle, or ring finger, it is classified as central polydactyly. Finally, postaxial polydactyly occurs on the little finger. Preaxial polydactyly is the most common type. It is more common in males and occurs approximately twice as frequently on the right side than on the left side. It usually shows sporadic inheritance. Postaxial polydactyly shows an autosomal dominant inheritance and is often bilateral. It can be associated with polydactyly of the feet or syndactyly. Central polydactyly is very rare and shows an autosomal dominant inheritance [5,21].
The Wassel classification system is most commonly used for classifying preaxial polydactyly. Wassel classified thumb polydactyly into seven types based on the level of duplication observed on radiographic images (Fig. 3). The type IV, where both the proximal and distal phalanges are separated, is the most common type of preaxial polydactyly [22]. Postaxial polydactyly is commonly classified by using the system proposed by Temtamy and McKusick [23]. Type A indicates a well-formed extra digit that articulates with the 5th or 6th metacarpal or phalangeal bone and has major structures including flexor tendons, extensor tendons, and neurovascular bundles. In type B, the extra digit is not properly formed and is only connected to the digit through small connections containing neurovascular structures. Central polydactyly commonly presents in association with syndactyly, often in the form of polysyndactyly, or cleft hand, with the highest prevalence in the fourth digit, followed by the third and second digits in order of frequency [24,25].
The timing of the surgical intervention follows the general surgical principles for other congenital hand anomalies but can be adjusted based on the extent of deformity and the general condition of the patient. Many authors recommend surgical intervention around the age of 12 months, a time when the risks associated with anesthesia are lower; the child has not yet developed the ability for a functional pinch, and considering the progression of the deformity [26]. For type B postaxial polydactyly, where the extra digit is attached only to the skin, surgery can be performed as early as around 6 months of age. The goal of the operation is to obtain the proper length and bulk of the finger; align the joints and physes along its longitudinal axis; ensure joint stability, adequate motion, and balanced tendon tension; and avoid nail deformities [27].
For preaxial polydactyly, the radial digit is usually excised because it is typically more hypoplastic than the ulnar digit. In addition, it is beneficial to preserve the ulnar collateral ligament (UCL), which is essential for pinch and grasp functions, on the ulnar side digit. The basic surgical procedure is as follows: the neurovascular bundles of the extra digits are ligated; the extensor and flexor tendons of the extra digit are cut or transferred to the remnant digit. Transferring the tendon from an extra digit can prevent off-axis pulling from the original tendon of the remaining digit. For patients with duplication at the metacarpophalangeal (MCP) level or more proximally, the abductor pollicis longus insertion should be preserved and transferred to the remaining proximal phalanx. If the patient had a duplication through the joint, the joint capsule and the collateral ligament attached to the extra digit were sutured to the remnant thumb with appropriate tension. If there is a significant angulation deformity, a wedge ostectomy or oblique osteotomy can be performed, and Kirschner wires (K-wires) can be used for immobilization for 6 to 8 weeks [2,5,26,28]. Fat grafts obtained from the surrounding area and extra digits to the depressed area can result in cosmetic improvement [28]. For patients with similar-sized thumbs in Wassel types I and II, which have duplication only in the distal phalanx, the Bilhaut-Cloquet procedure can be performed by resecting the middle part of duplication and combining the ulnar and radial sides [29]. However, it is used in limited cases owing to the potential risk of stiffness, split nail deformity, epiphyseal damage, and growth arrest [30]. In cases where one finger has well-developed distal parts and the other has well-developed proximal parts, an on-top plasty procedure can be considered. This involves elevating the well-developed distal portion as a neurovascular flap and then cutting the distal part of the finger with the well-developed proximal aspects. The flap is then inserted on top and secured using K-wires [26].
The treatment of postaxial polydactyly is fundamentally similar to that of preaxial polydactyly. After removing an extra digit with an osseous connection, the widened articular surface is refined or the bifurcated head of the metacarpal or phalangeal bone is trimmed. If the duplication originates from the MCP joint, the UCL and the abductor digiti minimi muscle are transferred at the base of the proximal phalanx of the remnant digit. The flexor and extensor tendons from the extra digits are transferred to maintain balance [31]. However, in the case of type B, it is possible to attempt ligation rather than surgical removal. This approach has the advantage of avoiding general anesthesia and surgery. However, if the inner neurovascular bundle is not ligated perfectly, a neuroma can develop, and if ligation is not successful in the proximal area, it can leave small nipple-like marks. In addition, rare complications, such as infection and bleeding, can occur [32-34]. In cases of postaxial polydactyly, it is not uncommon for accompanying foot polydactyly and bilateral polydactyly involvement. In this case, it is common practice to perform surgery in two stages, dividing the hands or feet, with the less severe site operated on first (Fig. 4) [31].
Central polydactyly often presents in a complex form, accompanied by syndactyly including bone fusion. Owing to these complexities, it is difficult to achieve satisfactory functional results without postoperative joint stiffness or finger misalignment [24,25,31]. Surgical procedures generally follow the principles of typical syndactyly correction, with a focus on distributing flap tissues to the fused bone and exposed joints. If necessary, osteotomies may be performed for alignment correction; however, in some cases, it is essential to retain the fused bones to maintain joint stability [24,25,31].
Except for tissues that need to be preserved for the retained digit all excess tissue should be thoroughly removed. Small fragments of cartilage or skin tissue can continue to grow, resulting in persistent protruding deformities. In the most common Wassel type IV preaxial polydactyly, secondary deformities can occur as it grows, and approximately 20% of the cases may require secondary revision surgery [35]. Common causes for revision surgery include joint stiffness, joint instability, angulation deformity with skeletal growth, and other cosmetic reasons [5]. In central polydactyly, flexion contractures, and various deformities can occur, especially at the interphalangeal joints, even after primary surgery, because of congenitally underdeveloped joints and soft tissues. Therefore, in most cases, secondary revisions, including skin grafts and osteotomies, are necessary [31] (Fig. 5).
Syndactyly is one of the most common congenital hand anomalies, occurring at a frequency of approximately 1 in 1,000 to 3,000 individuals, with a male-to-female ratio of approximately 2:1. A familial history is reported in 15% to 40% of cases, and it often manifests bilaterally in approximately half of the cases. Syndactyly arises from abnormalities in the AER, leading to the failure of cell apoptosis in the interdigital space and impeding proper separation of the fingers. The third web space was the most common site for syndactyly (50%), followed by the fourth (30%), second (15%), and the first (5%). The first web space is the least commonly affected because it is the first to separate during embryonic development. Syndactyly can occur as an isolated condition in conjunction with other upper limb abnormalities or syndromic associations. It may also coexist with conditions, such as polydactyly or cleft hands [36].
Syndactyly is classified into two main types based on the extent of fusion within the web space. A complete type occurs when the entire web space is fused, whereas an incomplete type occurs when only a portion of the web space is fused. Syndactyly can be further categorized into simple and complex types. In the simple type, only the soft tissues are fused. The joints typically show no abnormalities and the flexor and extensor tendons remain unaffected. For neurovascular bundle, although it’s possible for the division of the common digital nerve and artery to occur beyond the normal commissure level, they generally remain intact [37]. Complex syndactyly indicates abnormalities in the bone and/or joint structures, with the most prevalent form involving the fusion of the distal phalanx tufts. As the complexity of syndactyly increases, the likelihood of encountering anomalies in tendons and neurovascular structures increases [38] (Fig. 6). In cases such as Apert syndrome or central polysyndactyly with complex manifestations, where the fingers are intricately entangled, making a simple classification based on a side-to-side relationship impractical, it is termed a complicated type of syndactyly [5,39].
Considering the cosmetic and functional implications of syndactyly, surgical intervention is recommended in the majority of cases. Surgery is typically recommended at around the age of 1 year [5]. However, if syndactyly causes asymmetrical hand growth or hinders grasping movements, early surgical intervention is necessary. In cases of syndactyly between the first and second fingers as well as between the fourth and fifth fingers, where there is a significant difference in length, early surgery is advised to prevent growth disturbances and deformities. Syndactyly between fingers of varying lengths can lead to angular deformities in the longer digit that is pulled distally by the shorter digit [40]. This can result in the development of fixed clinodactyly or camptodactyly in the previously flexible rays [41]. In complex or complicated types where the phalangeal bones are fused, early surgery is recommended to avoid both growth disturbances and functional impairments. For simple syndactyly between the third and fourth fingers, there is no urgency for early surgery. Surgery is not recommended in complex cases with insufficient osteoarticular tissue to create stable and mobile independent digits because separation could potentially worsen hand function [40].
Considering the course of the neurovascular bundle of the digit, it is recommended that the adjacent web spaces not be separated simultaneously to avoid vascular compromise. Recently, however, some surgeons have reported safe results after simultaneously separating the adjacent web spaces [40]. Hynes et al. [42] described four cases of one-stage division of whole finger syndactyly, and there was no digital ischemia Tian et al. [43] also reported 31 cases of single-stage division of multiple syndactyly, including adjacent web spaces, without digital ischemia or necrosis. The staged release was necessary for only one patient, attributed to an abnormal vascular anatomy.
The general surgical technique for syndactyly division is as follows. A triangular or trapezoidal flap is designed on the dorsum of the hand, starting from the metacarpal head to approximately two-thirds of the proximal phalanx, and zigzag incisions are made to the attached fingertips. Similarly, zigzag incisions are made from the volar side to ensure complete separation of the fused spaces between the fingers [44-48]. For the dorsal flap, many variations have been proposed for the dorsal flap, such as hourglass, omega, and gullwing-shaped flaps [47-50]. Instead of a dorsal flap, the use of a palmar flap, alone or combined with a dorsal flap has also been reported [51-57]. Following the incision and elevation of the flaps, all fibrous connections of the web space are released proximally until the intended commissure level is reached. During dissection, care should be taken to avoid damaging the neurovascular bundles. After the release of the fibrous connection, the dorsal triangular or trapezoidal flap is pulled towards the palmar side to create an interdigital commissure and slope, and the triangular flaps created by zigzag incisions are sutured onto the sides of the fingers. To prevent contractures and preserve sensation in the fingertips, it is essential to cover the nail area and distal tip with a paronychial flap and pulp flaps [58-60]. The remaining defect area is covered with a full-thickness skin graft. Many donor sites have been utilized for full-thickness skin grafts, such as the inguinal, malleolar, hypothenar, wrist flexion creases, plantar, and retroauricular areas. Although the inguinal area is the most commonly utilized donor site, its main drawbacks include the risk of hyperpigmentation and hair growth. The skin of the medial malleolus showed a good color match. Performing a thick split-thickness skin graft from the non-weight-bearing medial plantar area resulted in fewer pigmentations and properties similar to those of the palm. However, it is difficult to consistently detach it with an even thickness, including a sufficient portion of the dermis, and harvesting a substantial amount. No single donor site has been proven superior to other donor sites [5]. Split-thickness skin grafts combined with dermal substitutes also showed results comparable to those of full-thickness skin grafts [61]. Split-thickness skin grafts alone showed acceptable results in syndactyly division, whereas slightly better functional results and skin stability were observed with full-thickness grafts [62]. The use of hyaluronic acid scaffolds also showed encouraging short-term outcomes, albeit without the results of long-term data [63].
The use of a skin graft or operating without a skin graft is one of the main debates in syndactyly surgery [40]. Although many satisfactory results without skin grafts have been reported, they have some limitations because they were not comparative or retrospective studies [64-67]. Yuan et al. [68] showed better cosmetic results when using skin grafts, with fewer revision surgeries, in their comparative study between a dorsally based rectangular flap with a skin graft from the groin and a dorsal pentagonal advancement flap without skin grafting. According to Ferrari and Werker [69], there was no significant difference in patient satisfaction between procedures involving skin grafts and those without. In a prospective matched comparative study involving 14 patients undergoing division with and without skin grafts, Wang and Hutchinson [70] observed enhanced cosmetic results and reduced web creep associated with the use of the skin graft technique.
Major postoperative complications include web creep, flexion contracture, and skin grafting-related complications. Web creep refers to the partial recurrence of syndactyly when hypertrophic scars develop in the triangular flap inserted into the interdigital space, requiring reoperation in approximately 8% of cases. Flexion contracture, which occurs postoperatively in approximately 13% of cases, can arise due to growth after syndactyly surgery. In patients with complex syndactyly, postoperative finger deformities, joint instability, and incomplete recovery of hand function may occur. Complications of skin grafting include graft failure, hypertrophic scarring, hyperpigmentation, and hair growth [1,2,5,40].
Apert syndrome is an autosomal dominant chromosomal disorder caused by a mutation in the fibroblast growth factor receptor 2 (FGFR2) gene, located on the long arm of chromosome 10 (10q26). It is characterized by craniosynostosis, midface retrusion, acrosyndactyly, and symphalangism. Based on these features, Apert syndrome is referred to as acrocephalosyndactyly. The severity of craniofacial abnormalities is inversely related to the extent of hand deformities, with more severe facial abnormalities being associated with milder hand abnormalities [2]. Syndactyly in Apert syndrome is classified according to the Upton classification, which is based on the degree of soft tissue and bone involvement. The detailed features of each type are listed in Table 5 (Fig. 7). In some cases, both feet were affected simultaneously [5].
The sequence for separating web spaces varied among the authors. Zucker et al. [71] recommended performing the first web space division, followed by the simultaneous division of the second and fourth web spaces, and finally, the division of the third web space. Chang et al. [72] proposed an initial bilateral separation of the first and fourth web spaces (border digit), followed by subsequent unilateral division of the middle syndactyly mass, with additional procedures such as thumb osteotomy and bone grafting performed as necessary. Fearon [73] suggested initially dividing the first and third web spaces, followed by releasing the second and fourth web spaces, or vice versa, to complete all the finger divisions in two operations. Guero [74] proposed the same sequence for patients with Upton types 1 and 2. However, patients with Upton type 3 are recommended to initially divide the first and fourth web spaces, followed by releasing the second web space and performing fourth-ray amputation.
As you can see, most authors prioritize the separation of the first web space first because of the functional issues. To maximize the thumb function, widening the first web space is crucial. This can be achieved through Z-plasty using four or five triangular flaps or using a large dorsal flap. Additionally, if necessary, the firm fascia around the adductor muscle should be sufficiently released. Ulnar deviation of the thumb is caused by the abnormal triangular shape of the proximal phalanx and abnormal attachment of the abductor pollicis brevis muscle on the ulnar side. Surgical correction including wedge osteotomy and ligament correction is necessary to address these abnormalities [2,5]. Taghinia et al. [75] reported the self-reported assessment of disability and measured functional data from 22 adults with Apert syndrome, and the results showed that the patients showed favorable self-reported outcomes despite their significant functional deficits.
Macrodactyly is an extremely rare congenital malformation, accounting for approximately 0.5% to 0.9% of all congenital hand anomalies [76]. What distinguishes it from secondary hypertrophy due to neoplasms is that in macrodactyly, all tissues of the affected digit, including fat, tendons, ligaments, bones, nerves, and blood vessels, are uniformly hypertrophic. Macrodactyly occurs sporadically and is recognized as a component of the PIK3CA-related overgrowth spectrum (PROS) arising from a somatic gain-of-function mutation in PIK3CA within mammalian target of rapamycin (mTOR) pathway [77]. While it is characterized as a tumorous condition, there have been no documented occurrences of malignant transformation to date [78]. Usually, peripheral nerves that innervate the affected digit show fibrofatty proliferation and hypertrophy, suggesting that overgrowth is mediated by nerves [79-82].
Macrodactyly presents with a diverse range of clinical phenotypes, with significant variations in the location, extent of overgrowth, and growth rate among patients. Several classification systems have been proposed for macrodactyly [82]. Among them, the most commonly used classification is based on the growth rate. This classification system includes both the progressive and static types. In the static type, growth occurs in proportions similar to those of the other digits, whereas in the progressive type, there is often more rapid enlargement [80].
Macrodactyly typically presents unilaterally, and cases involving simultaneous overgrowth of two or more digits are approximately 2 to 3 times more common than those affecting only one digit. Most cases of macrodactyly originate within the territory of a single digital nerve, with the index and middle fingers most commonly affected. If it arises from the ulnar side digital nerve, the affected finger tends to twist towards the radial side, showing a clinodactyly pattern. If it occurs in the common palmar digital nerve, the radial digit of the adjacent finger shows overgrowth on the ulnar side, whereas the other ulnar digit shows overgrowth on the radial side, resulting in a divergent pattern. In this context, as the digital nerves are located on the volar side, overgrowth of the phalanx tends to cause curvature towards the dorsal side [1,5].
Hypertrophy of macrodactyly is not uniform for each element. Overgrowth becomes more prominent toward the distal side, exhibiting a more severe clinical presentation as it progresses from the metacarpal to the proximal, middle, and distal phalanges [80-83]. If there is involvement of the median and ulnar nerves, swelling may occur in the tissue around the wrist, leading to compressive neuropathy [84]. Carpal tunnel syndrome can arise from median nerve compression, whereas cubital tunnel syndrome can arise from ulnar nerve compression [84,85]. Median nerve involvement is more common than ulnar nerve involvement [82,86]. The thickening of the flexor tendon sheath can also result in a trigger finger [5]. About 10% of cases may be combined with syndactyly [82,87,88]. Associated syndromes include Ollier disease, Maffucci syndrome, Klippel-Trenaunay-Weber syndrome, and Proteus syndrome [82,89-91].
Macrodactyly has a very low incidence, and because of its diverse clinical presentations, there is a lack of clearly defined treatment protocol or long-term follow-up results. Surgical treatment for macrodactyly should be based on individual clinical status, including the growth potential of the patients and their growth rate.
In children with remnant growth potential, epiphysiodesis can be performed to prevent longitudinal overgrowth [82,83,92,93]. The clinical effects of epiphysiodesis in macrodactyly patients are still controversial. Ishida and Ikuta [92] and Topoleski et al. [93] demonstrated successful inhibition of longitudinal overgrowth after epiphysiodesis, and Woo et al. [83] also reported successful control of longitudinal growth in macrodactyly patients, including both progressive and static types. In contrast, Tsuge [81] proposed that bony overgrowth is not a result of overgrowth from the epiphyseal plate but is attributed to the hypertrophy of all tissues. Therefore, he suggested that epiphysiodesis alone might be insufficient for longitudinal overgrowth control. Epiphysiodesis is relatively less invasive; however, this method cannot restrict volume growth, and there is a risk of recurrence and joint stiffness after surgery. Topoleski et al. [93] reported increased width of the toe metaphysis after epiphysiodesis. Woo et al. [83] also reported an increased width ratio during the postoperative follow-up. For the timing of epiphysiodesis, Cerrato et al. [82] recommended surgery when the affected digit reached the expected adult length, as determined by comparing it with one of the parents’ hands. Woo et al. [83] established an operative indication when the total length of the affected digit reached 75% or more of the corresponding digit in the same-sex parent (Fig. 8).
In patients with fully developed digits, the surgical goal is to reduce girth and volume. Soft-tissue debulking surgery is one of the most common procedures for patients with macrodactyly. Debulking surgery usually requires multiple procedures and alternate debulking between the radial and ulnar sides of the digit is recommended to prevent vascular compromise of the finger [82]. Ostectomy and mid-phalanx shortening can also be performed to reduce the length and circumference, although they may lead to potential complications such as compromised blood supply or joint stiffness [80,81]. If the affected digit is curved in the coronal plane, wedge ostectomy can be performed to correct the longitudinal axis. Ray amputation can also be considered in patients with uncontrollable progressive overgrowth, fingers without function, or macrodactyly hindering overall hand function [5]. Cerrato et al. [82] performed digit or toe transfers after ray amputation and showed good functional and aesthetic results. Recently, since isolated limb overgrowth was related to a somatic gain-of-function mutation in PIK3CA within the mTOR pathway, sirolimus has been considered an adjuvant treatment [94]. Correcting macrodactyly to achieve normal function and appearance is challenging, and surgical outcomes are often unsatisfactory. Therefore, it is crucial to emphasize the prognosis of macrodactyly and the necessity for multiple procedures before surgery.
Constriction band syndrome, also called amniotic band syndrome, is a rare congenital anomaly characterized by the inward wrapping of the skin and subcutaneous tissue due to constrictive fibrous bands, leading to a condition similar to binding with a rubber band. It is a rare congenital deformity that occurs in approximately 1 in 1,200 to 15,000 individuals [5,95,96]. Constriction band syndrome often involves swelling of the distal part of the constriction band, and it is common to observe acrosyndactyly or cases where the distal part is absent. In addition, constriction band syndrome sometimes occurs with other conditions, such as club feet, facial clefts, renal cysts, gastroschisis, or imperforated anus [97]. Histologically, the constriction band consists of intact, full-thickness skin structures, including a complete basement membrane and enhanced collagen deposition in the subcutaneous layer. They had a normal subcutaneous space with intact vital vessels and nerves [98].
The exact mechanism of constriction band syndrome is not precisely understood, and no genetic factors, genetic predispositions, or gender or race associations have been identified [99]. It is generally accepted that this occurrence is attributed to an extrinsic factor known as the amniotic band, which is formed after intrauterine amniotic rupture; entwining between this amniotic band and body parts causes constriction band syndrome deformities. This extrinsic mechanism is based on observations of amputated digits found within the uterus [100]. It has also been hypothesized that it arises from vascular disruption caused by developmental abnormalities in the blastoderm, which is known as the intrinsic mechanism [101]. The suggested risk factors for constriction band syndrome include low birth weight, premature birth, amniocentesis, trauma, hemorrhage, maternal drug use, and maternal illness [95,100,102].
Constriction band syndrome of the extremities can be categorized using the Patterson classification, which grades patients according to the severity of involvement of the extremities. Type I refers to the presence of a simple constriction ring, and type II refers to ring constriction with extremity deformity or distal lymphedema. Type III refers to the presence of constriction rings and syndactyly. Type IV refers to patients who underwent intrauterine amputation. Among these, type II is the most common [103].
Except for very mild cases, surgical intervention is generally recommended for functional and cosmetic improvement. Surgery is typically performed between the ages of 2 and 4 years; however, immediate surgery may be necessary if ischemic changes occur in the proximal part due to poor blood circulation caused by the congenital constriction band at birth. In addition to vascular compromise, the presence of neurological deficits or lymphedema due to band compression requires early surgical intervention [96,104]. The common surgical approach for constriction band syndrome is removing and releasing the skin and connective tissue at the band site and using Z-plasty or W-plasty techniques to elongate the tissue (Fig. 9). Traditionally, constriction band release has been performed in two or more stages to avoid any vascular compromise of the distal part of the constriction band. Recently, many surgeons have performed single-stage release of the constriction band and have demonstrated good outcomes without vascular compromise, such as venous congestion or ischemia. Jiang et al. [98] pathologically showed that constriction band structures have a normal subcutaneous space with intact, unaffected, deep vital vessels and nerves. From this point, they conducted single-stage surgery with complete removal of the constriction band, triangular flap technique, and fascial flap reduction and showed good aesthetic contour and functional results. Dufournier et al. [105] also showed good functional and aesthetic results without neurologic or vascular complications using single-stage circumferential resection of the constriction band and direct closure. Furthermore, Habenicht et al. [106] conducted a single-stage complete circular constriction band resection and circumferential skin closure and showed good functional and aesthetic results without recurrence. Additionally, Mutaf and Sunay [107] introduced a single-stage resection of the constriction band and reconstruction using a superior- or inferior-based alternating de-epithelialized rectangular dermofat flap folded into the deep side of the opposite rectangular cutaneous flap to fill the dead space formed after constriction band resection. This method successfully prevented sandglass depression deformities. Finally, Inglesby et al. [95] modified the Mutaf and Sunay method [107] using only subcutaneous fat flap turnover without including the dermis to maintain the volume and ensure an adequate blood supply to the skin edge. All of these techniques emphasize complete, radical, and whole-depth excision of the constriction band tissue to minimize recurrence. Castro-Govea et al. [108] reported good results with multiple perpendicular and circumferential subcisions of the constriction band and fat grafting.
Thumb hypoplasia can manifest in various forms, ranging from a slightly smaller thumb without any functional problems to a complete absence of the thumb. Often, it may be accompanied by other congenital hand anomalies such as polydactyly, cleft hand, brachydactyly, transverse deficiencies and symbrachydactyly, ulnar longitudinal deficiency, and constriction band syndrome [109,110]. Therefore, before initiating treatment, a thorough examination of the size, position, relationship with other fingers, status of bones and joints, intrinsic and extrinsic muscles, status of the first web space, any issues with adjacent skeletal structures such as the radius, and any associated deformities should be carefully assessed for a correct diagnosis [2]. The incidence of thumb hypoplasia is 1 in 100,000 live births worldwide [109]. According to reports by Entin [110], thumb hypoplasia is found in approximately 16% of all congenital hand anomalies, and according to Flatt [76], it is present in approximately 3.6% of the cases. Thumb hypoplasia can be associated with syndromes such as VACTERL (vertebral anomalies, anal atresia, cardiovascular anomalies, tracheoesophageal fistula, renal anomalies, limb abnormalities), Fanconi anemia, Holt-Oram syndrome, and TAR syndrome (thrombocytopenia with absent radius). Various radial longitudinal deficiencies can occur owing to genetic or environmental factors; therefore, genetic counseling should be sought when necessary.
As mentioned previously, thumb hypoplasia exhibits various presentations of the affected elements. Manske and McCarroll [112] introduced a modified Blauth classification system, categorizing thumb hypoplasia into types I to V, which has gained widespread acceptance for grading the severity of hypoplastic thumbs. This classification is based on the intrinsic muscles, extrinsic tendons, first web space, and the carpometacarpal joint stability of the thumb [112,113].
Type I is characterized by a thumb that is nearly normal in size and shape but is underdeveloped overall. The metacarpal bone and phalanges are thin and small but have an intact distal radius, including the styloid process, scaphoid, and trapezoid. Intrinsic muscles are all present, but the abductor pollicis brevis, lateral head of the flexor pollicis brevis, and opponens pollicis muscles are slightly hypoplastic [2,112,113].
Type II lacks the median nerve innervated intrinsic muscles of the thumb, resulting in hypoplasia of the thenar eminence and a narrow first web space. In addition, the UCL of the thumb exhibits laxity and instability. The scaphoid, trapezium, trapezoid, and sometimes lunate may show hypoplastic features. Narrowing the first web space restricts thumb abduction. Underdevelopment of the thenar muscles restricts opposition movements. UCL insufficiency leads to MCP joint instability [2,109]. Various tendon and muscle abnormalities have been identified in type II or IIIA patients. The muscle belly and tendon of the flexor pollicis longus may exhibit abnormalities, such as proximal duplications or a distal insertion towards the radial side [114-117]. In certain cases, the muscle may originate from the transverse carpal ligament, flexor digitorum profundus tendon of the index finger, or the fascia of the intrinsic thenar muscle and be inserted into the flexor tendon sheath or extensor mechanism [114,115,118]. Flexor pollicis longus muscle may be completely absent in certain instances [119-121]. Sometimes the radial wrist extensors or thumb abductors show abnormalities, rather than malformed or mispositioned thumb flexors [122]. On occasion, a small anomalous muscle, referred to as the “musculus lumbricalis pollicis,” may extend from the origin of the thumb across the first web space and insert into the index finger flexor system [123]. The extrinsic extensors may exhibit abnormal insertions, extend over the MCP joint in a noncentralized position, and establish unusual connections with the extrinsic flexor [122-124]. These atypical insertions of both flexors and extensors, along with their altered course, collaborate to make both tendons primarily function as radial deviators rather than as primary flexors or extensors. Tupper termed this condition “pollex abductus” and observed that when these muscles contract, there is no flexion or extension at the IP joint; instead, there is only abduction or radial deviation of the thumb [122].
Type III builds upon type II issues, with additional deformities in the extrinsic muscles and tendons. A significantly more pronounced degree of skeletal shortening and narrowing is observed at the metacarpal level. The trapezium is typically very small, and the scaphoid is often missing. The distal radius shows a blunt shape owing to the hypoplastic feature of the radius and the absence of the styloid process. The intrinsic muscles innervated by the median nerve are either significantly underdeveloped or completely absent. However, if present, they may actively flex MCP joints. The collateral ligaments, volar plate, flexor retinaculum, and major pulleys are hypoplastic or absent [1,2]. Type III is further subdivided into A and B based on the stability of the thumb carpometacarpal joint. Type IIIA shows a stable carpometacarpal joint, whereas type IIIB is unstable. In type IIIB, the fibrous band may link the underdeveloped metacarpal to a cartilaginous mass, which can represent either a trapezium or the base of the metacarpal.
Type IV involves a thumb attached to a soft tissue bridge containing neurovascular bundles or a “floating thumb” configuration. There is no metacarpal bone and two hypoplastic phalangeal bones are present in the floating thumb. The absence of the trapezium is common and, less frequently, the scaphoid is also absent [1,2].
Type V is the most severe form, in which the thumb is completely absent. Radius deficiency can occur in approximately half of patients with type V disease [112,126]. If the radius is normal, the index finger also shows normal features and exhibits good abduction at the MCP joint, due to the presence of a robust first dorsal interosseous muscle. However, if there is a radial deficiency, the index finger tends to be shorter, and stiffer, and frequently syndactyly occurs with the middle finger [126].
The treatment goals for thumb hypoplasia include achieving a stable and mobile carpometacarpal joint with an intact metacarpal; a scarless first web space possessing sufficient depth and width covered with full-thickness skin; at least two mobile joints out of the carpometacarpal, MCP, or interphalangeal joints; a stable MCP joint, especially of the UCL; sufficient strength for robust flexion and extension at either the MCP or interphalangeal joint; and the capability to be positioned in a palmar abducted state for effective pinch and grasp maneuvers [2,127].
There is a debate regarding the optimal timing of surgical treatment. Those advocating early surgical intervention provide the following rationale: releasing tethered musculotendinous units and addressing joint contractures will enable unhindered growth, with the reconstructed thumb adapting physiologically to both growth and functional use. From a cognitive perspective, early surgery permits the development of a child with a reconstructed thumb before thumb corticalization, a process that typically occurs at approximately 18 months of age. In slightly older patients, the hypoplastic thumb becomes larger, alleviating potential problems related to osteotomies, growth alteration, skeletal fixation, joint reconstruction, and the risk of compromising blood supply. Moreover, with older patients, the surgeon can evaluate the child’s functional requirements more effectively. Some authors recommend pollicization when the child is between 12 and 18 months of age. For thumb reconstruction without pollicization, some surgeons recommend performing surgery around the age of 2 to 3 years when the structures have sufficiently developed, allowing for safe operation on the bones, joints, and ligaments [2,116,128].
The surgical treatment approach for thumb hypoplasia varies according to its type (Table 6). Because type I patients generally do not show functional deficits, they do not require surgical treatment. Augmentation procedures can be performed to improve cosmetic outcomes.
For types II and IIIA, these problems should be addressed individually. The five main problems in these patients included narrowing of the first web space, MCP joint instability, poor IP joint motion, inadequate palmar abduction and opposition, and thumb abduction posturing [2]. Widening of the first web space is essential for thumb growth and function. The first web space was widened using local flaps. Four-flap Z-plasty, double-opposing Z-plasty, and five-flap Z-plasty are commonly used procedures that provide good aesthetic contour and release. In contrast, basic two-flap Z-plasty does not provide the appropriate shape and typically results in a central depression at the base of the first web space [109]. Skin graft can also be used with local flap transposition techniques. For a more severely narrowed first web space, the first dorsal interosseous artery flap or distally based radial forearm flap can be utilized [129]. Free fasciocutaneous flap transfer or the use of a tissue expander can also be an option [130]. During the first web space release, it is important to release the taut investing fascia sufficiently over the first dorsal interosseous muscles, adductor muscles, and intermetacarpal band to minimize adduction contracture [131].
For uniaxial MCP joint instability, Manske and McCarroll [112] recommended that if the nearby tissue is deemed sufficient, imbrication of the capsuloligamentous structures may offer satisfactory stability to the UCL. Hovius et al. [132] and de Kraker et al. [133] observed that local tissue imbrication tends to undergo stretching and eventual failure over time. They reported utilization of the flexor digitorum superficialis (FDS) tendon slip to reconstruct the UCL. Tonkin [110] recommended incorporating a 2- to 3-mm strip of distally based volar plate to complement the imbrication of local tissue. Free tendon graft reconstruction, such as that using the palmaris longus, can also be utilized [129]. For biaxial instability, both radial and UCLs can be reconstructed using two slips of the FDS tendon. In cases of multiplanar instability, addressing MCP joint hyperextension can be achieved through the advancement of the volar plate. The volar plate is disconnected at the proximal end and moved proximally toward the head-neck junction of the metacarpal [110]. When the volar surface of the metacarpal head lacks two well-defined condyles, an inadequately developed flat proximal phalanx articular surface is prone to uncontrolled movement. In such cases, soft-tissue stabilization is ineffective, necessitating arthrodesis [109,110,131].
To restore opposition function due to the hypoplasia or absence of intrinsic muscles, opponensplasty is performed using abductor digiti minimi transfer or FDS of ring finger or long finger transfer [132-137]. To achieve effective opponensplasty results, sufficient release of the first web space, stability of the MCP joint, and mobility of the carpometacarpal joint are necessary. For abductor digiti minimi muscle transfer, an incision is made at the junction of the volar and dorsal skin, starting from the pisiform to the proximal phalanx. The insertion of the muscle is detached from the base of the proximal phalanx. For harvesting with sufficient length, the tendon contribution to the extensor mechanism can also be harvested [110]. The abductor digiti minimi origin at the pisiform can be detached partially for additional length [132] or remain intact [110,131]. The abductor digiti minimi can be transferred through a subcutaneous tunnel above the palmar fascia. Skin islands can also be included as myocutaneous flaps. This technique offers the benefit of addressing soft tissue deficiency in the palm while reducing tunnel tightness, which can lead to muscle ischemia [131,136,137]. After transposition, the tendon is inserted into the head-neck junction of the thumb metacarpal, the radial collateral ligament at the MCP joint or the remnant structure of the abductor pollicis brevis [109]. The use of the FDS tendon for opponensplasty allows simultaneous reconstruction of the UCL, as described earlier [110,132,133]. Typically, the FDS of the ring finger is utilized because independent flexion of the proximal interphalangeal joint of the ring finger is seldom required for daily activities, although it may weaken the power grip [132,134]. To ensure adequate pronation of the thumb and prevent the tendon from migrating radially, the FDS transfer necessitates the creation of a pulley system. Several options, such as the transverse carpal ligament, palmar aponeurosis, or flexor carpi ulnaris, have been suggested for the pulley [110,134,138,139]. Vuillermin et al. [134] reported that there is no significant difference in pinch and grip strength and Kapandji score between the pulleys of the transverse carpal ligament and flexor carpi ulnaris. After tendon transfer, the tendon attachment method is the same as the abductor digiti minimi transfer technique. To reinforce the UCL, a drill hole is made in the head of the metacarpal bone and, by passing the transferred tendon from the radial to the ulnar side, it can be fixed at the base of the proximal phalanx on the ulnar side. The transfer of the FDS can concurrently reconstruct the UCL and offer an enhanced palmar abductor, whereas the transfer of the abductor digiti minimi can contribute volume to the thenar eminence and offer superior pronation for the thumb [109].
Several procedures can be performed to restore interphalangeal joint motion. Abnormal connections between the flexor pollicis longus and extensor mechanism (i.e., the pollex abductus) should be divided. Dorsal capsulotomy and extrinsic tendon recentralization should be performed [2]. Abnormal insertion of the extrinsic flexor and extensor muscles can be corrected. The pulley system can be reinforced by using a segment of the transferred tendon or extensor retinaculum. Tendon transfers to restore the function of the extrinsic flexors or extensors may occasionally be considered, although achieving an effective range of motion can be challenging [109,110,138,140]. For flexion, the FDS of the ring finger was the most appropriate choice for flexion, while the extensor indicis proprius was optimal for extension. Secondary transfer of the brachioradialis or extensor carpi radialis longus along with a tendon graft to the extensor pollicis longus can also be utilized [2,126].
For type IIIB, due to instability of the carpometacarpal joint, functional outcomes remain suboptimal even after thumb reconstruction surgery. Therefore, thumb removal is considered and pollicization using the index finger is performed to create a new thumb. Many different pollicization approaches have been described [127,141-143]. The main principles of pollicization include follows: (1) incision design as Y-to-V advancement with a back cut to form a normal, wide first web space; (2) transposition of the index finger as a neurovascular island flap; (3) metacarpal shortening with proximal dorsally angulated oblique osteotomy at the metacarpal base and distal metacarpal head osteotomy through the physis and complete ablation of the growth plate to ensure growth halt. The metacarpal head is rotated, recessed, and positioned anterior to the oblique osteotomy line at the base with hyperextension of the MCP joint. The recommended position for the new thumb is approximately 45° abduction and 135° pronation; (4) rebalance of the intrinsic and extrinsic muscles and tendons; (5) tensionless adequate skin closure for web reconstruction [127,144]. After the pollicization, the structures in the original index finger that corresponded to the structures of the new thumb after pollicization are shown in Fig. 10 and Table 7 [2]. Although pollicization significantly improves function, surgeons should remember that there are some limitations to the new thumb. The newly formed carpometacarpal joint corresponds to the index MCP joint and, as a result, lacks the same degree of freedom as the normal carpometacarpal saddle joint of the thumb. Owing to the absence of a normal cone of the thenar muscles, the strength, and stability of these thumbs in both the pinch and grasp maneuvers do not reach normal levels [2,127]. In cases of types IV and V, pollicization was also performed.
For patients with type IIIB or IV who do not wish to undergo pollicization, several methods have been introduced to create a five-digit hand. Schneider et al. [145] reported a case of free vascularized partial second metatarsal bone transfer in a patient with type IIIB thumb hypoplasia. Chow et al. [146] reported thumb reconstruction using a nonvascularized partial fourth metatarsal bone graft. Zhong et al. [147] described a nonvascularized iliac bone graft in patients with type IIIB thumb hypoplasia. These techniques required several staged operations to create a stable and mobile thumb but limited functional results have eventually been reported. Vascularized second toe transfer, vascularized fourth toe transfer, and vascularized second metatarsophalangeal joint transfer were also reported [148-151]. Ozols et al. [152,153] described second toe transplantation using the MTP joint arthrodesis technique for patients with type IIIB, IV, and V thumb hypoplasia. Arthrodesis of the metatarsophalangeal joint is performed to form a stable metacarpal bone of sufficient length, with the proximal interphalangeal joint assuming the role of the MCP joint in the new thumb. They reported functional outcomes similar to those of pollicization while preserving a five-digit hand.
Congenital hand anomalies are common malformations. Because many congenital hand anomalies present with other anomalies, a thorough examination is important to make an accurate diagnosis. These anomalies not only impact aesthetic appearance but also influence an individual’s hand function, ultimately lowering their quality of life. Surgical treatment should address these problems to provide patients with more natural and functional hands. Furthermore, surgeons should be aware of treatment principles beyond surgery to ensure that parents receive proper counseling and that children are referred to other specialists when necessary.
Over decades, significant advances in the treatment of congenital hand anomalies have occurred, and recent technological developments have improved and refined existing surgical techniques and addressed many previously unresolved issues. However, many challenges remain for surgeons. Building upon the current knowledge and research on congenital anomalies, continued efforts with active further research and investigation should be made to surpass the current limitations of the treatment of congenital hand anomalies.
References
1. Wolfe SW, Pederson WC, Kozin SH, Cohen MS. Green’s operative hand surgery. 8th ed. Philadelphia: Elsevier;2021.
2. Chang J, Neligan PC. Plastic surgery. Volume 6. Hand and upper limb. 4th ed. London: Elsevier;2017.
3. Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993; 75:1401–16.

4. Bamshad M, Watkins WS, Dixon ME, et al. Reconstructing the history of human limb development: lessons from birth defects. Pediatr Res. 1999; 45:291–9.

5. Korean Society of Plastic and Reconstructive Surgeons. Standard plastic and reconstructive surgery. 3rd ed. Paju: Koonja Publishing;2019.
6. Oberg KC, Greer LF, Naruse T. Embryology of the upper limb: the molecular orchestration of morphogenesis. Handchir Mikrochir Plast Chir. 2004; 36:98–107.

7. Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994; 371:609–12.

8. Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994; 79:993–1003.

9. Sun X, Lewandoski M, Meyers EN, Liu YH, Maxson RE, Martin GR. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000; 25:83–6.

10. Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995; 83:631–40.

11. Vogel A, Rodriguez C, Warnken W, Izpisúa Belmonte JC. Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature. 1995; 378:716–20.

12. Woods CG, Stricker S, Seemann P, et al. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Hum Genet. 2006; 79:402–8.

13. Yang Y, Niswander L. Interaction between the signaling molecules WNT7a and SHH during vertebrate limb development: dorsal signals regulate anteroposterior patterning. Cell. 1995; 80:939–47.

14. Raspopovic J, Marcon L, Russo L, Sharpe J. Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science. 2014; 345:566–70.

15. Sheth R, Marcon L, Bastida MF, et al. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science. 2012; 338:1476–80.

16. Oberg KC, Ros MA, Goldfarb CA. New insights on upper limb development: digitizing the hand. IFSSH Ezine. 2014; 4:16–22.
17. Swanson AB, Barsky AJ, Entin MA. Classification of limb malformations on the basis of embryological failures. Surg Clin North Am. 1968; 48:1169–79.

18. Oberg KC, Feenstra JM, Manske PR, Tonkin MA. Developmental biology and classification of congenital anomalies of the hand and upper extremity. J Hand Surg Am. 2010; 35:2066–76.

19. Finley WH, Gustavson KH, Hall TM, Hurst DC, Barganier CM, Wiedmeyer JA. Birth defects surveillance: Jefferson County, Alabama, and Uppsala County, Sweden. South Med J. 1994; 87:440–5.

20. Kim D, Park SK, Kim DC, Oh SJ, Yoo KY. Nationwide estimation for incidence at birth of congenital polydactyly and syndactyly in Korean. J Korean Soc Plast Reconstr Surg. 2003; 30:24–32.
21. Flatt AE. A test of a classification of congenital anomalies of the upper extremity. Surg Clin North Am. 1970; 50:509–16.

22. Wassel HD. The results of surgery for polydactyly of the thumb. A review. Clin Orthop Relat Res. 1969; 64:175–93.
23. Temtamy SA, McKusick VA. The genetics of hand malformations. Birth Defects Orig Artic Ser. 1978; 14:i–xviii, 1-619.
25. Tada K, Kurisaki E, Yonenobu K, Tsuyuguchi Y, Kawai H. Central polydactyly: a review of 12 cases and their surgical treatment. J Hand Surg Am. 1982; 7:460–5.
28. Kim BJ, Choi JH, Kwon ST. Oblique osteotomy for the correction of the zigzag deformity of Wassel type IV polydactyly. Plast Reconstr Surg. 2017; 140:1220–8.

29. Bilhaut M. The curing of a bifid thumb by a new operative procedure (translated from the original French by HE Tonkin and MA Tonkin). Hand Surg. 1997; 2:75–7.

30. Townsend DJ, Lipp EB, Chun K, Reinker K, Tuch B. Thumb duplication, 66 years’ experience: a review of surgical complications. J Hand Surg Am. 1994; 19:973–6.
31. Kim BJ, Choi JH, Kwon ST. Surgical treatment of axial polysyndactyly and postaxial polydactyly of the hand in Korean: a clinical analysis of 24 cases. J Korean Soc Surg Hand. 2017; 22:20–6.

32. Watson BT, Hennrikus WL. Postaxial type-B polydactyly. Prevalence and treatment. J Bone Joint Surg Am. 1997; 79:65–8.
33. Singer G, Thein S, Kraus T, Petnehazy T, Eberl R, Schmidt B. Ulnar polydactyly: an analysis of appearance and postoperative outcome. J Pediatr Surg. 2014; 49:474–6.
34. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2010; 27:39–42.

35. Stutz C, Mills J, Wheeler L, Ezaki M, Oishi S. Long-term outcomes following radial polydactyly reconstruction. J Hand Surg Am. 2014; 39:1549–52.

36. Jordan D, Hindocha S, Dhital M, Saleh M, Khan W. The epidemiology, genetics and future management of syndactyly. Open Orthop J. 2012; 6:14–27.

37. Mantero R, Ferrari GL, Ghigliazza GB, Auxilia E. [Syndactyly: an angiographic study]. Ann Chir Main. 1983; 2:62–5. French.
38. Samson P, Salazard B. [Syndactyly]. Chir Main. 2008; 27 Suppl 1:S100–14. French.
39. Tonkin MA. Classification of congenital anomalies of the hand and upper limb. J Hand Surg Eur Vol. 2017; 42:448–56.

40. Le Hanneur M, Cambon-Binder A, Bachy M, Fitoussi F. Treatment of congenital syndactyly. Hand Surg Rehabil. 2020; 39:143–53.

41. Kozin SH, Zlotolow DA. Common pediatric congenital conditions of the hand. Plast Reconstr Surg. 2015; 136:241e–57e.

42. Hynes SL, Harvey I, Thomas K, Copeland J, Borschel GH. CT angiography-guided single-stage release of adjacent webspaces in non-Apert syndactyly. J Hand Surg Eur Vol. 2015; 40:625–32.

43. Tian X, Xiao J, Li T, Chen W, Lin Q, Chim H. Single-stage separation of 3- and 4-finger incomplete simple syndactyly with contiguous gull wing flaps: a technique to minimize or avoid skin grafting. J Hand Surg Am. 2017; 42:257–64.

45. Toledo LC, Ger E. Evaluation of the operative treatment of syndactyly. J Hand Surg Am. 1979; 4:556–64.

46. Keret D, Ger E. Evaluation of a uniform operative technique to treat syndactyly. J Hand Surg Am. 1987; 12(5 Pt 1):727–9.

47. Barabás AG, Pickford MA. Results of syndactyly release using a modification of the Flatt technique. J Hand Surg Eur Vol. 2014; 39:984–8.

48. De Smet L, Van Ransbeeck H, Deneef G. Syndactyly release: results of the Flatt technique. Acta Orthop Belg. 1998; 64:301–5.
49. D’Arcangelo M, Gilbert A, Pirrello R. Correction of syndactyly using a dorsal omega flap and two lateral and volar flaps. A long-term review. J Hand Surg Br. 1996; 21:320–4.
50. Uzunismail A. Utility of the “seagull” flap for unoperated simple complete syndactyly in adults. Br J Plast Surg. 1988; 41:548–50.

51. Delord M, Forli A, Aribert M, Moutet F, Corcella D. [Results of Blauth Palmar flap in congenital syndactyly: long-term outcome in a 31 webs study]. Ann Chir Plast Esthet. 2020; 65:204–12. French.
52. Killian JT, Neimkin RJ. Syndactyly reconstruction by a modified Cronin method. South Med J. 1985; 78:414–8.

53. Lewis RC, Nordyke MD, Duncan KH. Web space reconstruction with a M-V flap. J Hand Surg Am. 1988; 13:40–3.

54. Karacaoglan N, Velidedeoglu H, Ciçekçi B, Bozdogan N, Sahin U, Türkgüven Y. Reverse W-M plasty in the repair of congenital syndactyly: a new method. Br J Plast Surg. 1993; 46:300–2.

55. Savaci N, Hoŝnuter M, Tosun Z. Use of reverse triangular V-Y flaps to create a web space in syndactyly. Ann Plast Surg. 1999; 42:540–4.

56. Mericli AF, Black JS, Morgan RF. Syndactyly web space reconstruction using the tapered M-to-V flap: a single-surgeon, 30-year experience. J Hand Surg Am. 2015; 40:1755–63.

57. Karamese M, Akdag O, Selimoglu MN, Unal Yıldıran G, Tosun Z. V-Y and rectangular flap combination for syndactyly repair. J Plast Surg Hand Surg. 2016; 50:102–6.

58. Bulic K. Long-term aesthetic outcome of fingertip reconstruction in complete syndactyly release. J Hand Surg Eur Vol. 2013; 38:281–7.

59. Golash A, Watson JS. Nail fold creation in complete syndactyly using Buck-Gramcko pulp flaps. J Hand Surg Br. 2000; 25:11–4.

60. Buck-Gramcko D. Congenital malformations of the hand and forearm. London: Churchill Livingstone;1998.

61. Duteille F, Truffandier MV, Perrot P. ‘Matriderm’ dermal substitute with split-thickness skin graft compared with full-thickness skin graft for the coverage of skin defects after surgical treatment of congenital syndactyly: results in 40 commissures. J Hand Surg Eur Vol. 2016; 41:350–1.

62. Deunk J, Nicolai JP, Hamburg SM. Long-term results of syndactyly correction: full-thickness versus split-thickness skin grafts. J Hand Surg Br. 2003; 28:125–30.

63. Landi A, Garagnani L, Leti Acciaro A, Lando M, Ozben H, Gagliano MC. Hyaluronic acid scaffold for skin defects in congenital syndactyly release surgery: a novel technique based on the regenerative model. J Hand Surg Eur Vol. 2014; 39:994–1000.

64. Wang S, Zheng S, Li N, Feng Z, Liu Q. Dorsal hexagon local flap without skin graft for web reconstruction of congenital syndactyly. J Hand Surg Am. 2020; 45:63.

65. Widerberg A, Sommerstein K, Dahlin LB, Rosberg HE. Long-term results of syndactyly correction by the trilobed flap technique focusing on hand function and quality of life. J Hand Surg Eur Vol. 2016; 41:315–21.

66. Ni F, Mao H, Yang X, Zhou S, Jiang Y, Wang B. The use of an hourglass dorsal advancement flap without skin graft for congenital syndactyly. J Hand Surg Am. 2015; 40:1748–54.

67. Dong Y, Wang Y. The use of a dorsal double-wing flap without skin grafts for congenital syndactyly treatment: a STROBE compliant study. Medicine (Baltimore). 2017; 96:e7639.
68. Yuan F, Zhong L, Chung KC. Aesthetic comparison of two different types of web-space reconstruction for finger syndactyly. Plast Reconstr Surg. 2018; 142:963–71.

69. Ferrari BR, Werker PM. A cross-sectional study of long-term satisfaction after surgery for congenital syndactyly: does skin grafting influence satisfaction? J Hand Surg Eur Vol. 2019; 44:296–303.

70. Wang AA, Hutchinson DT. Syndactyly release: a comparison of skin graft versus graftless techniques in the same patient. J Hand Surg Eur Vol. 2019; 44:845–9.

71. Zucker RM, Cleland HJ, Haswell T. Syndactyly correction of the hand in Apert syndrome. Clin Plast Surg. 1991; 18:357–64.

72. Chang J, Danton TK, Ladd AL, Hentz VR. Reconstruction of the hand in Apert syndrome: a simplified approach. Plast Reconstr Surg. 2002; 109:465–71.

73. Fearon JA. Treatment of the hands and feet in Apert syndrome: an evolution in management. Plast Reconstr Surg. 2003; 112:1–12.

75. Taghinia AH, Yorlets RR, Doyle M, Labow BI, Upton J. Long-term functional upper-extremity outcomes in adults with Apert syndrome. Plast Reconstr Surg. 2019; 143:1136–45.

76. Flatt AE. The care of congenital hand anomalies. St. Louis: Quality Medical;1994.
77. Rios JJ, Paria N, Burns DK, et al. Somatic gain-of-function mutations in PIK3CA in patients with macrodactyly. Hum Mol Genet. 2013; 22:444–51.

78. Ezaki M, Beckwith T, Oishi SN. Macrodactyly: decision-making and surgery timing. J Hand Surg Eur Vol. 2019; 44:32–42.

79. Tsuge K, Ikuta Y. Macrodactyly and fibro-fatty proliferation of the median nerve. Hiroshima J Med Sci. 1973; 22:83–101.
82. Cerrato F, Eberlin KR, Waters P, Upton J, Taghinia A, Labow BI. Presentation and treatment of macrodactyly in children. J Hand Surg Am. 2013; 38:2112–23.
83. Woo SJ, Jung JH, Choi JH, Kim Y, Kwon ST, Kim BJ. The effect of epiphysiodesis on the longitudinal bone growth of hands or feet in children with macrodactyly based on long-term quantitative analysis. J Pediatr Orthop. 2023; 43:e363–9.

84. Shen XF, Gasteratos K, Spyropoulou GA, Yin F, Rui YJ. Congenital difference of the hand and foot: pediatric macrodactyly. J Plast Reconstr Aesthet Surg. 2022; 75:4054–62.

85. Stern PJ, Nyquist SR. Macrodactyly in ulnar nerve distribution associated with cubital tunnel syndrome. J Hand Surg Am. 1982; 7:569–71.

86. Al-Qattan MM. Lipofibromatous hamartoma of the median nerve and its associated conditions. J Hand Surg Br. 2001; 26:368–72.

87. Waters PM, Bae DS. Pediatric hand and upper limb surgery: a practical guide. Philadelpia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2012.
88. Wu J, Tian G, Ji Y, Higgins JP, Lee WP. Clinical characteristics of 90 macrodactyly cases. J Hand Surg Am. 2020; 45:982.
89. Kalb JP, Suarez DA, Herrera AM. Bilateral macrodactyly of the halluces in an adolescent girl corrected with shortening osteotomies of the first metatarsal and the phalangeal bones: a case report. JBJS Case Connect. 2018; 8:e58.
90. Temtamy SA, Rogers JG. Macrodactyly, hemihypertrophy, and connective tissue nevi: report of a new syndrome and review of the literature. J Pediatr. 1976; 89:924–7.

91. Wiedemann HR, Burgio GR, Aldenhoff P, Kunze J, Kaufmann HJ, Schirg E. The Proteus syndrome. Partial gigantism of the hands and/or feet, nevi, hemihypertrophy, subcutaneous tumors, macrocephaly or other skull anomalies and possible accelerated growth and visceral affections. Eur J Pediatr. 1983; 140:5–12.
92. Ishida O, Ikuta Y. Long-term results of surgical treatment for macrodactyly of the hand. Plast Reconstr Surg. 1998; 102:1586–90.

93. Topoleski TA, Ganel A, Grogan DP. Effect of proximal phalangeal epiphysiodesis in the treatment of macrodactyly. Foot Ankle Int. 1997; 18:500–3.

94. Badawy M, Ma Y, Baldrighi C, Oestreich K, Jester A. Efficacy of mTOR inhibitors (sirolimus) in isolated limb overgrowth: a systematic review. J Hand Surg Eur Vol. 2022; 47:698–704.

95. Inglesby DC, Janssen PL, Graziano FD, Gopman JM, Rutland JW, Taub PJ. Amniotic band syndrome: head-to-toe manifestations and clinical management guidelines. Plast Reconstr Surg. 2023; 152:338e–46e.

96. Chiu DT, Patel A, Sakamoto S, Chu A. The impact of microsurgery on congenital hand anomalies associated with amniotic band syndrome. Plast Reconstr Surg Glob Open. 2018; 6:e1657.

97. Cignini P, Giorlandino C, Padula F, Dugo N, Cafà EV, Spata A. Epidemiology and risk factors of amniotic band syndrome, or ADAM sequence. J Prenat Med. 2012; 6:59–63.
98. Jiang Y, Mao H, Yang X, et al. Single-stage resection of type II constriction rings in limbs on the basis of histologic and magnetic resonance imaging observations: a retrospective study of 21 consecutive patients. Plast Reconstr Surg. 2016; 138:164–73.

99. Mukherjee PM, Natera M, Drew H, Creanga A. Amniotic band syndrome: a multidisciplinary care approach to the treatment of a rare case. Cleft Palate Craniofac J. 2019; 56:105–9.

100. Torpin R. Amniochorionic mesoblastic fibrous strings and amnionic bands: associated constricting fetal malformations or fetal death. Am J Obstet Gynecol. 1965; 91:65–75.
101. Van Allen MI, Siegel-Bartelt J, Dixon J, Zuker RM, Clarke HM, Toi A. Constriction bands and limb reduction defects in two newborns with fetal ultrasound evidence for vascular disruption. Am J Med Genet. 1992; 44:598–604.

102. Syvänen J, Raitio A, Nietosvaara Y, et al. Risk factors and prevalence of limb deficiencies associated with amniotic band sequence: a population-based case-control study. J Pediatr Orthop. 2021; 41:e94–7.

104. Weinzweig N, Barr A. Radial, ulnar, and median nerve palsies caused by a congenital constriction band of the arm: single-stage correction. Plast Reconstr Surg. 1994; 94:872–6.
105. Dufournier B, Guero S, de Tienda M, et al. One-stage circumferential limb ring constriction release and direct circular skin closure in amniotic band syndrome: a 14-case series. Orthop Traumatol Surg Res. 2020; 106:1353–9.

106. Habenicht R, Hülsemann W, Lohmeyer JA, Mann M. Ten-year experience with one-step correction of constriction rings by complete circular resection and linear circumferential skin closure. J Plast Reconstr Aesthet Surg. 2013; 66:1117–22.
107. Mutaf M, Sunay M. A new technique for correction of congenital constriction rings. Ann Plast Surg. 2006; 57:646–52.

108. Castro-Govea Y, Vela-Martinez A, Treviño-Garcia LA. Lipoinjection and multiple internal cuts for congenital constriction bands: a new treatment approach. Aesthetic Plast Surg. 2017; 41:375–80.

109. Mende K, Suurmeijer JA, Tonkin MA. Surgical techniques for reconstruction of the hypoplastic thumb. J Hand Surg Eur Vol. 2019; 44:15–24.

110. Tonkin M. Surgical reconstruction of congenital thumb hypoplasia. Indian J Plast Surg. 2011; 44:253–65.

111. Entin MA. Reconstruction of congenital aplasia of phalanges in the hand. Surg Clin North Am. 1968; 48:1155–68.

112. Manske PR, McCarroll HR. Reconstruction of the congenitally deficient thumb. Hand Clin. 1992; 8:177–96.

113. Blauth W, Schneider-Sickert FR. Congenital deformities of the hand: an atlas of their surgical treatment. Berlin, Heidelberg: Springer;2012.

114. Blair WF, Buckwalter JA. Congenital malposition of flexor pollicis longus: an anatomy note. J Hand Surg Am. 1983; 8:93–4.
115. Blair WF, Omer GE. Anomalous insertion of the flexor pollicis longus. J Hand Surg Am. 1981; 6:241–4.

116. Graham TJ, Louis DS. A comprehensive approach to surgical management of the type IIIA hypoplastic thumb. J Hand Surg Am. 1998; 23:3–13.

117. Dellon AL, Rayan G. Congenital absence of the thenar muscles. Report of two cases. J Bone Joint Surg Am. 1981; 63:1014–5.

118. Fitch RD, Urbaniak JR, Ruderman RJ. Conjoined flexor and extensor pollicis longus tendons in the hypoplastic thumb. J Hand Surg Am. 1984; 9:417–9.

119. Köster G. Isolated aplasia of the flexor pollicis longus: a case report. J Hand Surg Am. 1984; 9:870–1.

120. Tsuchida Y, Kasai S, Kojima T. Congenital absence of flexor pollicis longus and flexor pollicis brevis: a case report. Hand. 1976; 8:294–7.

121. Arminio JA. Congenital anomaly of the thumb: absent flexor pollicis longus tendon. J Hand Surg Am. 1979; 4:487–8.

122. Tupper JW. Pollex abductus due to congenital malposition of the flexor pollicis longus. J Bone Joint Surg Am. 1969; 51:1285–90.

123. Lister G. Pollex abductus in hypoplasia and duplication of the thumb. J Hand Surg Am. 1991; 16:626–33.

124. Neviaser RJ. Congenital hypoplasia of the thumb with absence of the extrinsic extensors, abductor pollicis longus, and thenar muscles. J Hand Surg Am. 1979; 4:301–3.

125. Kobayashi A, Ohmiya K, Iwakuma T, Mitsuyasu M. Unusual congenital anomalies of the thumb extensors. Report of two cases. Hand. 1976; 8:17–21.

126. Upton J. Treatment of congenital forearm and hand anomalies. In : McCarthy JG, May JW, Littler JW, editors. Pleasetic surgery. Philadelphia: WB Saunders;1990. p. 5352–6.
127. Taghinia AH, Littler JW, Upton J. Refinements in pollicization: a 30-year experience. Plast Reconstr Surg. 2012; 130:423e–33e.
128. Manske PR, Rotman MB, Dailey LA. Long-term functional results after pollicization for the congenitally deficient thumb. J Hand Surg Am. 1992; 17:1064–72.

129. Upton J, Havlik RJ, Coombs CJ. Use of forearm flaps for the severely contracted first web space in children with congenital malformations. J Hand Surg Am. 1996; 21:470–7.

130. Coombs CJ, Mutimer KL. Tissue expansion for the treatment of complete syndactyly of the first web. J Hand Surg Am. 1994; 19:968–72.

131. Smith P, Sivakumar B, Hall R, Fleming A. Blauth II thumb hypoplasia: a management algorithm for the unstable metacarpophalangeal joint. J Hand Surg Eur Vol. 2012; 37:745–50.

132. Hovius SE, Versnel SL, Zuidam JM. Tendon transfers in the congenital hand. In : Friden J, editor. Tendon transfers in reconstructive hand surgery. London: CRC Press;2005. p. 121–32.
133. de Kraker M, Selles RW, Zuidam JM, Molenaar HM, Stam HJ, Hovius SE. Outcome of flexor digitorum superficialis opponensplasty for Type II and IIIA thumb hypoplasia. J Hand Surg Eur Vol. 2016; 41:258–64.

134. Vuillermin C, Butler L, Lake A, Ezaki M, Oishi S. Flexor digitorum superficialis opposition transfer for augmenting function in types II and IIIA thumb hypoplasia. J Hand Surg Am. 2016; 41:244–9.

135. Manske PR, McCarroll HR. Abductor digiti minimi opponensplasty in congenital radial dysplasia. J Hand Surg Am. 1978; 3:552–9.

136. Ogino T, Minami A, Fukuda K. Abductor digiti minimi opponensplasty in hypoplastic thumb. J Hand Surg Br. 1986; 11:372–7.

137. Upton J, Taghinia AH. Abductor digiti minimi myocutaneous flap for opponensplasty in congenital hypoplastic thumbs. Plast Reconstr Surg. 2008; 122:1807–11.

139. Christen T, Dautel G. Type II and IIIA thumb hypoplasia reconstruction. J Hand Surg Am. 2013; 38:2009–15.

140. Abdel-Ghani H, Amro S. Characteristics of patients with hypoplastic thumb: a prospective study of 51 patients with the results of surgical treatment. J Pediatr Orthop B. 2004; 13:127–38.

141. Buck-Gramcko D. Pollicization of the index finger. Method and results in aplasia and hypoplasia of the thumb. J Bone Joint Surg Am. 1971; 53:1605–17.
142. Foucher G, Medina J, Lorea P, Pivato G. Principalization of pollicization of the index finger in congenital absence of the thumb. Tech Hand Up Extrem Surg. 2005; 9:96–104.

143. Kozin SH, Weiss AA, Webber JB, Betz RR, Clancy M, Steel HH. Index finger pollicization for congenital aplasia or hypoplasia of the thumb. J Hand Surg Am. 1992; 17:880–4.

144. Ozols D, Butnere MM, Petersons A. Methods for congenital thumb hypoplasia reconstruction. A review of the outcomes for ten years of surgical treatment. Medicina (Kaunas). 2019; 55:610.

145. Schneider W, Reichert B, Pallua N, Meyer H. Correction of hypoplastic thumb by free transfer of metatarsal bone: a case report. Microsurgery. 1993; 14:468–71.

146. Chow CS, Ho PC, Tse WL, Hung LK. Reconstruction of hypoplastic thumb using hemi-longitudinal metatarsal transfer. J Hand Surg Eur Vol. 2012; 37:738–44.

147. Zhong W, Tian W, Zhao J, et al. Nonvascularized iliac crest bone graft for reconstruction of the first metacarpal in type IIIB thumb hypoplasia: a radiographic follow-up study. J Hand Surg Am. 2023; 48:196.

148. O'Brien BM, Black MJ, Morrison WA, MacLeod AM. Microvascular great toe transfer for congenital absence of the thumb. Hand. 1978; 10:113–24.
149. Ozols D, Zarins J, Petersons A. Novel technique for toe-to-hand transplantation: the fourth-toe as an alternative option for toe-to-hand transplantation for pediatric patients. Tech Hand Up Extrem Surg. 2019; 23:74–80.

150. Foucher G, Medina J, Navarro R. Microsurgical reconstruction of the hypoplastic thumb, type IIIB. J Reconstr Microsurg. 2001; 17:9–15.

151. Tu YK, Yeh WL, Sananpanich K, et al. Microsurgical second toe-metatarsal bone transfer for reconstructing congenital radial deficiency with hypoplastic thumb. J Reconstr Microsurg. 2004; 20:215–25.

Fig. 1.
Signaling centers and coordinate axes of the limb bud. Pr, proximal; Di, distal; Rad, radial; Uln, ulnar; Do, dorsal; Vo, volar; AER, apical ectodermal ridge; ZPA, zone of polarizing activity; PZ, progress zone. Modified from Oberg et al. [6] with permission of Georg Thieme Verlag KG.
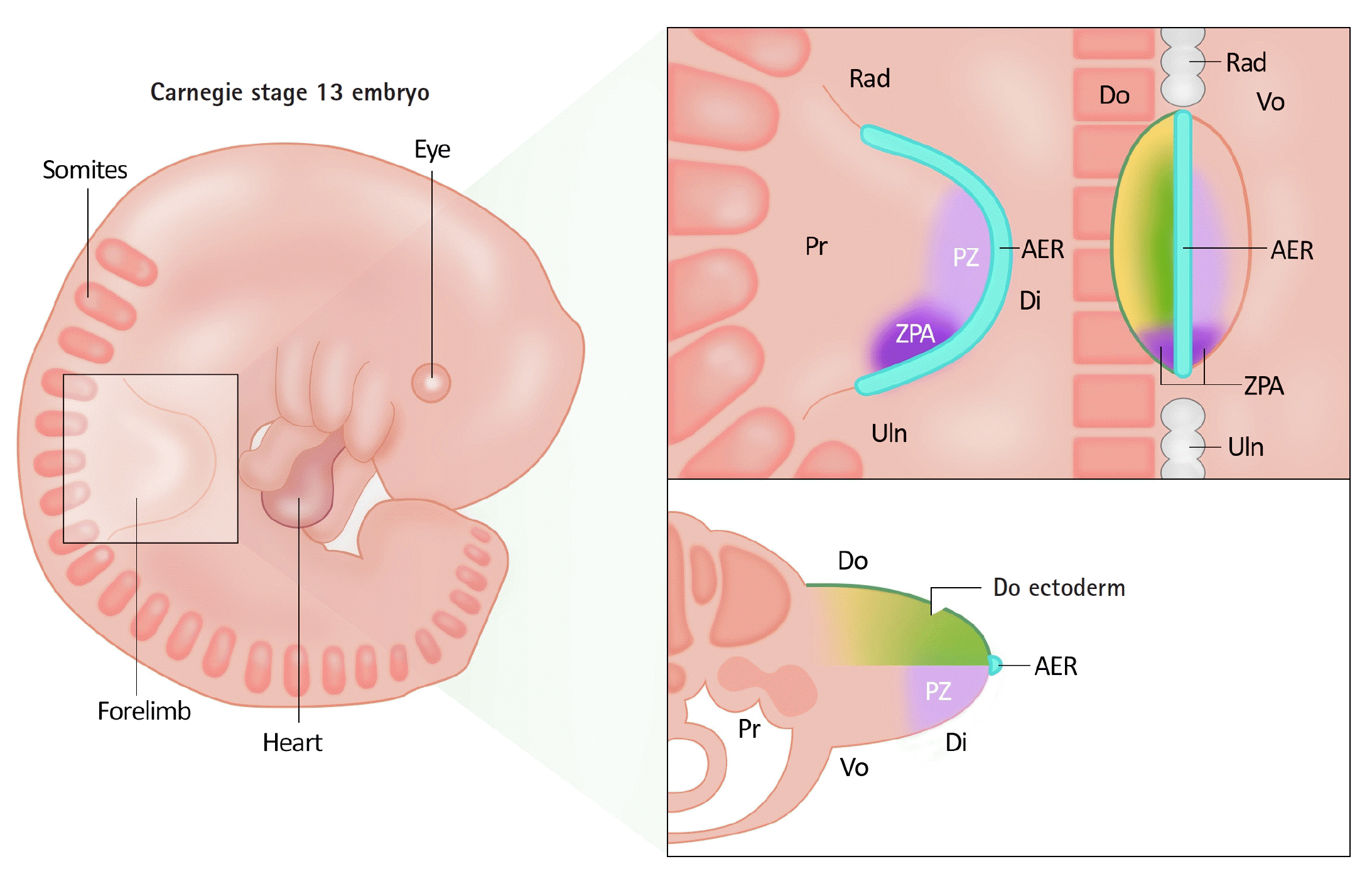
Fig. 2.
Mechanism of determining the number of digits. Modified from Oberg et al. [16] with permission of International Federation of Societies for Surgery of the Hand.
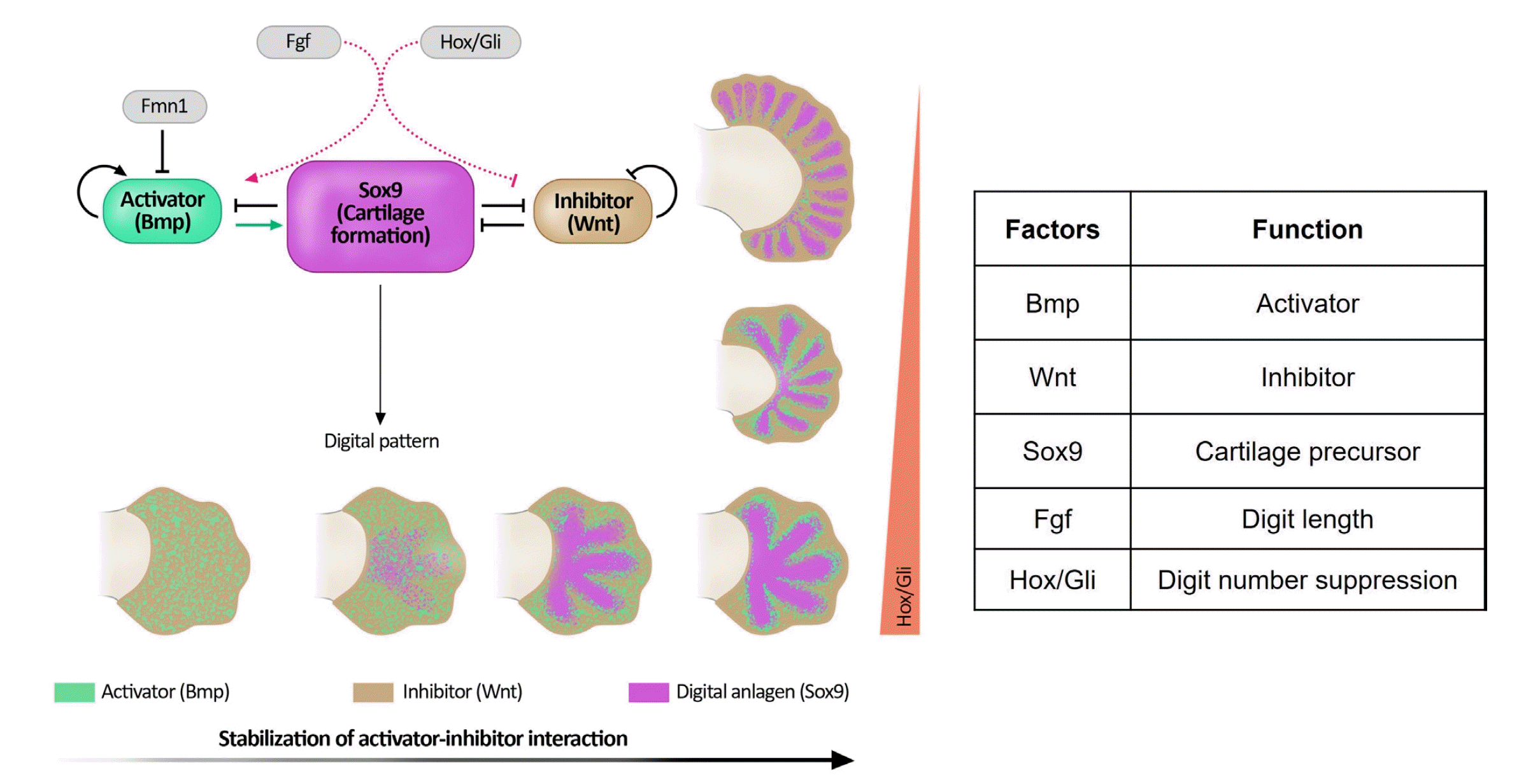
Fig. 4.
The left hand shows postaxial polydactyly type B. The light hand and both feet show postaxial polydactyly type A. Postaxial polydactyly often affects both hands and feet and is associated with an autosomal dominant genetic inheritance. (Sourced from the Department of Plastic and Reconstructive Surgery at Seoul National University Hospital, with informed consent from the legal guardians of the patients.)
Fig. 5.
Preoperative X-ray and clinical photograph of a case of central polydactyly. (A) X-ray and clinical photo after several operations. (B) Despite multiple surgeries, many patients still experience issues, such as finger deformities and joint stiffness. (Sourced from the Department of Plastic and Reconstructive Surgery at Seoul National University Hospital, with informed consent from the legal guardians of the patients.)
Fig. 6.
Simple incomplete-type syndactyly (A) and complex complete-type syndactyly (B, C). (Sourced from the Department of Plastic and Reconstructive Surgery at Seoul National University Hospital, with informed consent from the legal guardians of the patients.)
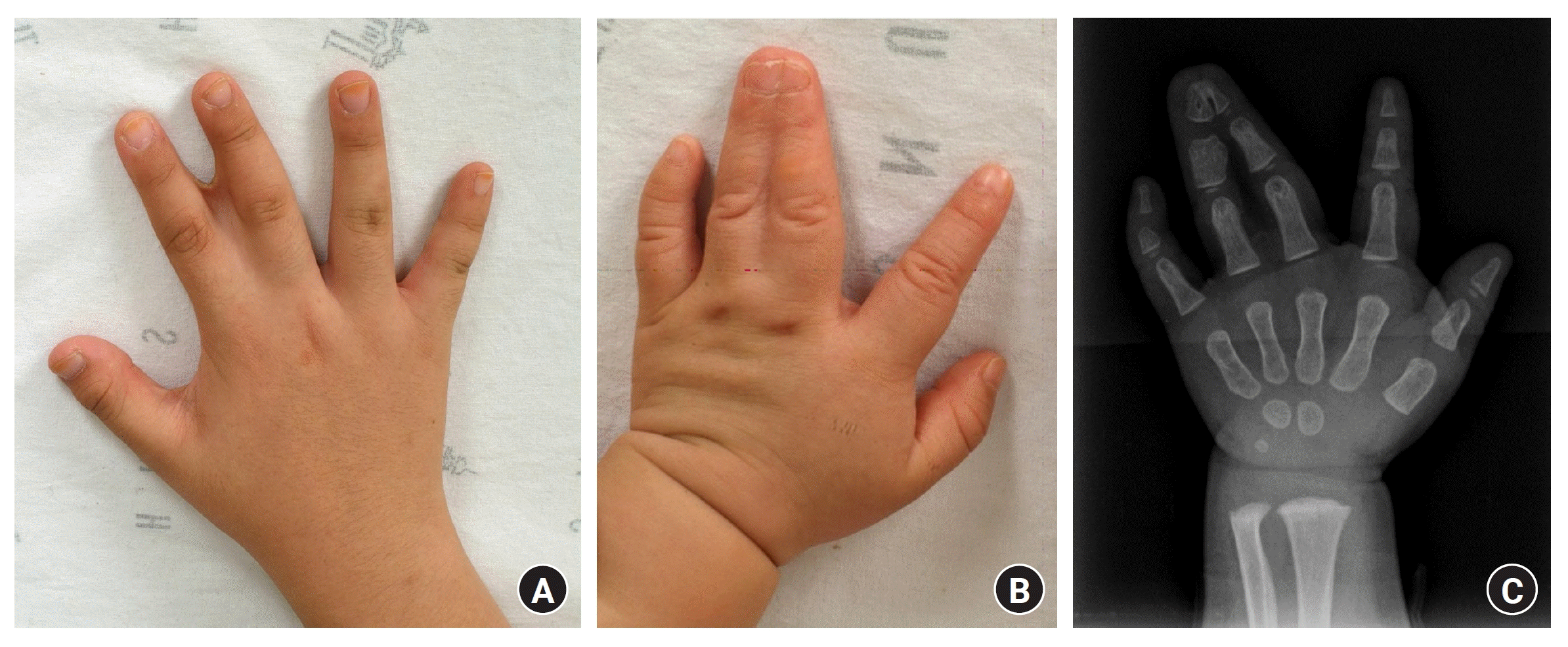
Fig. 7.
Clinical photographs and X-rays of syndactyly in Apert syndrome. (A) Upton type I, (B) type II, and (C) type III. (Sourced from the Department of Plastic and Reconstructive Surgery at Seoul National University Hospital, with informed consent from the legal guardians of the patients.)
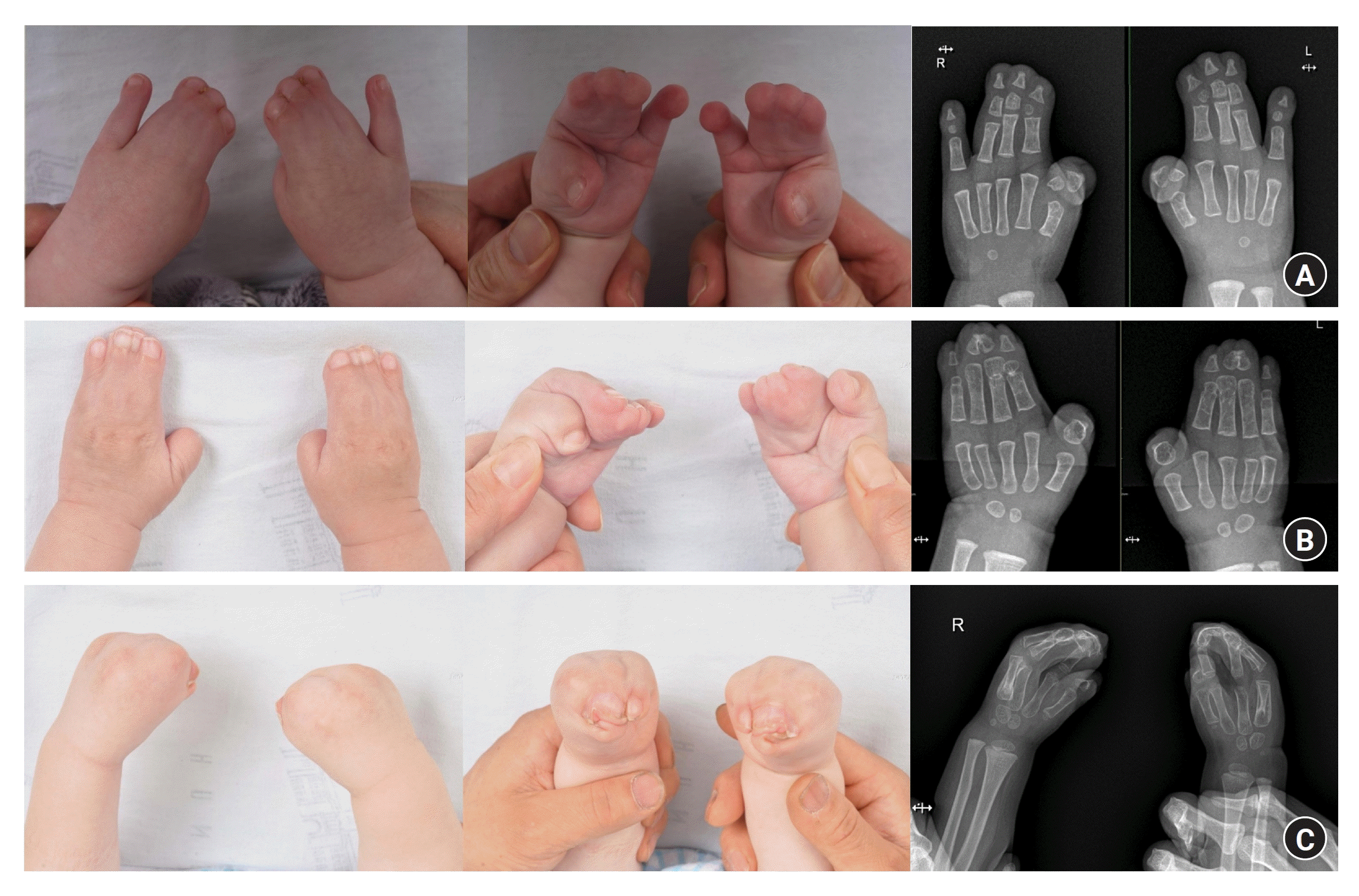
Fig. 8.
Results of epiphysiodesis in left index finger macrodactyly. (A) A preoperative hand X-ray. (B) A hand X-ray taken at postoperative 7 years. Note that longitudinal growth is well controlled, but circumferential growth is not controlled. (Sourced from the Department of Plastic and Reconstructive Surgery at Seoul National University Hospital, with informed consent from the legal guardians of the patients.)
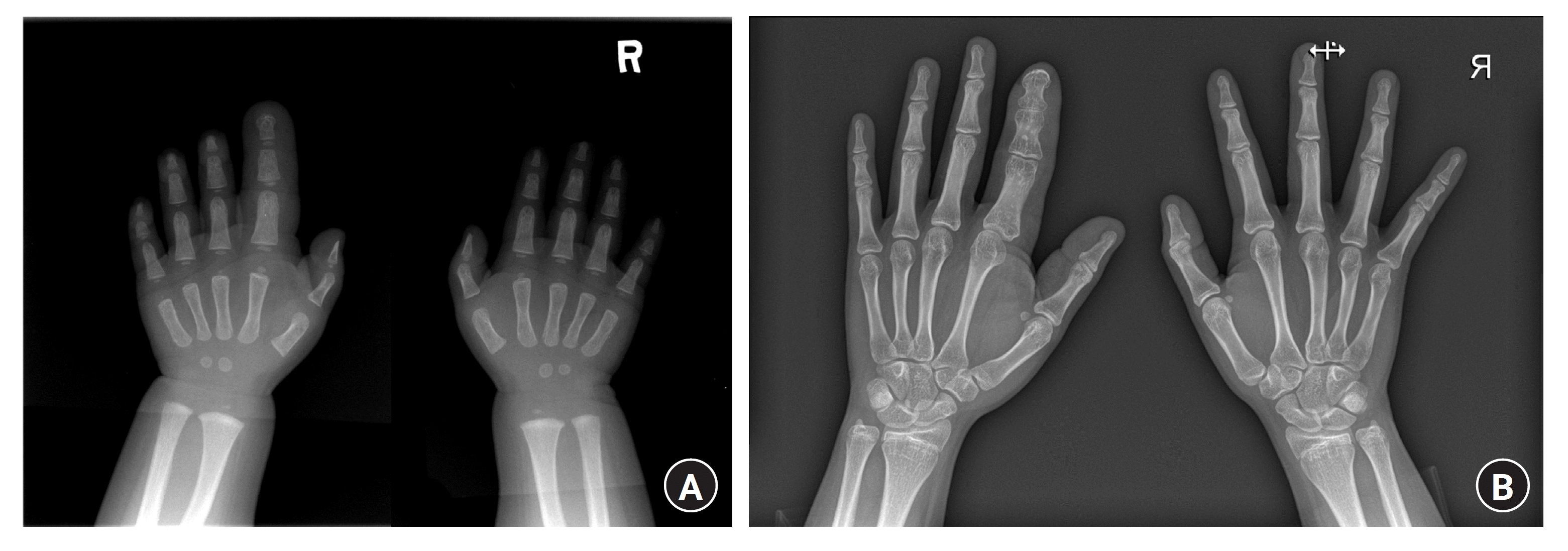
Fig. 9.
A patient with constriction band syndrome and syndactyly. (A) Preoperative clinical photograph. (B) The results after Z- and W-plasty, and syndactyly division with a skin graft. (Sourced from the Department of Plastic and Reconstructive Surgery at Seoul National University Hospital, with informed consent from the legal guardians of the patients.)
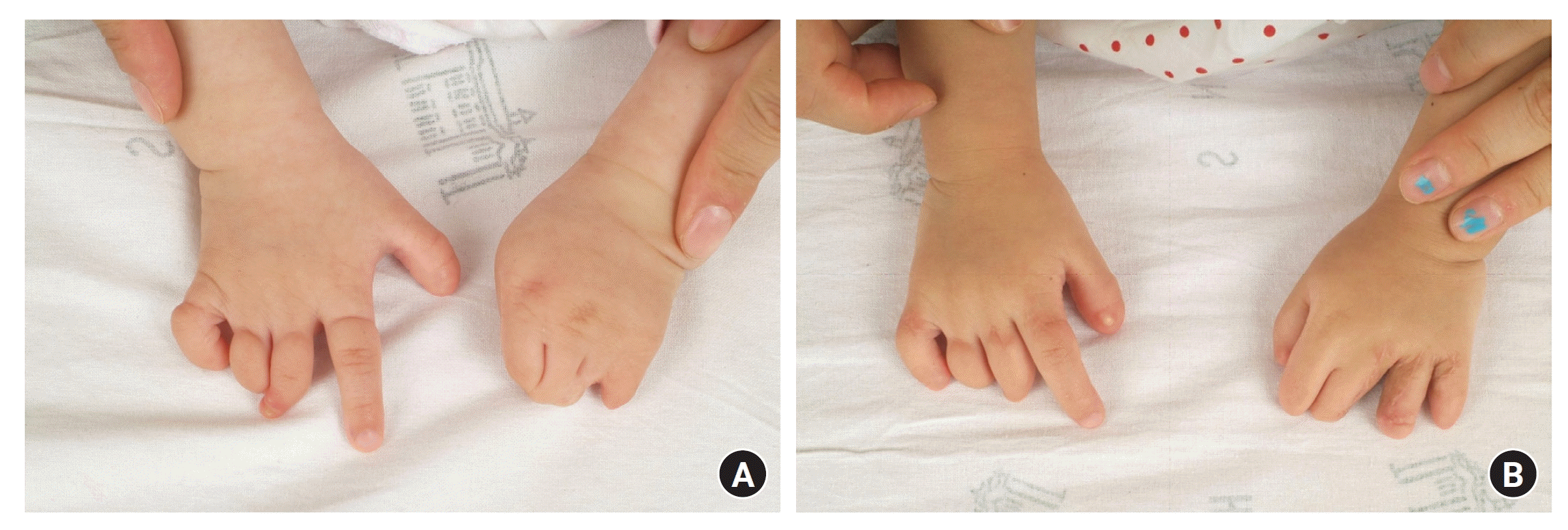
Fig. 10.
After the pollicization, the structures in the original index finger that correspond to the structures of the new thumb are described in Fig. 10 and Table 7. DP, distal phalanx; DIPJ, distal interphalangeal joint; IPJ, interphalangeal joint; MP, middle phalanx; PP, proximal phalanx; PIPJ, proximal interphalangeal joint; MPJ, metacarpophalangeal joint; M, metacarpal bone; CMCJ, carpometacarpal joint; Trapez, trapezium; PI, palmal interossei; AddP, adductor pollicis; DI, dorsal interossei; AbPB, abductor pollicis brevis; EIP, extensor indicis proprius; EPL, extensor pollicis longus; EDC, extensor digitorum communis; AbPL, abductor pollicis longus. Modified from Chang et al. [2] with permission of Elsevier.
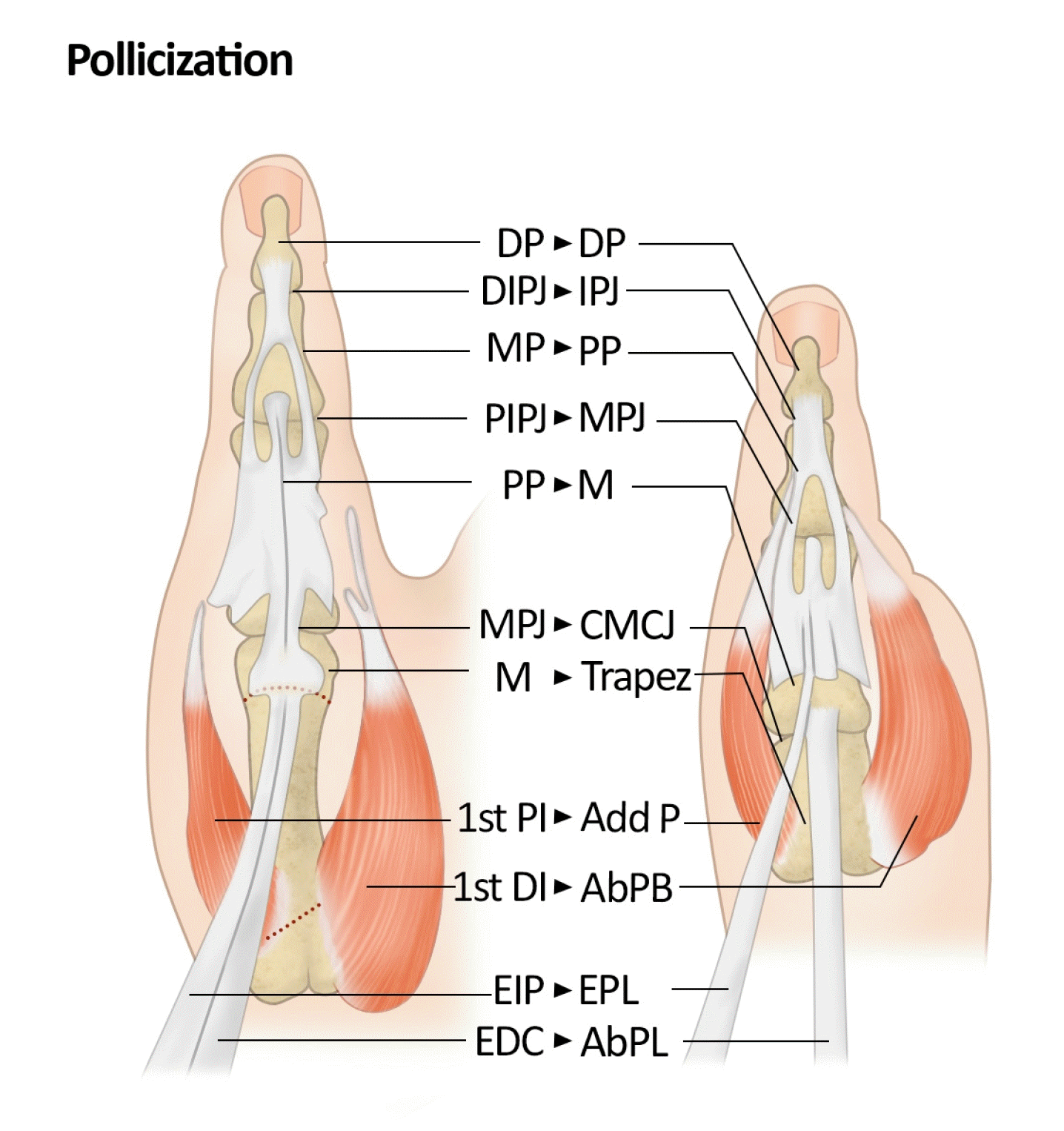
Table 1.
Development of the upper limb
Table 2.
Signal centers, associated proteins, and function of the limb bud
Table 3.
Swanson/IFSSH’s classification of congenital upper limb anomalies (1983)
Table 4.
The classification of congenital upper limb anomalies by Oberg, Manske, and Tonkin (OMT classification)
Table 5.
Upton classification for syndactyly in Apert syndrome
Table 6.
Classification of thumb hypoplasia and its treatment
Table 7.
Pollicization




 PDF
PDF Citation
Citation Print
Print



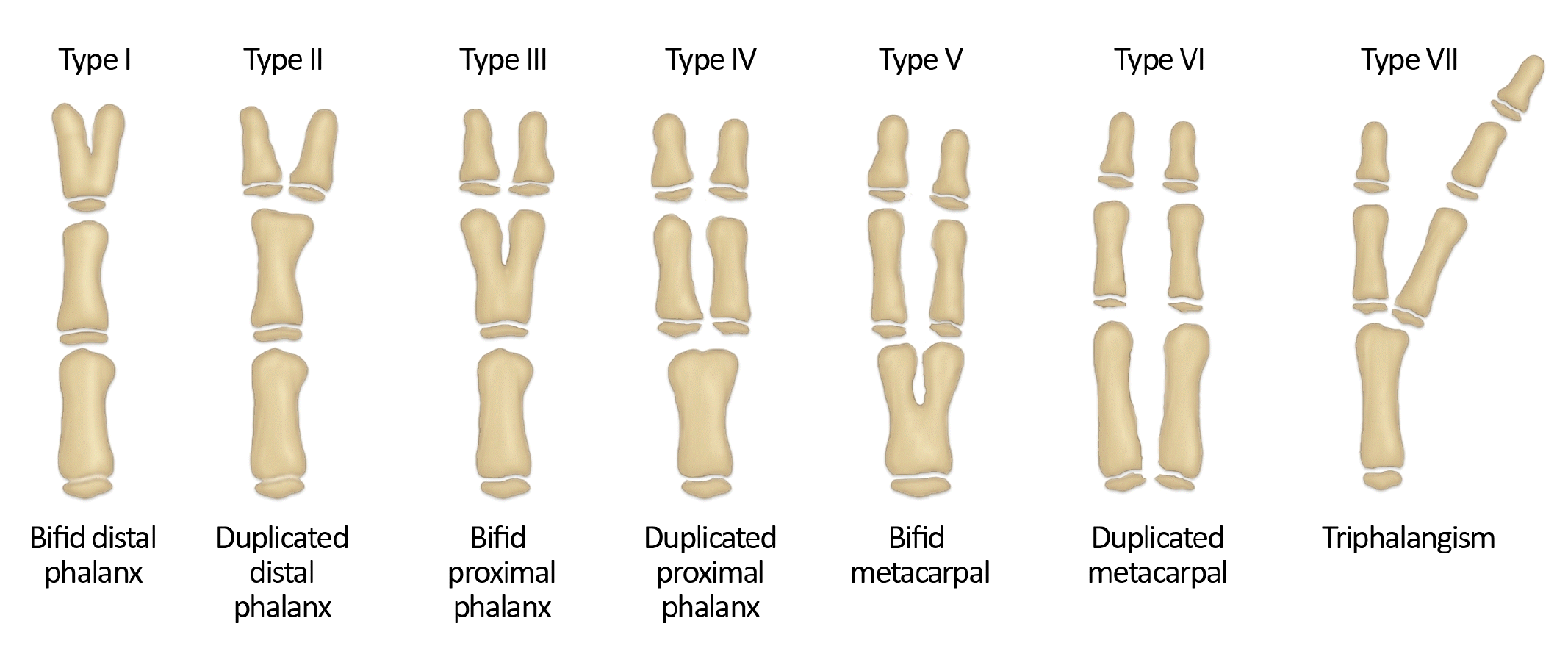
 XML Download
XML Download