1. Darcey J, Roudsari RV, Jawad S, Taylor C, Hunter M. Modern endodontic principles. Part 5: Obturation. Dent Update. 2016; 43:114–116. 119–120. 123–126 passim. PMID:
27188127.
2. Hammad M, Qualtrough A, Silikas N. Evaluation of root canal obturation: a three-dimensional
in vitro study. J Endod. 2009; 35:541–544. PMID:
19345801.
3. Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M. Physical properties of 5 root canal sealers. J Endod. 2013; 39:1281–1286. PMID:
24041392.
4. Gasner NS, Brizuela M. Endodontic materials used to fill root canals. Treasure Island, FL: StatPearls;2023.
5. Huang FM, Tai KW, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002; 35:153–158. PMID:
11843970.
6. Viapiana R, Baluci CA, Tanomaru-Filho M, Camilleri J. Investigation of chemical changes in sealers during application of the warm vertical compaction technique. Int Endod J. 2015; 48:16–27. PMID:
24697552.
7. Raghavendra SS, Jadhav GR, Gathani KM, Kotadia P. Bioceramics in endodontics - a review. J Istanb Univ Fac Dent. 2017; 51(Supplement 1):S128–S137. PMID:
29354316.
8. Donnermeyer D, Bunne C, Schäfer E, Dammaschke T. Retreatability of three calcium silicate-containing sealers and one epoxy resin-based root canal sealer with four different root canal instruments. Clin Oral Investig. 2018; 22:811–817.
9. Zaki DY, Zaazou MH, Khallaf ME, Hamdy TM.
In vivo comparative evaluation of periapical healing in response to a calcium silicate and calcium hydroxide based endodontic sealers. Open Access Maced J Med Sci. 2018; 6:1475–1479. PMID:
30159080.
10. Mohammadi Z, Shalavi S. Clinical applications of glass ionomers in endodontics: a review. Int Dent J. 2012; 62:244–250. PMID:
23106837.
13. Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. 2015; 31:609–614. PMID:
25975276.
14. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1. PMID:
25554246.
15. Rifi R, Matar M, Ghazi M, Abboud C, El Masri J, Al Majdalany D, et al. Current state of clinical trials regarding lung transplant rejection. Transpl Immunol. 2022; 74:101668. PMID:
35842078.
16. El Masri J, El Ayoubi LM, Zreika B, Adhami F, El Masri D, El Hage S, et al. Current state of clinical trials regarding liver transplant rejection. Transpl Immunol. 2022; 70:101522. PMID:
34954324.
17. El Masri J, Afyouni A, Ghazi M, Baroud T, Al Majdalany D, Saleh A, et al. Current state of clinical trials on xenograft. Xenotransplantation. 2023; 30:e12801. PMID:
37144505.
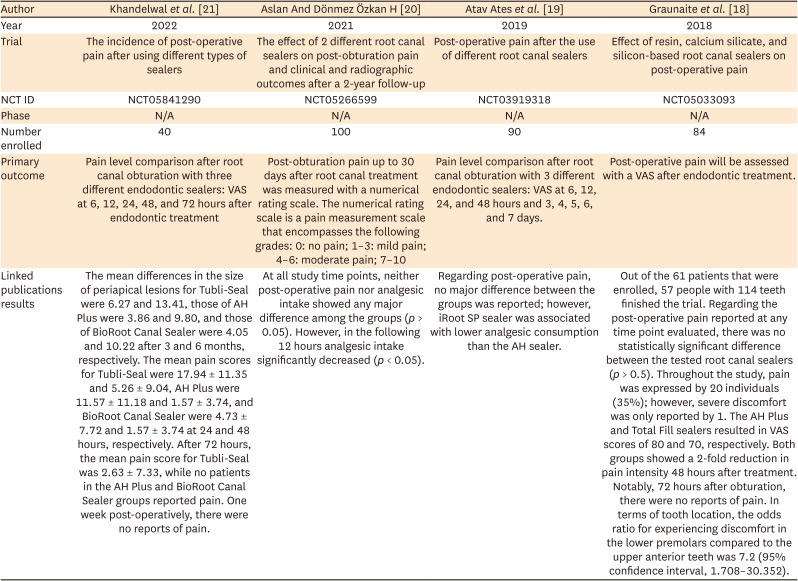
18. Graunaite I, Skucaite N, Lodiene G, Agentiene I, Machiulskiene V. Effect of resin-based and bioceramic root canal sealers on postoperative pain: a split-mouth randomized controlled trial. J Endod. 2018; 44:689–693. PMID:
29571915.
19. Atav Ates A, Dumani A, Yoldas O, Unal I. Post-obturation pain following the use of carrier-based system with AH Plus or iRoot SP sealers: a randomized controlled clinical trial. Clin Oral Investig. 2019; 23:3053–3061.
20. Aslan T, Dönmez Özkan H. The effect of two calcium silicate-based and one epoxy resin-based root canal sealer on postoperative pain: a randomized controlled trial. Int Endod J. 2021; 54:190–197. PMID:
32929721.
21. Khandelwal A, Jose J, Teja KV, Palanivelu A. Comparative evaluation of postoperative pain and periapical healing after root canal treatment using three different base endodontic sealers - a randomized control clinical trial. J Clin Exp Dent. 2022; 14:e144–e152. PMID:
35173897.
22. León-López M, Cabanillas-Balsera D, Martín-González J, Montero-Miralles P, Saúco-Márquez JJ, Segura-Egea JJ. Prevalence of root canal treatment worldwide: a systematic review and meta-analysis. Int Endod J. 2022; 55:1105–1127. PMID:
36016509.
23. El-Hagrassy MM, Duarte DGG, Thibaut A, Lucena MFG, Fregni F. Principles of designing a clinical trial: optimizing chances of trial success. Curr Behav Neurosci Rep. 2018; 5:143–152. PMID:
30467533.
24. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015; 372:1031–1039. PMID:
25760355.
25. Jones CW, Handler L, Crowell KE, Keil LG, Weaver MA, Platts-Mills TF. Non-publication of large randomized clinical trials: cross sectional analysis. BMJ. 2013; 347:f6104. PMID:
24169943.
26. Vanderbeek AM, Rahman R, Fell G, Ventz S, Chen T, Redd R, et al. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro-oncol. 2018; 20:1034–1043. PMID:
29518210.
27. Silveira CM, Pinto SC, Zedebski RA, Santos FA, Pilatti GL. Biocompatibility of four root canal sealers: a histopathological evaluation in rat subcutaneous connective tissue. Braz Dent J. 2011; 22:21–27. PMID:
21519643.
28. Mergoni G, Ganim M, Lodi G, Figini L, Gagliani M, Manfredi M. Single versus multiple visits for endodontic treatment of permanent teeth. Cochrane Database Syst Rev. 2022; 12:CD005296. PMID:
36512807.
29. Tibúrcio-Machado CS, Michelon C, Zanatta FB, Gomes MS, Marin JA, Bier CA. The global prevalence of apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2021; 54:712–735. PMID:
33378579.
30. Aminoshariae A, Kulild JC, Fouad AF. The impact of endodontic infections on the pathogenesis of cardiovascular disease(s): a systematic review with meta-analysis using grade. J Endod. 2018; 44:1361–1366.e3. PMID:
30078571.
31. Rosen J, Sancheti P, Fierlinger A, Niazi F, Johal H, Bedi A. Response to: Important considerations when determining the cost-effectiveness of viscosupplements in the treatment of knee osteoarthritis. Adv Ther. 2017; 33:2273–2276. PMID:
27778299.
32. Jakovljevic A, Sljivancanin Jakovljevic T, Duncan HF, Nagendrababu V, Jacimovic J, Aminoshariae A, et al. The association between apical periodontitis and adverse pregnancy outcomes: a systematic review. Int Endod J. 2021; 54:1527–1537. PMID:
33908039.
33. Niazi SA, Bakhsh A. Association between endodontic infection, its treatment and systemic health: a narrative review. Medicina (Kaunas). 2022; 58:931. PMID:
35888650.
34. Dos Santos Costa FM, Fernandes MH, Batistuzzo de Medeiros SR. Genotoxicity of root canal sealers: a literature review. Clin Oral Investig. 2020; 24:3347–3362.
35. Parirokh M, Zarifian A, Ghoddusi J. Choice of treatment plan based on root canal therapy versus extraction and implant placement: a mini review. Iran Endod J. 2015; 10:152–155. PMID:
26213535.
36. Hamasha AA, Hatiwsh A. Quality of life and satisfaction of patients after nonsurgical primary root canal treatment provided by undergraduate students, graduate students and endodontic specialists. Int Endod J. 2013; 46:1131–1139. PMID:
23560436.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download