I. Introduction
II. Materials and Methods
1. Sialolith, tonsillolith, and antrolith specimen collection
2. Specimen selection and grouping
Table 1
3. Micro-computed tomography (micro-CT) analysis
4. Histopathological analysis
5. Scanning electron microscopy (SEM) analysis
6. Energy dispersive X-ray spectroscopy (EDS) analysis
7. Transmission electron microscopy (TEM) analysis
8. Statistical analysis
III. Results
1. Patient characteristic and demographic data
2. Sialolith, tonsillolith, and antrolith specimen data
3. Micro-CT analysis sialolith, tonsillolith, and antrolith
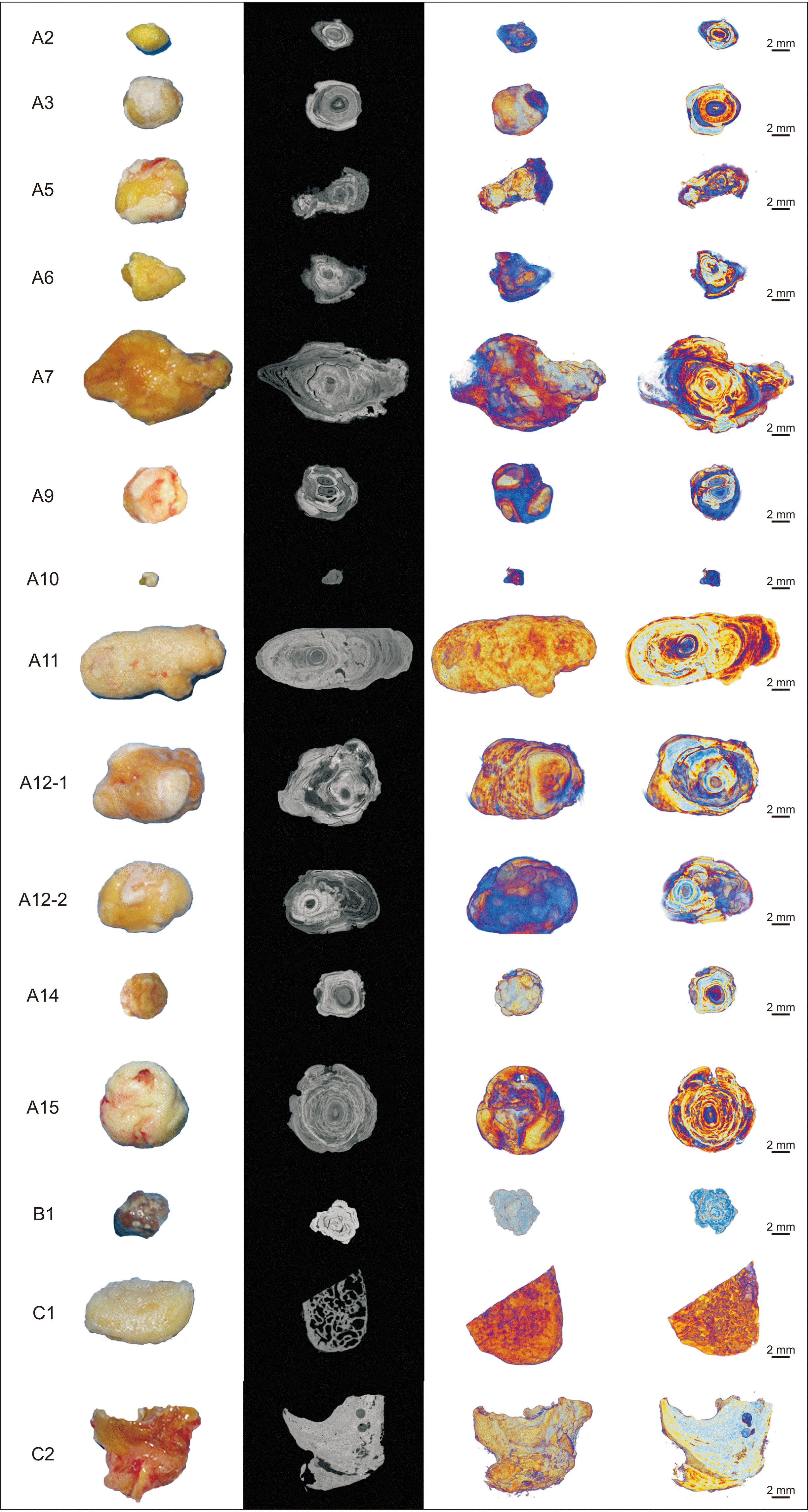 | Fig. 1Representative clinical, two-dimensional micro-computed tomography cross section and three-dimensional reconstructed images of specimen A2, A3, A5, A6, A7, A9, A10, A11, A12-1, A12-2, A14, A15, B1, C1 and C2. Sialoliths show an onion-like concentric lamellar structure. Brighter regions represent higher mineralization and dark regions represent organic substance. Scale bars=2 mm. |
Table 2
(TV: total VOI [volume of interest] volume, Obj.V: object volume, Obj.V/TV: percent object volume, TS: total VOI surface, Obj.S: object surface, Obj.S/Obj.V: object surface/volume ratio, Obj.S/ TV: object surface density, St.Th: structure thickness, St.Sp: structure separation, Po(tot): total porosity)
4. Histopathological findings
1) Histological findings in group A
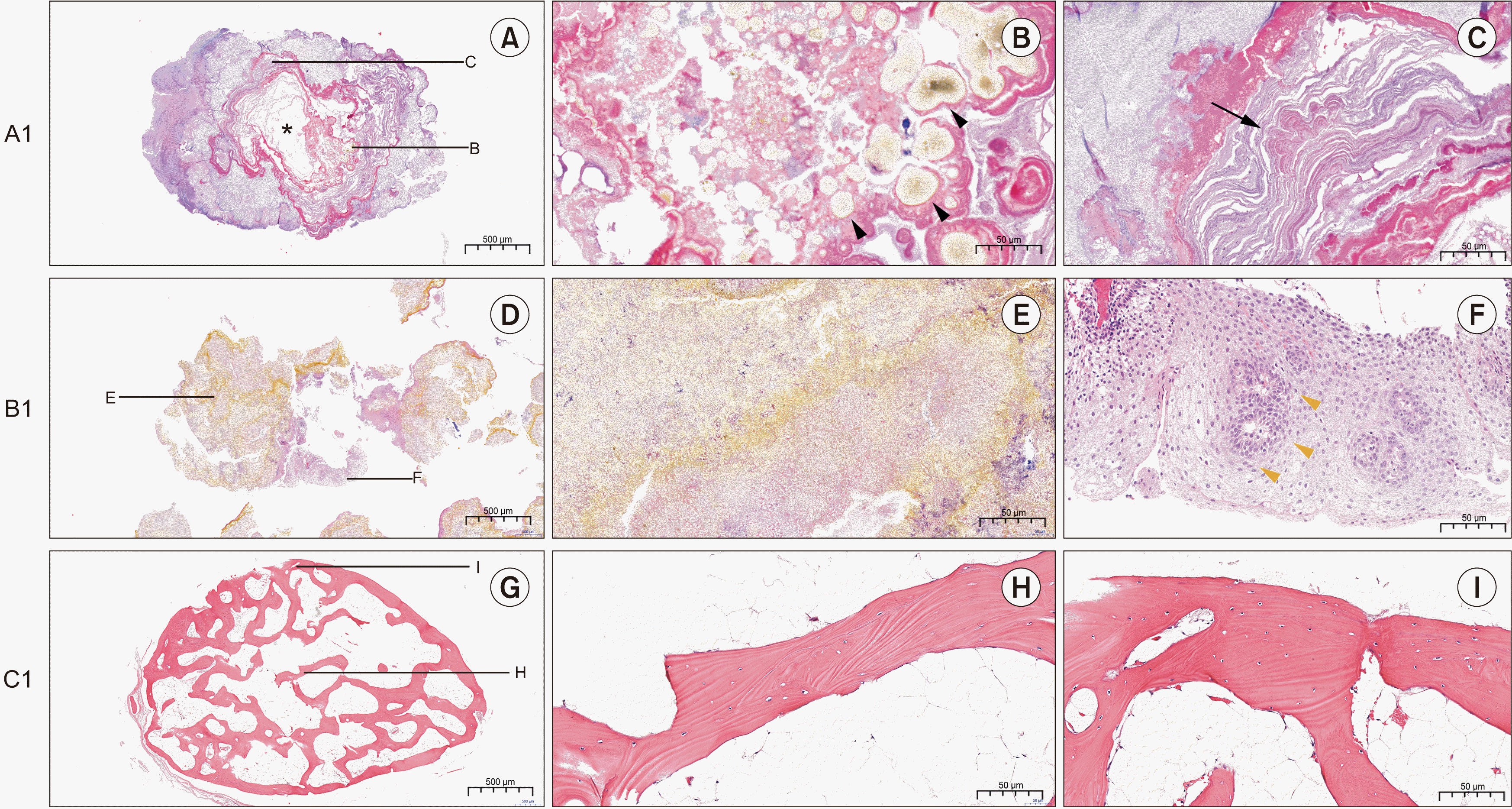 | Fig. 2Representative histological findings of specimen A1, B1, and C1. A. Sialolith A1 with a single organic core in which the core was lost during the histological slide preparation (H&E staining, 4×). Scale bar=500 μm. B. Globular lipid particles found near the core of the sialolith (black arrowheads), (H&E staining, 20×). Scale bar=50 μm. C. Mineralized nodules were found in the outer layers of the core showing irregular mineralization (black arrow) (H&E staining, 20×). Scale bar=50 μm. D. Tonsillolith specimen (B1) (H&E staining, 4×). Scale bar=500 μm. E. The overall specimen exhibits a crystalline structure of organic material at a mature stage of its development (H&E staining, 20×). Scale bar=50 μm. F. A duct-like structure was observed at the center of squamous epithelium indicating the minor salivary gland duct (yellow arrowheads) (H&E staining, 20×). Scale bar=50 μm. G. Antrolith specimen (C1) (H&E staining, 4×). Scale bar=500 μm. H. The lesion was not encapsulated and showed a homogeneous lamellar bone with fibrous marrow cavities (H&E staining, 4×). Scale bar=50 μm. I. The woven bone was replaced by lamellar bone with Haversian canals at the periphery (H&E staining, 4×). Scale bar=50 μm. |
2) Histological findings in group B
3) Histological findings in group C
5. SEM findings of sialolith, tonsillolith, and antrolith specimens
1) Ultrastructural findings of group A
2) Ultrastructural findings in group B
3) Ultrastructural findings in group C
6. Chemical composition of sialolith, tonsillolith, and antrolith specimens
Table 3
 | Fig. 4Representative mapping of elemental distribution and a spectrum of the representative points with energy dispersive X-ray spectroscopy (EDS) results in tonsillolith (B1). Scanning electron microscopy (SEM) image, 10,000× magnification. EDS analysis was carried out at five representative points of interest on the peripheral, middle, and core layers. |
Table 4
7. TEM analysis
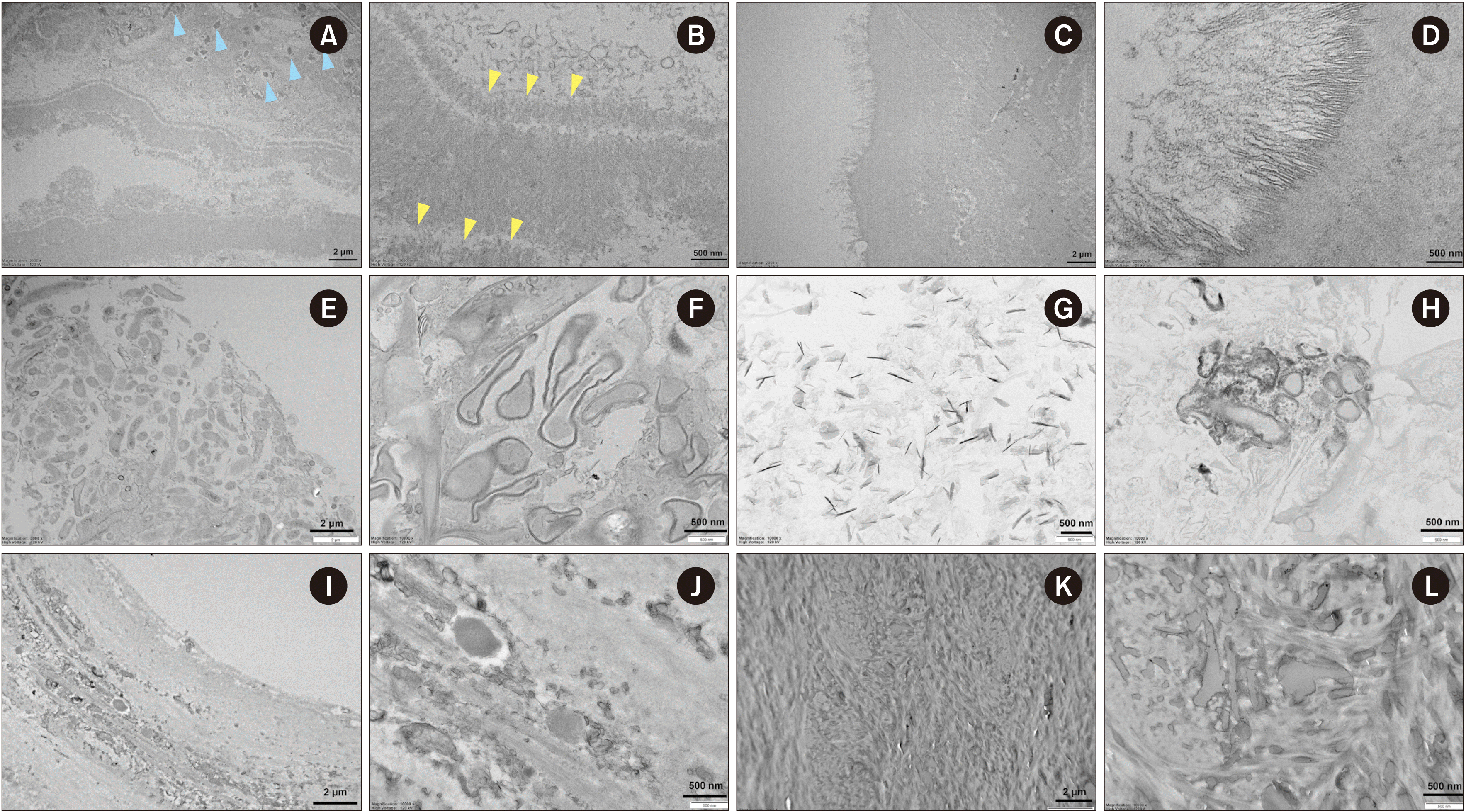 | Fig. 5Representative transmission electron microscopy (TEM) images of A4 sialolith (A-D), B1 tonsillolith (E-H), and C2 antrolth (I-L). Layered appearance of the sialolith showing the globular mineralized structure in internal lamella, (blue arrowheads), while the crystalline needle-like pattern was heterogeneous (yellow arrowheads), magnification 2,000×, 10,000× (A, B). Needle-like filamentary crystals, magnification 2,000×, 20,000× (C, D). Representative TEM images of B1 tonsillolith. The B1 tonsillolith had stratified squamous epithelium in its peripheral area, magnification 2,000×, 10,000× (E, F). Needle-like crystals in the core region and extra-vesicular deposition of inorganic material were also observed, magnification 10,000× (G, H). Representative TEM images of C2 antrolith. Outer layer showing osteoblastic rimming, magnification 2,000×, 10,000× (I, J). Dense, mature, predominantly lamellar bone in the middle area of the specimen, magnification 2,000×, 10,000× (K, L). |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download