Carcinoma cuniculatum (CC) usually affects the plantar aspect of the foot, but it has been reported at other sites as well, including the oesophagus, abdomen, palm, leg, penis, and cervix
2-9. Flieger and Owiński
10, who first described CC in the oral cavity in 1977, highlighted its aggressive behaviour and unique features. The incidence of OCC is very rare, with fewer than 60 cases ever reported
11, and it accounts for 2.7% of all oral squamous carcinomas
12. Its etiology remains unclear, although trauma, tobacco use, alcohol consumption, chronic inflammation, and human papilloma virus (HPV) have been implicated as potential risk factors
11,13,14. The most common location of OCC is the alveolar mucosa and gingiva, followed by the tongue, hard palate, mandible, and buccal mucosa
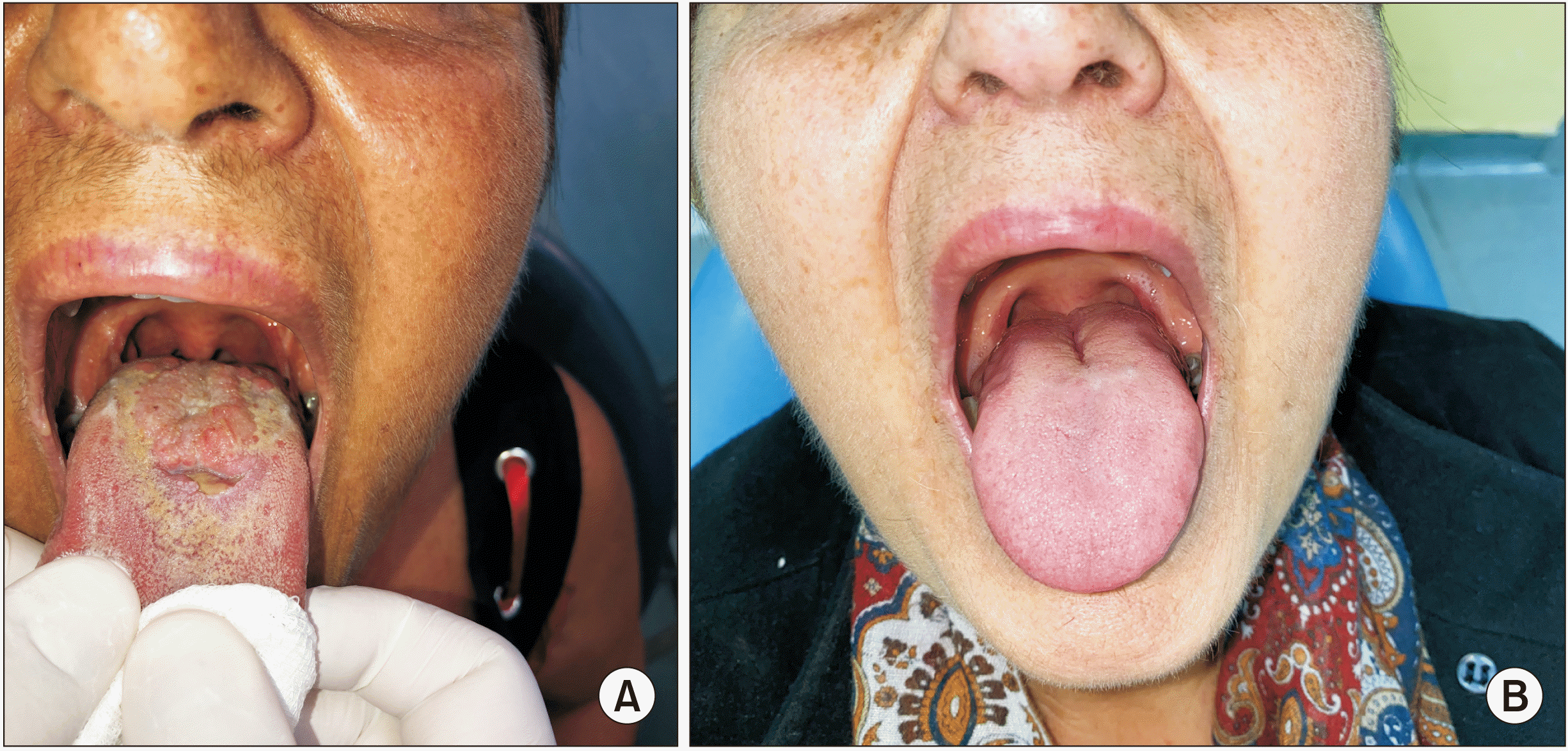
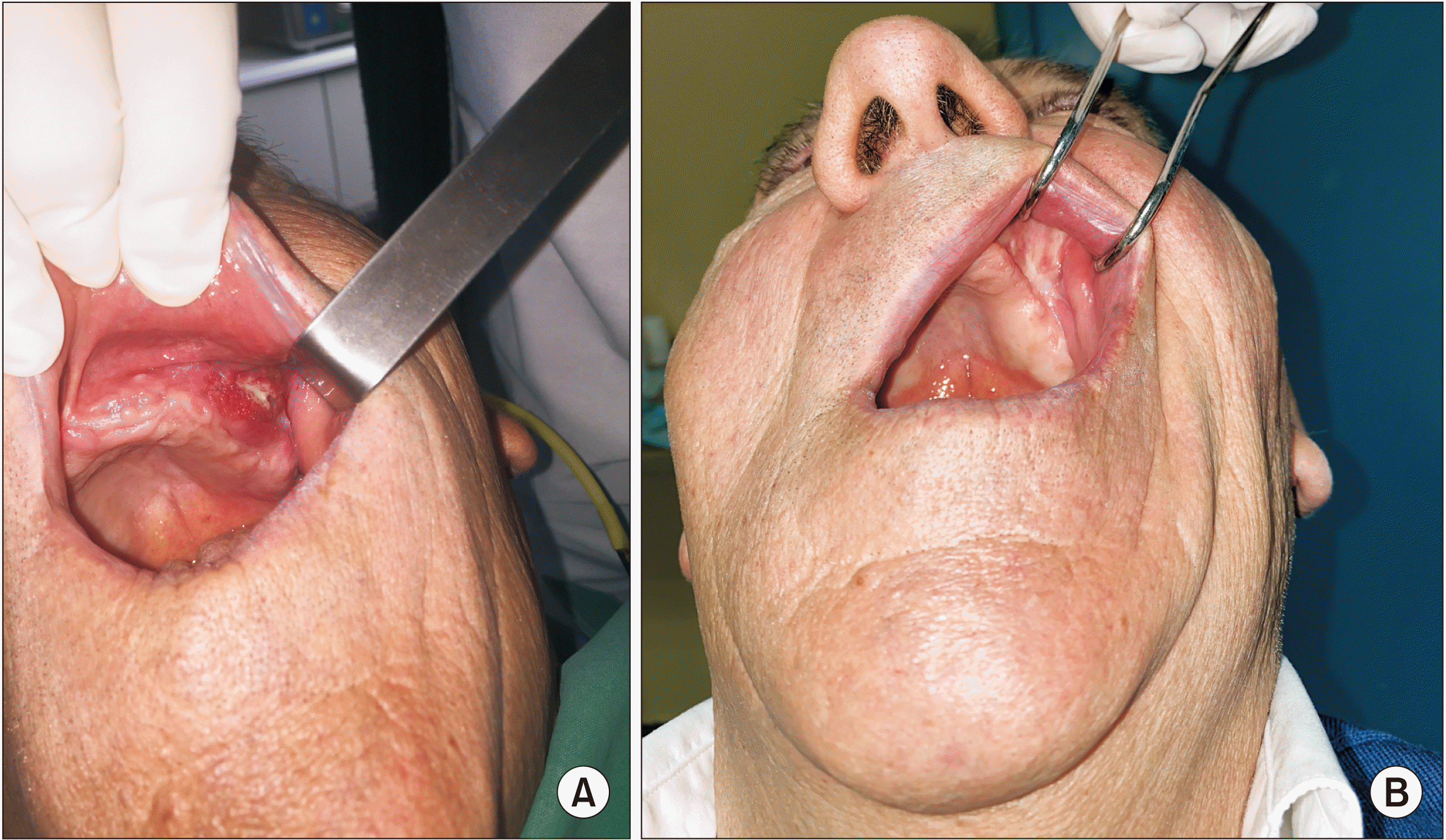
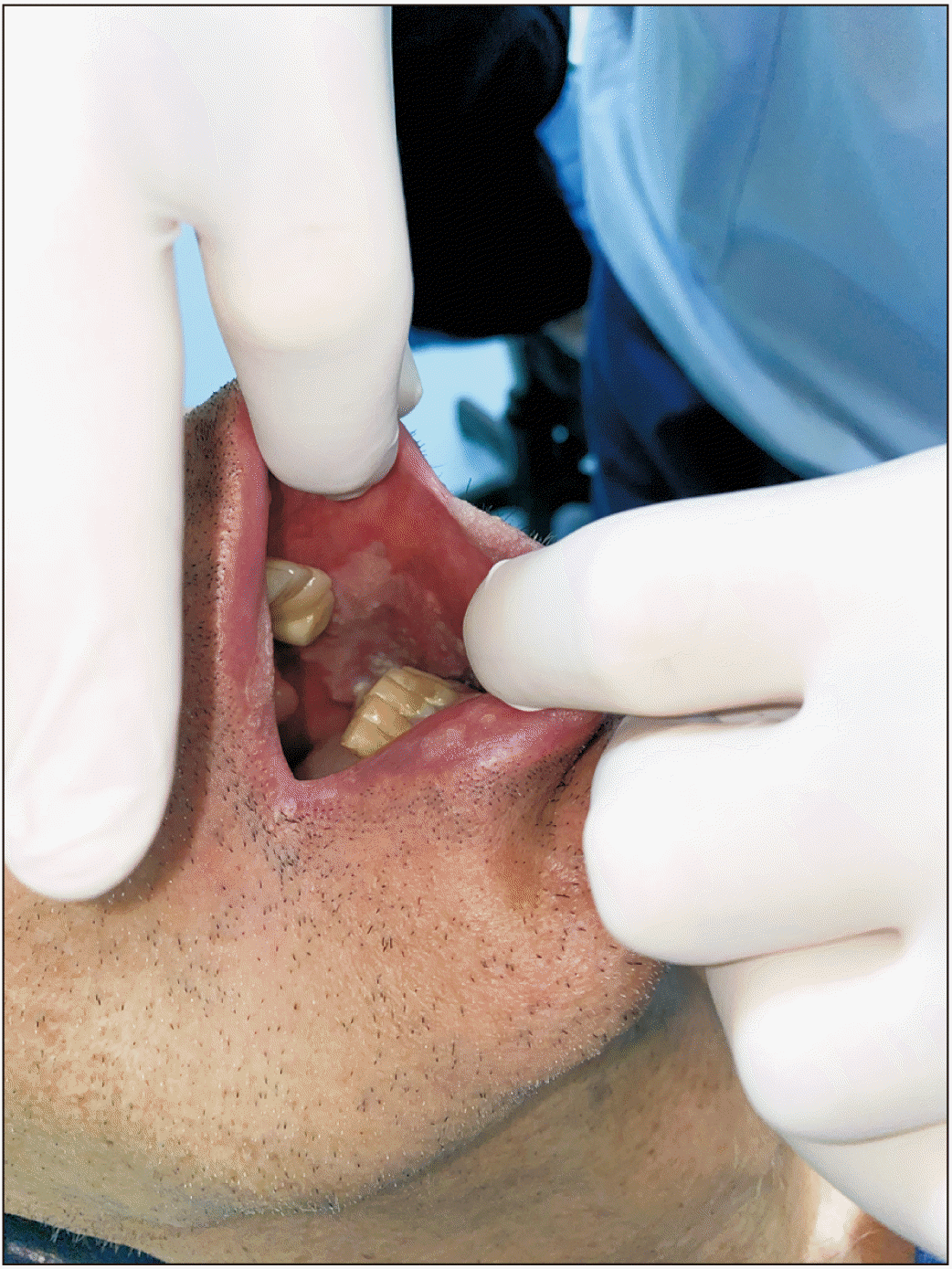
11. It can present with a variety of clinical manifestations such as exophytic induration, verrucous or cauliflower mass, erythroleukoplakia, or as a non-healing ulcer with or without bone exposure
11. Histologically, OCC is characterized by well-differentiated epithelial cells that are organized into ramified sinuses and keratin-filled crypts that resemble rabbit burrows (cuniculatum in Latin) with a notable lack of cytological atypia, unlike SCC. This may be the reason it is often not identified as a malignancy, resulting in treatment delays
15. Most clinicians are unaware of its distinct existence, which makes its diagnosis challenging and potentially missed
16. In this article we present a series of four OCC cases that was referred to our department. Our goal was to demonstrate its unique features and discuss its diagnosis and management.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download