Introduction
Despite continuous advances in pharmacotherapy and myocardial revascularization, myocardial infarction (MI) remains still challenging because of post-infarct myocardial remodeling, a non-contractile fibrotic scar which reduces ventricular contractile (
1). Adipose-derived stem cells (ASCs) could differentiate into endothelial cells and participate in vessel formation (
2). ASCs secrete cytokines and growth factors including vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) (
3). We and others have shown that intramyocardial transplantation of ASCs can augment angiogenesis, improve cardiac function, and reduce infarct size (
4,
5). However, ASC therapy has limitations due to the poor survival and insufficient homing efficacy of implanted cells within ischemic myocardium (
6,
7).
Integrins are transmembrane receptors that bridges for cell-extracellular matrix (ECM) interaction. When activated, integrins transduce chemical signal to the interior, stimulates chemical composition and mechanical behaviors of ECM, and regulates cell functions including proliferation, migration, and cytokine secretion (
8). Vascular cell adhesion molecule-1 (VCAM-1) is substantially up-regulated in endothelial cells under hypoxic conditions and interacts specifically with the binding partner of integrin α
4 on the surface of stem cells (
9,
10). VCAM-1/integrin α
4 interaction builds a biological bonding between stem cells and ischemic myocardium and facilitates transplanted cells to attach, migrate and engraft. Enhanced cell retention is essential for long-term survival and engraftment, and can translate into augmented therapeutic benefits.
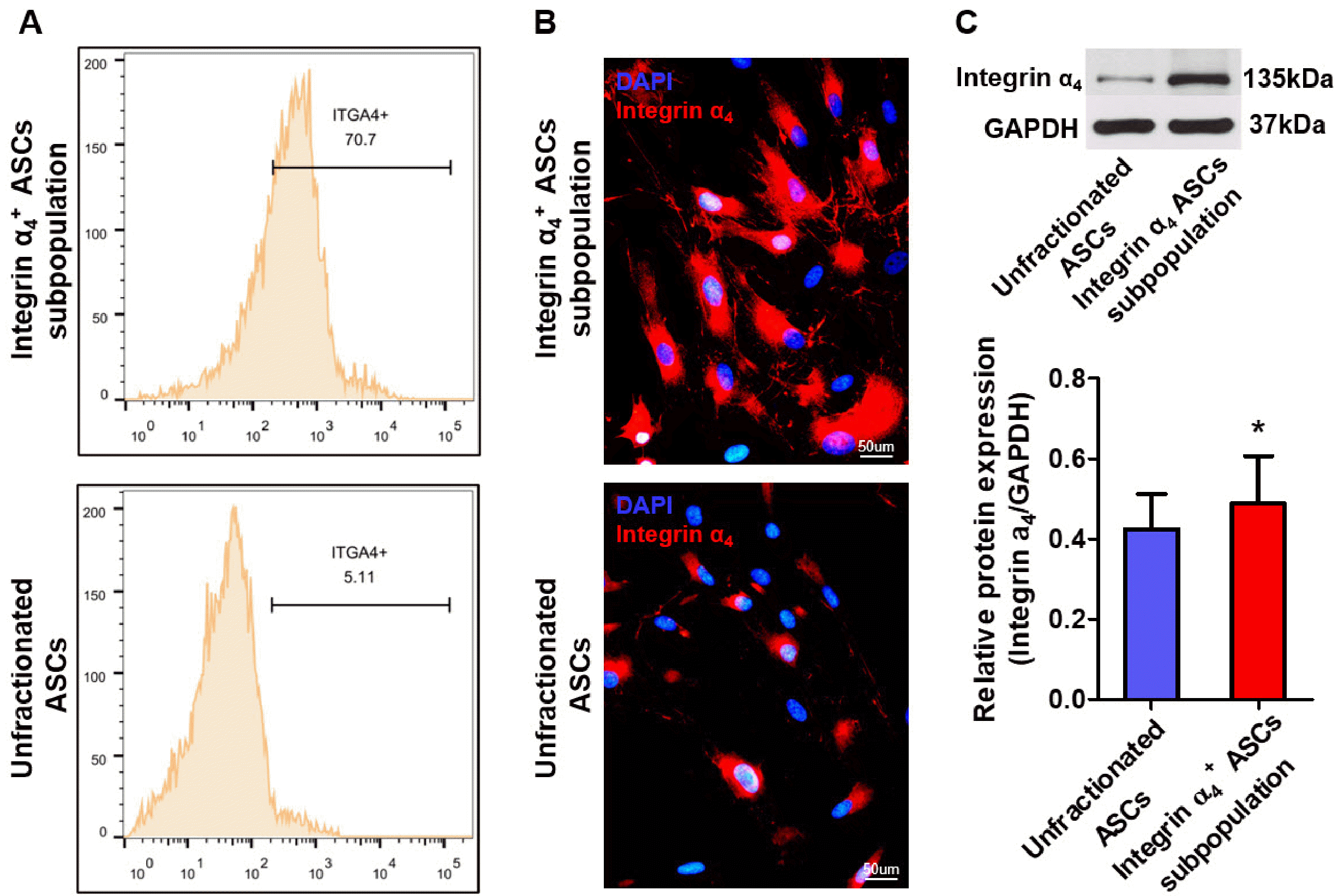
ASCs typically express insufficient integrin α4, and the percentage of integrin α4+ cells were approximately 5.11%, which limits their homing and engraftment after trans-plantation. Thus, in vitro cultured ASCs were sort-purified using fluorescence-activated cell sorting (FACS) to harvest integrin α4+ subpopulation. We examined whether integrin α4 enhanced the migration, cytokine secretion, and anti-apoptotic properties of ASCs in vitro. Moreover, we implanted a subpopulation of integrin α4+ ASCs into infarcted myocardium and investigated whether integrin α4+ ASCs subpopulation exhibited more robust engraftment and were better able to treat MI than did the unfractionated ASCs.
Materials and Methods
This study was approved by the Institutional Animal Care and Use Committee of Huazhong University of Science and Technology. All animal experiments complied with the ARRIVE guideline (Animal Research: Reporting of In Vivo Experiments) and were carried out on accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH publications No. 8023, revised 1978).
ASCs isolation and culture
ASCs were isolated from male green fluorescent protein (GFP) transgenic rats. Abdominal subcutaneous and inguinal adipose tissue are collected, minced, and digested at 37℃ for 20∼30 minutes with 0.2% (w/v) collagenase I. The digested tissue was sequentially filtered through 100-μm and 25-μm nylon screen and centrifuged at 300 rpm for 5 minutes. The cell pellets were then resuspended in cell-culture medium and maintained in a humidified atmosphere of 5% CO2 at 37℃. The culture medium was changed every day to remove dead cells and cell debris. The adherent cells were regarded as ASCs. The ASCs were in vitro cultured in complete medium and then cryopreserved at liquid nitrogen (−196℃). Immediately before cell transplantation, the ASCs were thawed, counted and resuspended in phosphate-buffered saline (PBS).
FACS
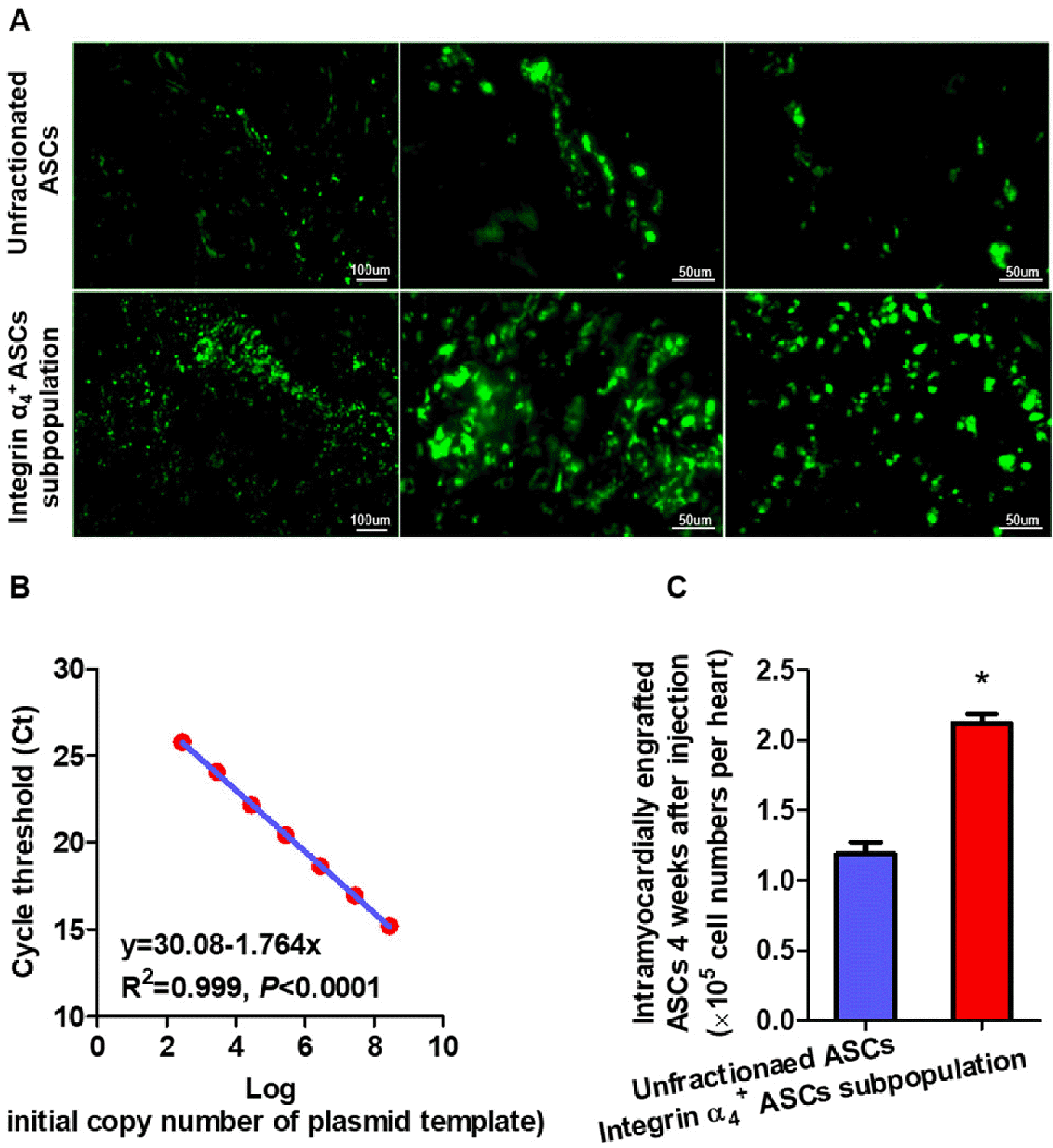
ASCs (1×107 cells/ml) were resuspended in PBS supplemented with 1% bovine serum albumin (BSA) and incubated with PE-conjugated integrin α4 monoclonal antibody (Clone R1-2; Thermo Fisher Scientific) at 4℃ for 30 minutes. Cellular suspension was centrifuged at 300 rpm for 5 minutes. After two washes with PBS, the ASCs were resuspended in cell-sorting medium at a concentration of 1×107 cells/ml. FACS were used for sorting target cell subpopulation. The fractions of purified samples typically are analyzed by the same technique for purity check. Cells harvested by FACS were immediately administered to the infracted hearts.
Cell migration assay
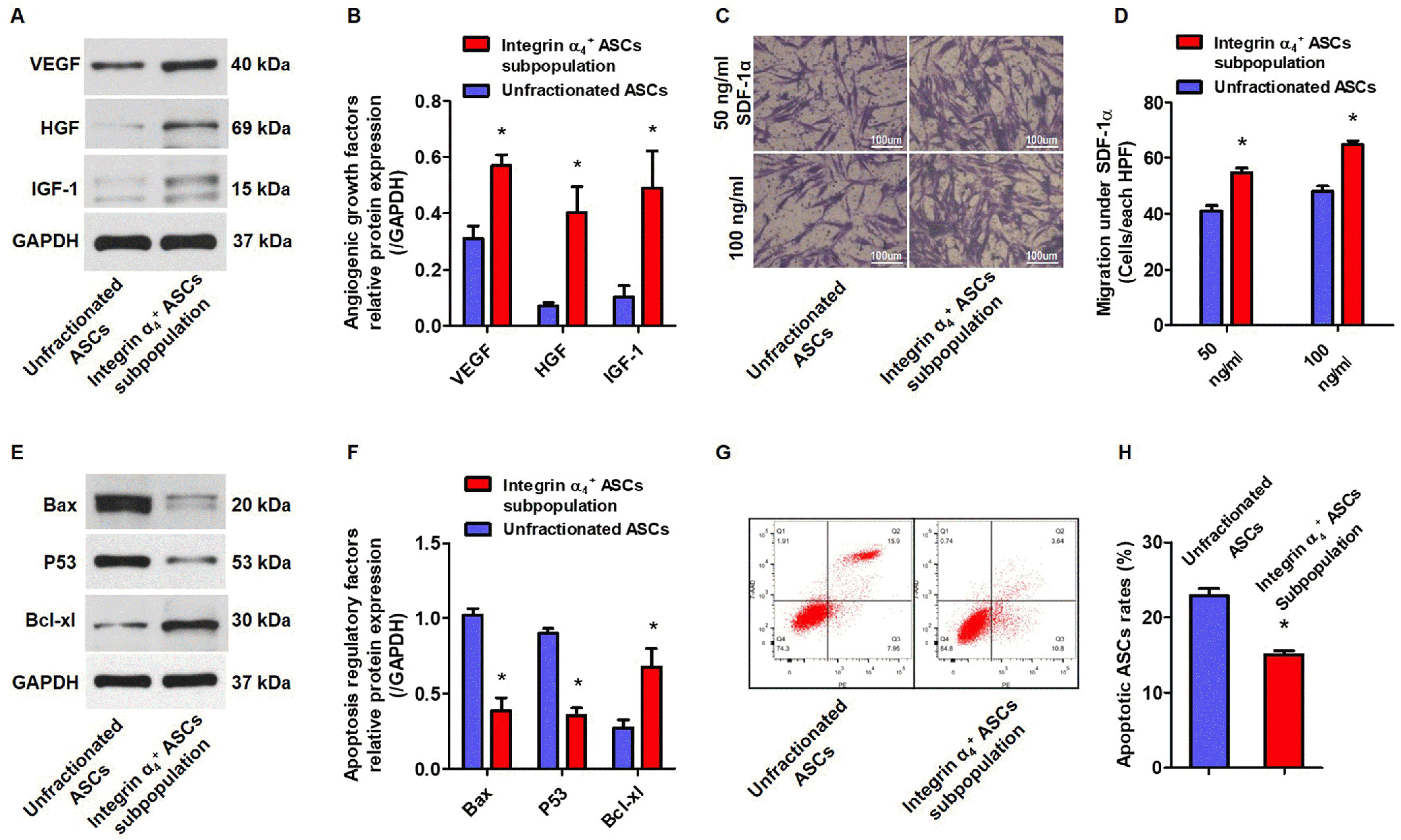
ASC migration was evaluated using Transwell plates with 8 μm pore size. The cells (5×105 cells/ml) were loaded in the upper chamber, and various concentrations of stromal cell-derived factor 1 alpha (SDF-1α) were added into the lower chamber filled with media. The cells in the transwell chambers were incubated at 37℃ for 24 hours to allow chemotactic migration toward a chemokine SDF-1α gradient. The cells that migrated into the lower surface of chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, whereas the non-migrating cells on the upper chamber were removed carefully using a cotton swab. The numbers of migrated cells were counted under an inverted microscope.
Western blot analysis
ASCs were washed with PBS and lysed on ice with RIPA lysis buffer containing protease inhibitor (Roche) and phosphates inhibitor cocktail (Sigma-Aldrich). After humanely sacrificing the animals, hearts were quickly removed and frozen in liquid nitrogen. Heart tissues were homogenized, and the lysates were centrifuged at 12,000 rpm for 15 minutes. The concentrations of protein samples were determined by Bradford protein assay.
The protein samples were separated by 10%∼15% SDS-PAGE gels, transferred to polyvinylidence difluoride membranes, and blocked with TBS containing 0.05% Tween-20 and 5% dry milk powder for 2 hours. The membrane for cell lysates was then incubated with primary anti-integrin α4 mouse polyclonal antibody (Cell signaling Technology). The membrances for tissue lysates were then incubated with the following specific primary mouse or rabbit polyclonal antibodies overnight at 4℃: VCAM-1, VEGF, HGF, insulin-like growth factor-1 (IGF-1), Bax, P53, and Bcl-xl (Proteintech). Equal loading in all lanes was verified by reprobing the nitrocellulose membrane with anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mouse mo-noclonal antibodies (Proteintech) overnight at 4℃. The membranes were sequentially treated with horseradish peroxidase-conjugated anti-mouse or rabbit secondary antibodies (Pierce) for 1 hour at room temperature. The intensities of the specific Western blot bands were quantified using the Software ImageJ and normalized against GAPDH.
Immunofluorescent staining
Adherent ASCs, grown in chamber slides, were fixed using 4% cold paraformaldehyde during 20 minutes at 4℃, and permeabilizated with ice-cold methanol for 5 minutes at −20℃. The cells were subsequently incubated with an anti-integrin α4 mouse polyclonal antibody 4℃ overnight. Next, the cells were incubated with an FITC-conjugated goat anti-mouse IgG secondary antibody at room temperature for 1 hour.
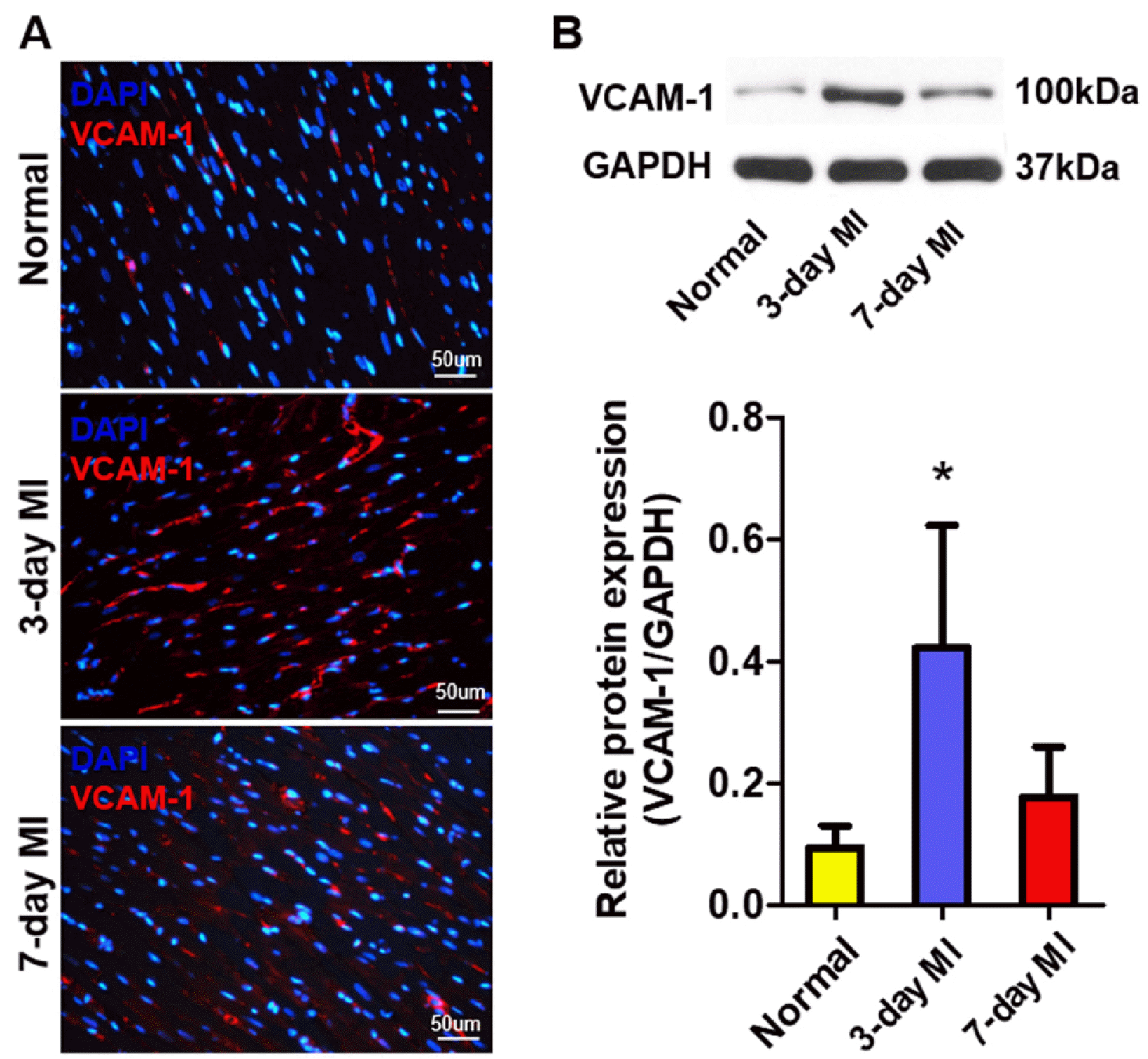
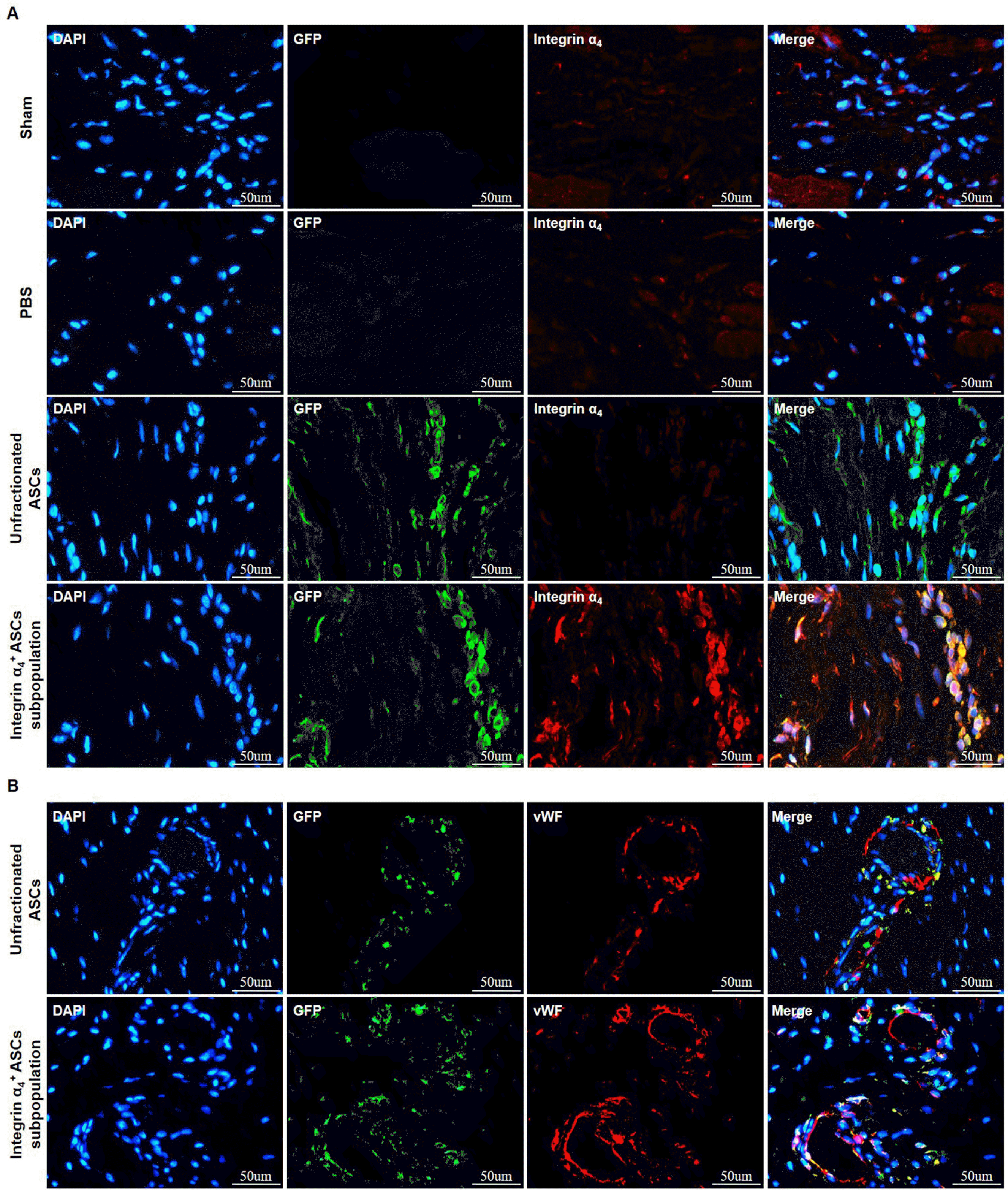
Hearts were then sliced coronally into 10-μm-thick sections at −20℃ on a cryotome. Tissue sections were fixed in cold methanol for 5 minutes, incubated in PBS with 0.1% Trton-X-100 for 15 minutes, and then blocked with 5% BSA for 30 minutes at room temperature. Finally, slides were incubated with primary antibodies for 1 hour at 37℃. VCAM-1, the ligand for integrin α4, was detected by an anti-VCAM-1 mouse polyclonal antibody (Proteintech). A mouse anti-integrin α4 antibody was used to detect the grafted integrin α4+ ASCs. von Willebrand Factor (vWF), an endothelial cell marker, was identified by an mouse anti-vWF antibody (Proteintech). After three more washes with PBS, slides were incubated with FITC-conjugated goat anti-mouse IgG for 45 minutes at 37℃. After three more washes with PBS, the slides were stained for nuclei using DAPI (4’,6-diamidino-2-phenylindole).
Cell apoptosis assay under oxygen glucose deprivation
ASCs were washed twice with serum-free and glucose-free medium and were incubated in the same medium at 37℃ in an air-tight anoxic chamber filled with 95% N2 and 5% CO2 for 24 hours. oxygen glucose deprivation (OGD) induces apoptosis in ASCs was evidenced by annexin V/propidium iodide (PI) double staining. Annexin V identified apoptotic cells while PI detected necrotic cells. The percentage of necrotic cells (PI positive/annexin negative) and apoptotic cells (PI negative or positive/an-nexin positive) was determined by FACS analysis.
MI model
Female rats (n=30) were endotracheally intubated, deeply anaesthetized with 2% isoflurane, and ventilated mechanically. The left anterior descending (LAD) coronary artery was completely ligated at 2∼3 mm distal to its origin. Sham-operated rats underwent the same cardiac exposure and manipulation without suture placement for LAD. At 3 and 7 days following coronary occlusion, six rats (n=3 per time-point) were euthanized, the hearts were carefully removed for the histological identification of VCAM-1.
Three days after coronary ligation, the rats (n=24) were randomly divided into four groups (sham, n=6; PBS, n=6; integrin α4+ ASCs, n=6; and unfractionated ASCs, n=6). In two ASC treatment groups, 150 μl of 1.5×106 GFP-positive ASCs were intramyocardially injected into four to six sites in the peri-infarct zone. The vehicle-treated group received an equal volume of PBS in the same regions. Four weeks after cell transplantation, the rats were subjected to positron emission tomography/computed tomography (PET/CT) examination, were then euthanized, and hearts were harvested for real-time polymerase chain reaction (PCR) and histological examination. The rats also underwent cardiac cine magnetic resonance examination at 4 weeks after PBS or cell treatment.
Quantitative real-time PCR
Real-time PCR was utilized to quantify engrafted male ASCs into the myocardium by measuring the amount of Y-chromosome-specific DNA. The genomic DNA was isolated from the infarcted myocardium with the QIAamp DNA Blood Mini Kit (QIAGEN). Real-time quantitative PCR reactions were performed using an ABI-PRISM 7700 Sequence Detection System (Applied Biosystems). Primers and probes for Y-chromosome-specific Sry3 gene and β-actin were designed and generated by Applied biosystems. Standard curves were prepared by serially diluting genomic DNA extracted from ASCs, which was mixed with genomic DNA obtained from an infracted heart to evaluate PCR efficiency and linear amplification. PCR was performed for 45 cycles with denaturation at 95℃ for 10 seconds, annealing at 60℃ for 1 minute, and extension at 72℃ for 15 seconds by using Master Mix (Applied Biosystems). β-Actin gene served as an internal control, and loading of DNA per reaction was normalized to the level of β-actin.
PET/CT imaging
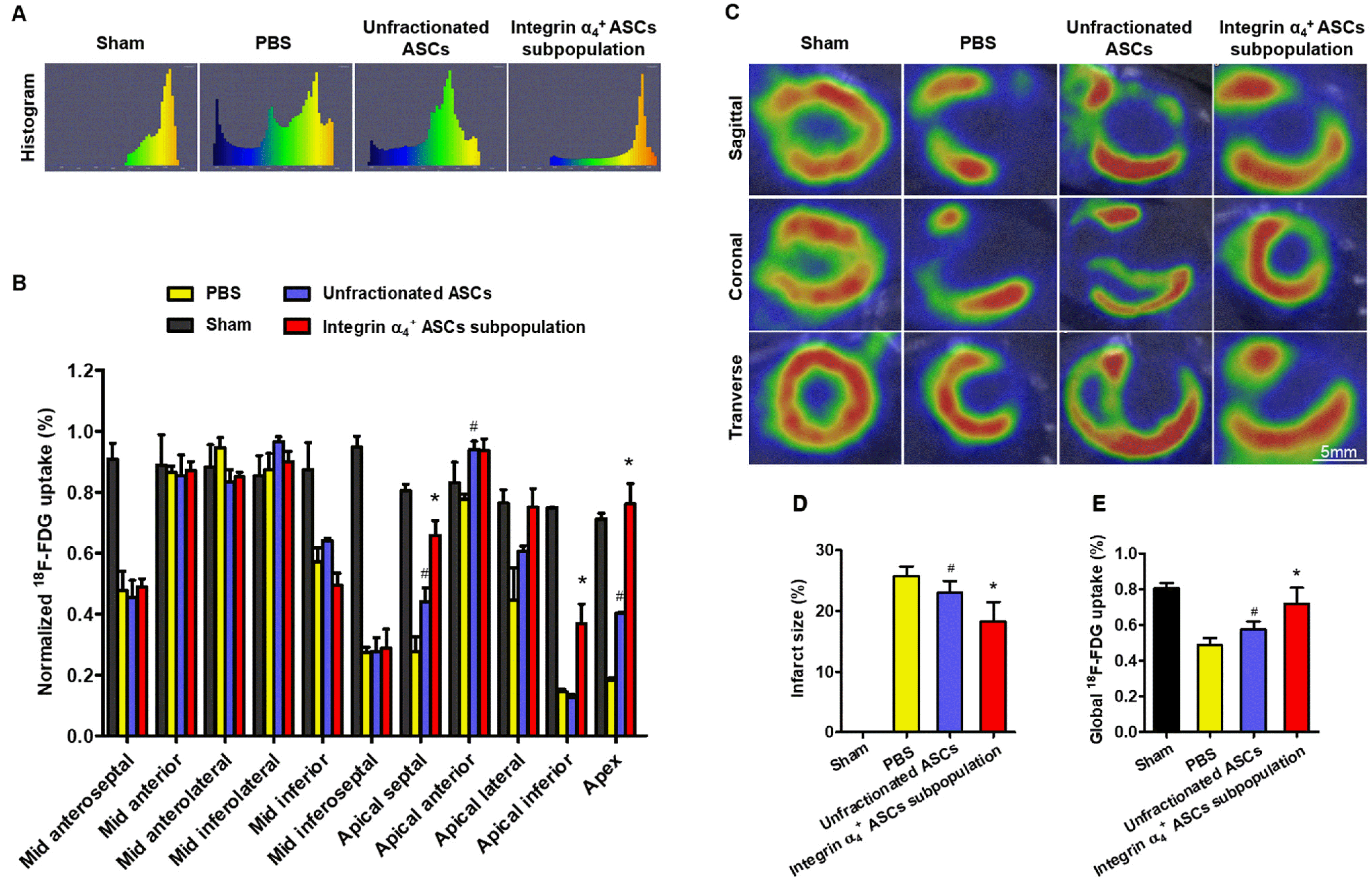
In vivo PET/CT scans were performed using a small animal dedicated PET/CT scanner 4 weeks posttransplantation. Rats were required to fast for 3 hours prior to injection of radioisotopic agents. 18F-FDG 500±25 μCi were then injected into the tail vein. At 45 to 60 minutes after injection, rats were anesthetized using inhaled 2% isoflurane. Images were acquired in prone position for 13 minutes. CT images were reconstructed using the standard Feld-kamp algorithm. The acquired PET images were reconstructed with a three-dimensional (3D) ordered subset expectation maximization method with a voxel size of 0.5×0.5×0.5 mm3. 18F-FDG PET images were automatically assembled into histograms.
Myocardium was divided into 17 segments according to the American Heart Association recommendation. Segmental heart analysis was conducted by segmentation of left ventricle (LV) PET images using a 17-segment model. The endo- and epicardial wall surfaces were visually traced based on morphological features. Standardized uptake value (%) of 18F-FDG for each LV segment was determined by dividing their values on the highest LV segmental acquisition. Myocardial infarct size was defined as the percent infarction area over the total LV myocardium.
Cardiac cine magnetic resonance imaging
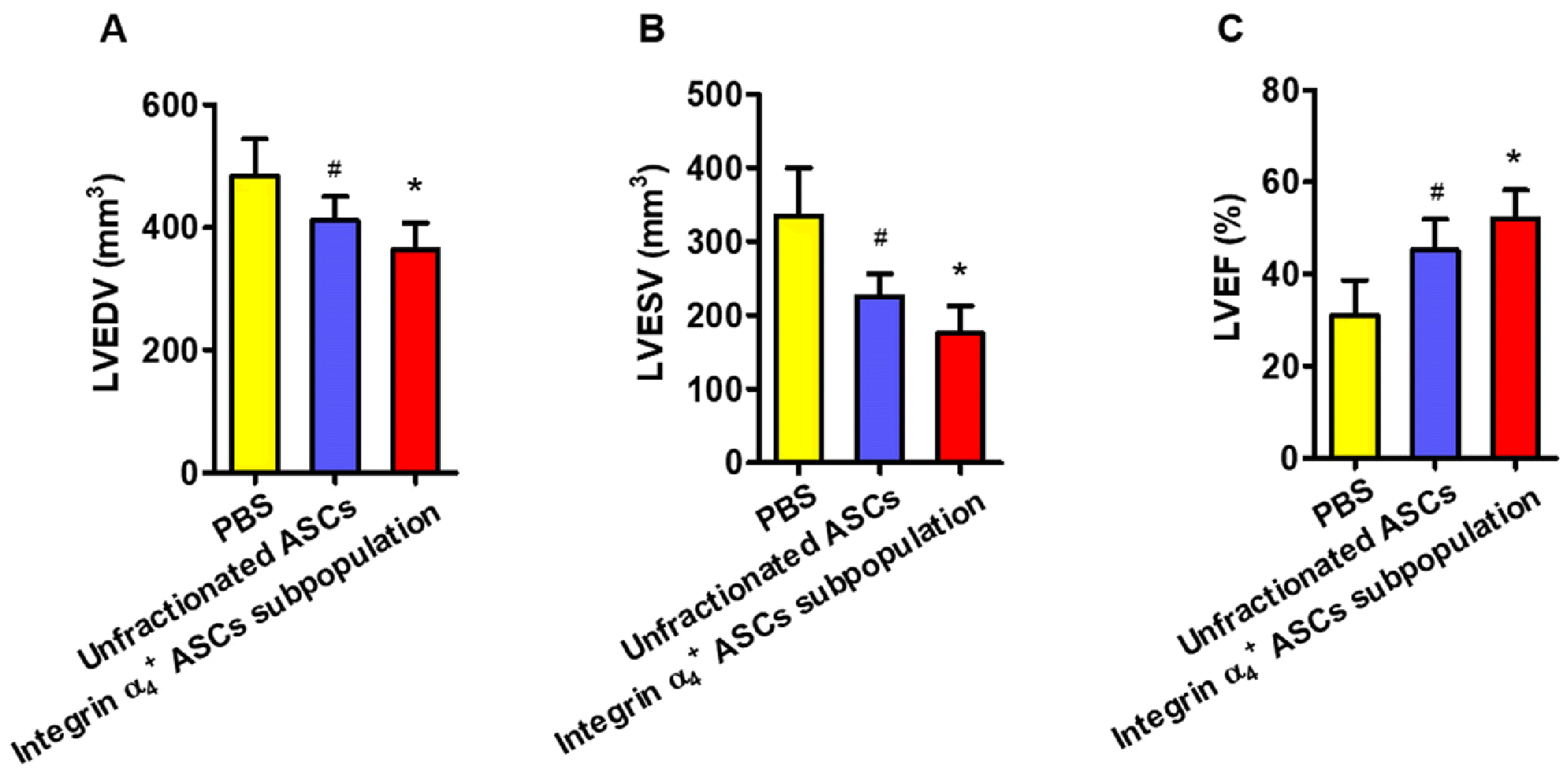
The anesthetized rats were placed in the prone position and fixed on a custom-built fiberglass cradle. A home-built quadrature surface coil was used as transceiver and then placed over the heat of the animals. In vivo cardiac cine images were obtained on a PharmaScan 7.0 T magnetic resonance imaging (MRI) scanner (Bruker) using a balanced steady-state free precession sequence: repetition time=9.2 msec, echo time=3.5 msec, field of view=8×8 cm2, matrix size=256×256. The cine images were acquired in consecutive short-axis views covering the LV from base to apex. The following parameters were determined: LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV ejection fraction (LVEF).
Statistical analysis
All of values were expressed as the mean±SD. Comparisons between two groups were performed with a two-tailed Student t-test. Comparisons among multiple groups were performed with two-way ANOVA. A p-value of <0.05 was considered statistically significant.
Discussion
We observed that integrin α
4 decreased the pro-apoptotic Bax and P53, increased the anti-apoptotic Bcl-xl, and eventually inhibited cell apoptosis
in vitro. We also confirm that integrin α
4+ ASCs subpopulation secreted a higher level of angiogenic growth factors and displayed a stronger migration potential as compared with unfractio-nated ASCs. Integrins, transmembrane receptors composed of α subunits and β subunits, modulate cell viability, proliferation, survival, and apoptosis through integrin-linked kinase (ILK) and focal adhesion kinase (FAK). ILK is a 59-kDa serine/threonine kinase that binds to the cyoplastic domain of integrin α
4 and regulates cell survival, migration and paracrine properties of stem cells (
11). FAK, an integrin-associated protein tyrosine kinase, activates its intrinsic kinase function, supports the survival and substrate adhesion and prevents the onset of apoptosis (
12). The role of integrin in stem cells has been in-depth investigated previously (
13-
15). Active integrin β
1 expression increased the proliferation and differentiation capacity of tonsil-derived mesenchymal stem cells (MSCs) (
13). Integrin α
5 forced expression enhanced the proliferation, migration, and osteogenic potential of periodontal ligament stem cells (
14). Integrin α
6 positive stem cells had a strong myogenic differentiation potential and an improved cell fusion capacity (
15).
We demonstrated that the integrin α
4+ ASCs subpopulation highly expressed integrin α
4 on their surface, and their binding partner of VCAM-1 was up-regulated in the infarcted myocardium. VCAM-1 expression peaked at 3 days post-MI and recovered to normalization 14 days post-MI (
16). VCAM-1 expression correlated well with the extent of bone marrow (BM)-MSCs retention at 3 days and 7 days post-MI (
16). Actually, we found that there were signi-ficantly higher numbers of integrin α
4+ ASCs engrafted and migrated into ischemic myocardium relative to the unfractionated ASCs, suggesting a crucial role of integrin α
4/VCAM-1 interaction in the survival and engraftment of implanted ASCs. Enhanced cell engraftment is translated into the functional benefits. According to our
in vivo PET-CT examination, transplantation of integrin α
4+ ASCs subpopulation resulted in a further improvement in segmental glucose metabolism and a further decrease in infarct size compared to unfractionated ASCs. Cardiac cine MRI demonstrated that integrin α
4+ ASCs subpopulation more effectively promoted LV contractile function recovery in infarcted hearts at four weeks posttransplantation. The enrichment of integrin α
4 positivity is the main cause of the enhanced engraftment and improved treatment efficacy after the transplantation of purified subpopulation. Lentivirus vector mediated integrin β
1 overexpression increased the survival of BM-MSCs and improved the efficacy of transplanted BM-MSCs for MI (
17). Intravenous injection of endothelial colony-forming cells genetically engineered to overexpress integrin β
1 effectively promote angiogenesis in ischemic mouse hindlimb (
18). Integrin α
4 overexpression by lentiviral transduction enhanced transendothelial migration of MSCs
in vitro (
19).
In conclusion, compared with unfractionated ASCs, integrin α4+ ASCs subpopulation releases more angiogenic growth factors, exhibits greater migratory capacity, and has stronger anti-apoptotic potential in vitro. Integrin α4+ ASCs subpopulation is more effective at engrafting into the infracted myocardium, reducing infarct size and improving cardiac function, representing a promising candidate for MI therapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download