Abstract
Human pluripotent stem cells (hPSCs) such as human embryonic stem cells (hESCs), induced pluripotent stem cells, and somatic cell nuclear transfer (SCNT)-hESCs can permanently self-renew while maintaining their capacity to differentiate into any type of somatic cells, thereby serving as an important cell source for cell therapy. However, there are persistent challenges in the application of hPSCs in clinical trials, where one of the most significant is graft rejection by the patient immune system in response to human leukocyte antigen (HLA) mismatch when transplants are obtained from an allogeneic (non-self) cell source. Homozygous SCNT-hESCs (homo-SCNT-hESCs) were used to simplify the clinical application and to reduce HLA mismatch. Here, we present a xeno-free protocol that confirms the efficient generation of neural precursor cells in hPSCs and also the differentiation of dopaminergic neurons. Additionally, there was no difference when comparing the HLA expression patterns of hESC, homo-SCNT-hESCs and hetero-SCNT-hESCs. We propose that there are no differences in the differentiation capacity and HLA expression among hPSCs that can be cultured in vitro. Thus, it is expected that homo-SCNT-hESCs will possess a wider range of applications when transplanted with neural precursor cells in the context of clinical trials.
Human pluripotent stem cells (hPSCs) have emerged as an attractive cell source for cell-based therapies aimed at restoring lost cell and tissue functions. These cells can permanently self-renew and differentiate into all cell types within the body. This has encouraged stem cell research in the context of drug development, cell regeneration, and gene therapy. Moreover, hPSCs provide a potential resource for cell replacement therapy for neurological diseases such as Parkinson’s disease. To achieve the complete cellular therapeutic potential of hPSCs, xenogeneic components from hPSC expansion and differentiation protocols must be removed. Defined and reproducible culture conditions must be achieved to generate hPSC derivatives that can support transplantation therapy (1).
hPSCs include human embryonic stem cells (hESCs), somatic cell nuclear transfer (SCNT)-hESCs, and human induced pluripotent stem cells (hiPSCs). hESCs are obtained from the inner cell mass of the blastocyst after fertilization of the oocyte and the formation of a diploid junction. These cells give rise to all somatic cell types within the embryo (2). Based on the concept of hESCs, SCNT-hESCs were created via the combination of a nucleus-removed oocyte and the nuclei of somatic cells. Therefore, SCNT-hESCs can produce patient-specific ESCs that reduce the likelihood of immune rejection (3-5). However, as the use of these cells invokes ethical considerations due to the requirement of human embryos, hiPSCs were subsequently developed as a replacement. hiPSCs are generated by optimal delivery of transcription factors to a variety of adult somatic cells with the same abilities as hESCs in addition to the advantage of producing patient- and disease-specific stem cells (6). Thus, hPSCs serve as a renewable and potentially unlimited cell source for transplantation in the treatment of multiple diseases (7-9).
One of the current challenges in using hPSCs prior to clinical use is the polymorphic nature of the human leukocyte antigen (HLA) that can induce powerful immune rejection (10, 11). Although allogeneic cell therapy targeting large patient populations is economically feasible, grafts are attacked through the recognition of non-self HLA Class I (HLA-A, -B, and -C) and HLA Class II (HLA-DR, -DP, and -DQ) by the CD8+ cytotoxic T cells and CD4+ hel-per T cells of recipients (12-14). In contrast, the lack of HLA Class I activates natural killer cells and can lead to pathogen infection or cell death (15-17). It is difficult to match the HLA haplotypes of the entire population.
Here, we used homozygous SCNT-hESCs (homo-SCNT-hESCs) that possess the same alleles of HLA-A, -B, and -DRB1 that are known to be the most polymorphic to reduce the possibility of immune rejection (18, 19). Evidence of transplant rejection was not observed even when only one HLA allele was matched, thus suggesting that using homo-SCNT-hESCs in cell therapy could help treat a relatively wide range of individuals. We also compared several hPSCs that could be cultured in vitro and observed no differences in the directed differentiation potential or HLA expression of all hPSCs in neurons.
The hESC culture protocol (HYI-17-137-6) was approved by the Institutional Review Board of Hanyang Uni-versity. The following hPSCs were used: CHA-hES15 (hESCs; CHA Stem Cell Institute); CHA-hiPS NT2-S4 (hiPSCs; CHA Stem Cell Institute); heterozygous CHA-FT-NT17 (hetero-SCNT-hESCs; Research Institute for Stem Cell Research, CHA Health Systems); homozygous CHA-FT-NT18 (homo-SCNT-hESCs; Research Institute for Stem Cell Research, CHA Health Systems). hPSCs were cultured in TeSRTM-E8TM basal medium (Stemcell Technologies) with TeSRTM-E8TM 25X supplement (Stemcell Technologies) on Vitronectin XFTM (Stemcell Technologies)-coated dishes under standard culture conditions (37℃, 5% CO2). The culture medium was changed daily, and the cells were passaged using ReLeSR (Stemcell Technologies).
hPSC colonies were detached with 1 mg/ml collagenase type IV (Sigma-Aldrich), and colonies were transferred onto uncoated bacterial dishes in Essential 6TM medium (Sigma-Aldrich) with 1% penicillin-streptomycin (10,000 U/ml, Thermo Fisher Scientific). The culture medium was changed every two days for five days.
Embryoid bodies (EBs) were transferred to culture dishes coated with 15 μg/ml poly-L-ornithine (PLO; Sigma-Aldrich) and 1 μg/ml fibronectin (FN; Sigma-Aldrich). The growth medium for the neural rosettes (1/2 N2 medium) was composed of serum-free medium supplemented with 0.5% 100X N2 supplement (Thermo Fisher Scientific). The 1/2 N2 medium was changed every two days for five days. After this period, the 1/2 N2 medium was replaced with another serum-free medium (N2bF medium) supplemented with 1% 100X N2 supplement and 40 ng/ml basic fibroblast growth factor (R&D System). The culture medium was changed every two days.
The generated neural rosettes were detached using a flame-pulled Pasteur pipette to maintain their mass form. The floating spherical neural masses (SNMs) were transferred to uncoated culture dishes containing the N2bF medium. The N2bF medium was changed every two days. For the purification of SNMs that grew 5∼7 days later, the SNMs were mechanically cut into 10∼15 pieces using a flame-pulled Pasteur pipette while removing the cystic portion within several minutes. Approximately 50 pieces of cut SNMs were transferred to uncoated culture dishes in N2bF medium. After 5∼7 days, the same method was repeated for passaging. The homogeneity of the SNM increased after to 3∼4 purification passages.
Approximately 50∼60 pieces of purified SNMs were collected and fragmented using 100 mesh (φ0.03×100×100 mm; The Nilaco Corporation) prior to being transferred onto PLO/FN-coated culture dishes in N2bF medium. The fragmented SNMs were expanded for several days to allow for adaptation. A few days later, to separate single cells the fragmented SNMs were dissociated using AccutaseTM (Sigma-Aldrich) for 5 minutes at 37℃ and centrifuged at 1,500 rpm for 3 minutes. The cell pellets were resuspended in N2bF medium and re-plated onto PLO/FN-coated culture dishes or onto glass coverslips (φ12 mm; Marienfeld Superior).
For spontaneous differentiation, neural precursor cells (NPCs) were seeded onto PLO/FN-coated dishes and cultured in serum-free differentiation medium (N2B27 medium) with 1% 100X N2 supplement, 50X B-27TM Supplement (Thermo Fisher Scientific), and 200 μM ascorbic acid (AA; Sigma-Aldrich).
For astrocyte differentiation, NPCs were cultured in N2B27 medium containing 10 ng/ml ciliary neurotrophic factor (CNTF; PeproTech).
A previously published protocol was applied (20). NPCs were plated onto PLO/FN-coated dishes in N2B27 medium containing 40 ng/ml triiodothyronine (T3; Sigma-Aldrich), 200 ng/ml sonic hedgehog (Shh; R&D Systems), 100 ng/ml noggin (R&D Systems), 100 ng/ml IGF (R&D Systems), and 50 nM dibutyryl-cAMP (db-cAMP; Sigma-Aldrich). All cell culture media were changed every 2∼3 days.
Cell differentiation was initiated using N2B27 medium to induce dopaminergic (DA) neurons after NPC expan-sion. After two days, the cells were cultured in N2B27 medium with 200 ng/ml Shh and 100 ng/ml fibroblast growth factor 8 (FGF8; PeproTech) for 2 days. For terminal differentiation, 200 μM AA, 50 nM db-cAMP, and 20 ng/ml glial cell-derived neurotrophic factor (GDNF; R&D System) were added to the N2B27 medium with Shh (without FGF8).
Cells were fixed in 4% paraformaldehyde (Sigma-Aldrich) for 15∼20 minutes at room temperature. Cells were blocked and permeabilized with 10% normal goat serum (Pel-Freez) and 0.3% Triton X-100 (Sigma-Aldrich) in 0.1% bovine serum albumin/phosphate buffered saline (BSA/PBS) for 1 hour. Samples were incubated with primary antibodies at 4℃ overnight. The samples were then washed three times with 0.1% BSA/PBS and incubated with secondary antibodies at room temperature for 1 hour. The cells were counterstained with 4’,6-diamidino-2-phenylin-dole (Vector Laboratories) and mounted using a mounting solution. Images were captured using an epifluorescence microscope (LEICA Microsystems). The primary antibodies used in this study were as follows: anti-NESTIN (1:500; BioLegend), anti-SOX2 (1:1,000; Sigma-Aldrich), anti-ZO1 (1:50; Thermo Fisher Scientific), anti-PAX6 (1:200; Novus Biologicals), anti-TUJ1 (1:2,000; BioLegend), anti-TH (1:2,000; Pel-Freez), anti-glial fibrillary acidic protein (GFAP, 1:2,000; DAKO), anti-OLIG2 antibody (1:1,000; Chemicon), anti-major histocompatibility complex (MHC) Class I+HLA A/B (1:200, MHC-I; Abcam), anti-MHC Class II (1:200, MHC-II; Abcam). The secondary antibodies used were Alexa Fluor 488 (1:500; Ther-mo Fisher Scientific), Rhodamine, or Cy3 (1:500; Jackson ImmunoResearch Laboratories).
Prior to staining, cells were fixed with 4% paraforma-ldehyde for 10 minutes at room temperature. Alkaline phosphatases (AP) staining was performed using an Alkaline Phosphatase Kit (CA# 86R-1KT; Sigma-Aldrich), according to the manufacturer’s protocol.
Total RNA was extracted using TRI REAGENT (Molecular Research Center, Inc.), and cDNA was reverse transcribed from 1 μg of RNA using the Superscript Kit (Sigma-Aldrich). Polymerase chain reaction (PCR) amplification and primer conditions are listed in Table 1.
All experiments were conducted at least three times. Cell counting for quantification was performed in 5∼10 microscopic fields per well with three wells per experimental condition. All quantified data were statistically analyzed and are presented as mean±SEM. Statistical analyses were performed using SigmaPlot for Windows version 10.0 (SystatSoftware GmbH).
We conducted the following experiments to identify the characteristics of hPSCs at the pluripotent stage, including EB formation. First, AP is known to be expressed at high levels in pluripotent stem cells such as hESCs, hiPSCs, and SCNT-hSCs. Similar to previous reports, AP expression in the hPSCs used in this study was confirmed by AP staining. Representative AP staining of hPSCs 5 days after plating is presented. All cell lines tested positive (Fig. 1A). Next, we confirmed the possibility of differentiating the three germ layers. The pluripotency marker OCT4 decreased after EB induction in addition to the expression of the ectodermal marker PAX6, the mesodermal marker LMO2, the endodermal marker GSC, and the housekeeping gene GAPDH as confirmed by reverse transcription (RT)-PCR analysis at 14 days (Fig. 1B, Table 1).
In this study, we developed an efficient protocol to generate NPCs from several hPSCs in a time-efficient manner on a large scale (Fig. 2A). First, multiple detached hPSC colonies were cultured on uncoated bacterial dishes to form EBs that are the three-dimensional aggregates formed in suspension of hESCs, hiPSCs, and SCNT-hESCs. (Fig. 2B-2I). To generate neural rosettes, EBs were transferred onto PLO/FN-coated culture dishes, cultured in 1/2 N2 medium for 5 days, and then cultured in N2bF medium for an additional 5 days (Fig. 2A, 2J, 2J-2M). After neural rosette selection, the SNMs were isolated via mechanical dissection and cultured in suspension in culture dishes. Following 3∼4 purification passages, the neural rosettes disappeared, and an increased number of SNMs resembling neurospheres were formed (Fig. 2A, 2N, 2N-2Q). To differentiate the SNMs into neurons, astrocytes, and oligodendrocytes, the SNM fragments were transferred to PLO/FN-coated culture dishes. Thereafter, the SNM fragments dissociated into single-cell NPCs that were transferred onto PLO/FN-coated glass coverslips (Fig. 2A, 2R-2U). Thus, this method proved to be equally applicable to all hPSCs with the typical NPC morphology observed in all cell lines.
The characteristics of the NPCs at each stage were identified, including the rosettes, SNMs, and NPCs. Experiments were performed to detect differences in the production efficiency of NPCs from each cell line. Immuno-staining revealed that the neural rosettes expressed a wide range of early neuroectoderm and neural rosette markers such as PAX6 and ZO-1. The rosettes also exhibited a morphological appearance (Fig. 3A). As detected by SNM immunostaining, most NESTIN-expressing cells (green) coexpressed SOX2 (red), a neural precursor cell marker in the central nervous system (CNS) (Fig. 3B). After single-cell dissociated cultures of NPCs, NESTIN, and SOX2 proteins were detected by immunostaining (Fig. 3C), thus suggesting that hPSC-derived NPCs possessed proliferative capacity in all cell lines.
We estimated the differentiation efficiency of the hPSC-derived NPCs. The hPSC-derived NPCs expressed TUJ1, a marker observed spontaneously in early neurons, was detected (Fig. 4A). NPCs were then cultured in a medium containing CNTF and T3 that promoted the formation of astrocytes and oligodendrocytes, respectively. In agreement with this, astrocyte and oligodendrocyte markers such as GFAP and OLIG2 were readily detectable (Fig. 4A). Overall, hPSC-derived NPCs were observed to possess efficient differentiation capabilities for neurons and glial cells; however, oligodendrocyte differentiation efficiencies were slightly lower (Fig. 4B).
The efficient generation of NPCs from hPSCs was observed in all cell lines in addition to their differentiation into neurons, astrocytes, and oligodendrocytes that constitute a large proportion of the CNS. To produce DA neuronal cultures, we optimized a DA neuron differentiation protocol (Fig. 5A) (1). The DA identity of the induced cells was confirmed by the expression of TUJ1 and the rate-limiting enzyme tyrosine hydroxylase (TH; Fig. 5B, 5C). As a result of DA differentiation induction, no difference in differentiation efficiency was observed between homo-SCNT-hESCs and the other cell lines. In conclusion, we expect homo-SCNT-hESCs to be useful, as they can be applied to a greater number of patients.
MHC expression was analyzed by differentiating hPSCs into neurons. In CHA15 hESC, hetero-SCNT-ESC, and homo-SCNT-ESC, almost no MHC expression was observed in the undifferentiated state; however, MHC expression began to increase from the EB stage. In differentiated neurons, MHC I and II expression was maintained (Fig. 6).
Over the last few decades, hPSCs have been extensively used in cellular research due to their potential as renewable cell sources for drug toxicity screening, human cell therapy, and regenerative medicine (21-23). However, the cell sources should be considered when using hPSCs, including allogeneic (non-self) and autologous (self) cells. For autologous cells, hiPSCs are generated from somatic cells using reprogramming factors, and SCNT-hESCs are generated by injecting their own nuclei. The use of autologous cells exhibits the advantage of low immune rejection; however, it is time-consuming, expensive, and inefficient. Allogeneic cells are eventually used for wider patient applications; however, these cells possess the potential for a strong immune rejection reaction (24-26).
HLA must be matched to avoid immune rejection; however, matching the HLA of an entire population is difficult, as HLAs are highly polymorphic (27, 28). Therefore, we used homo-SCNT-hESCs that possess the same alleles of HLA-A, -B, and -DRB1 genes that are known to be the most diverse among HLA (29, 30). When used for transplantation, immune rejection does not occur despite the recipient matching only one allele, thus increasing the potential use of homo-SCNT-hESCs in a wider range of individuals. It is a suitable cell source that saves time and money in addition to its usefulness in the context of cell therapy.
Several studies have targeted HLA in hiPSCs (31-33). hiPSCs prepared from either patients or selected donors with HLA matching are a notable source of cells from the immunological standpoint of cell therapy. Studies have demonstrated that immune rejection is reduced when autologous or syngeneic iPSC-derived grafts are used that are ideal sources from an immunological perspective (34, 35). However, in contrast to previous research, it has been reported that mouse and human iPSC-derived cells can be immunogenic in syngeneic or autologous recipients and in autonomous humanized mouse models despite the use of their own cells (36-38).
AP staining, RT-PCR, and immunocytochemistry were performed to identify the cell characteristics at each step. First, AP is highly expressed in pluripotent cells but is very low in differentiated cells. All cell lines were stained for AP in an undifferentiated proliferative state. Second, we confirmed that markers for each characteristic were expressed in the EB, rosettes, SNM, and NPCs. hPSC-derived NPCs were clonogenic and self-renewable. Third, we further confirmed that when cultured under each condition, neurons, astrocytes, oligodendrocytes, and DA neurons were produced in all cell lines and demonstrated the availability of these cells. MHC was not expressed in undifferentiated PSCs, thus indicating that undifferentiated PSCs were not immunogenic. However, as MHC expression increases as differentiation progresses, immunogenicity should be considered when NPC or differentiated cells are used for cell therapy. Therefore, the role of homo SCNT-ESCs are believed to be highlighted.
In conclusion, we successfully applied these protocols to all known pluripotent cell lines, including hESCs, hiPSCs, and SCNT-hESCs, that are capable of producing functional neurons. Additionally, we studied the basis for the use of homo-SCNT-hESCs in future clinical applications. We believe that this study will provide support for future cell replacement therapies in a wide range of patients.
Notes
Authors’ Contribution
Conceptualization: JSL, JEL, SHY, TC, DRL, CHP. Data curation: JSL, JEL, SHY, TC. Formal analysis: JSL, JEL, TC. Funding acquisition: CHP, DRL. Investigation: JSL, JEL, SHY. Methodology: JSL, JEL, TC. Project administration: DRL, TC, CHP. Resources: DRL, TC, CHP. Software: JSL, JEL, SHY. Supervision: DRL, TC, MYC, CHP. Validation: TC, DRL, MYC, CHP. Visualization: JSL, JEL, SHY. Writing – original draft: JSL, JEL, SHY, CHP. Writing – review and editing: DRL, CHP.
References
1. Cho MS, Hwang DY, Kim DW. 2008; Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc. 3:1888–1894. DOI: 10.1038/nprot.2008.188. PMID: 19008875.

2. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. Erratum in: Science 1998;282:1827. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.

3. Chung YG, Eum JH, Lee JE, et al. 2014; Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 14:777–780. DOI: 10.1016/j.stem.2014.03.015. PMID: 24746675.

4. Matoba S, Liu Y, Lu F, et al. 2014; Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 159:884–895. DOI: 10.1016/j.cell.2014.09.055. PMID: 25417163. PMCID: PMC4243038.

5. Chung YG, Matoba S, Liu Y, et al. 2015; Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell. 17:758–766. DOI: 10.1016/j.stem.2015.10.001. PMID: 26526725.

6. Takahashi K, Tanabe K, Ohnuki M, et al. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.

7. Schwartz SD, Hubschman JP, Heilwell G, et al. 2012; Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 379:713–720. DOI: 10.1016/S0140-6736(12)60028-2. PMID: 22281388.

8. Kim JH, Auerbach JM, Rodríguez-Gómez JA, et al. 2002; Dopa-mine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 418:50–56. DOI: 10.1038/nature00900. PMID: 12077607.

9. Laflamme MA, Chen KY, Naumova AV, et al. 2007; Cardiomyo-cytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 25:1015–1024. DOI: 10.1038/nbt1327. PMID: 17721512.

10. van Berlo JH, Molkentin JD. 2014; An emerging consensus on cardiac regeneration. Nat Med. 20:1386–1393. DOI: 10.1038/nm.3764. PMID: 25473919. PMCID: PMC4418535.

11. Williams RC, Opelz G, Weil EJ, McGarvey CJ, Chakkera HA. 2017; The risk of transplant failure with HLA mismatch in first adult kidney allografts 2: living donors, summary, guide. Transplant Direct. 3:e152. DOI: 10.1097/TXD.0000000000000664. PMID: 28573187. PMCID: PMC5441983.

12. Gottwald W. 1978; Neurologic and psychiatric syndromes in lupus erythematosus. Z Hautkr. 53:505–514. German.
13. Albert ML, Sauter B, Bhardwaj N. 1998; Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. DOI: 10.1038/32183. PMID: 9510252.

14. Doyle C, Strominger JL. 2010; Interaction between CD4 and class II MHC molecules mediates cell adhesion. 1987. J Immunol. 184:5935–5938. DOI: 10.1038/330256a0. PMID: 2823150.
15. Ichise H, Nagano S, Maeda T, et al. 2017; NK cell alloreactivity against KIR-ligand-mismatched HLA-haploidentical tissue derived from HLA haplotype-homozygous iPSCs. Stem Cell Reports. 9:853–867. DOI: 10.1016/j.stemcr.2017.07.020. PMID: 28867344. PMCID: PMC5599245. PMID: 55716d85db5e4dffa93d13d9157f0cc5.

16. Kruse V, Hamann C, Monecke S, et al. 2015; Human induced pluripotent stem cells are targets for allogeneic and autologous natural killer (NK) cells and killing is partly mediated by the activating NK receptor DNAM-1. PLoS One. 10:e0125544. DOI: 10.1371/journal.pone.0125544. PMID: 25950680. PMCID: PMC4423859. PMID: ef081892998f45fa9fea252226fc72b1.

17. McGranahan N, Rosenthal R, Hiley CT, et al. 2017; Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 171:1259–1271.e11. DOI: 10.1016/j.cell.2017.10.001. PMID: 29107330. PMCID: PMC5720478.
18. Lee KW, Oh DH, Lee C, Yang SY. 2005; Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 65:437–447. DOI: 10.1111/j.1399-0039.2005.00386.x. PMID: 15853898.

19. Yang G, Deng YJ, Hu SN, et al. 2006; HLA-A, -B, and -DRB1 polymorphism defined by sequence-based typing of the Han population in Northern China. Tissue Antigens. 67:146–152. DOI: 10.1111/j.1399-0039.2006.00529.x. PMID: 16441486.

20. Lee EH, Park CH. 2017; Comparison of reprogramming methods for generation of induced-oligodendrocyte precursor cells. Biomol Ther (Seoul). 25:362–366. DOI: 10.4062/biomolther.2017.066. PMID: 28605832. PMCID: PMC5499613.

21. Mandai M, Watanabe A, Kurimoto Y, et al. 2017; Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 376:1038–1046. DOI: 10.1056/NEJMoa1608368. PMID: 28296613.

22. Lipsitz YY, Timmins NE, Zandstra PW. 2016; Quality cell therapy manufacturing by design. Nat Biotechnol. 34:393–400. DOI: 10.1038/nbt.3525. PMID: 27054995.

23. Chakradhar S. 2016; An eye to the future: researchers debate best path for stem cell-derived therapies. Nat Med. 22:116–119. DOI: 10.1038/nm0216-116. PMID: 26845400.

24. Krystkowiak P, Gaura V, Labalette M, et al. 2007; Alloimmunisa-tion to donor antigens and immune rejection following foetal neural grafts to the brain in patients with Huntington's disease. PLoS One. 2:e166. DOI: 10.1371/journal.pone.0000166. PMID: 17245442. PMCID: PMC1764859. PMID: a940106c9c5c4ba7a428d87fe610e8b6.

25. Brundin P, Pogarell O, Hagell P, et al. 2000; Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain. 123(Pt 7):1380–1390. DOI: 10.1093/brain/123.7.1380. PMID: 10869050.

26. Barker RA, Drouin-Ouellet J, Parmar M. 2015; Cell-based therapies for Parkinson disease-past insights and future poten-tial. Nat Rev Neurol. 11:492–503. DOI: 10.1038/nrneurol.2015.123. PMID: 26240036.

27. Sette A, Sidney J. 1998; HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr Opin Im-munol. 10:478–482. DOI: 10.1016/S0952-7915(98)80124-6. PMID: 9722926.

28. Williams TM. 2001; Human leukocyte antigen gene polymor-phism and the histocompatibility laboratory. J Mol Diagn. 3:98–104. DOI: 10.1016/S1525-1578(10)60658-7. PMID: 11486048.

29. Gallardo D, Brunet S, Torres A, et al. 2004; Hla-DPB1 mismatch in HLA-A-B-DRB1 identical sibling donor stem cell transplantation and acute graft-versus-host disease. Transplantation. 77:1107–1110. DOI: 10.1097/01.TP.0000122225.10296.10. PMID: 15087781.

30. Park MH, Kim HS, Kang SJ. 1999; HLA-A,-B,-DRB1 allele and haplotype frequencies in 510 Koreans. Tissue Antigens. 53(4 Pt 1):386–390. DOI: 10.1034/j.1399-0039.1999.530412.x. PMID: 10323346.

31. Deuse T, Hu X, Gravina A, et al. 2019; Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 37:252–258. Erratum in: Nat Biotechnol 2022;40:1690. DOI: 10.1038/s41587-022-01426-8. PMID: 36114291. PMCID: PMC6419516.

32. Xu H, Wang B, Ono M, et al. 2019; Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 24:566–578.e7. DOI: 10.1016/j.stem.2019.02.005. PMID: 30853558.

33. Morizane A, Kikuchi T, Hayashi T, et al. 2017; MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 8:385. DOI: 10.1038/s41467-017-00926-5. PMID: 28855509. PMCID: PMC5577234. PMID: 37518cb36fd0402692f5bdd69bd784da.

34. Araki R, Uda M, Hoki Y, et al. 2013; Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 494:100–104. DOI: 10.1038/nature11807. PMID: 23302801.

35. Morizane A, Doi D, Kikuchi T, et al. 2013; Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports. 1:283–292. DOI: 10.1016/j.stemcr.2013.08.007. PMID: 24319664. PMCID: PMC3849265.

36. Zhao T, Zhang ZN, Rong Z, Xu Y. 2011; Immunogenicity of induced pluripotent stem cells. Nature. 474:212–215. DOI: 10.1038/nature10135. PMID: 21572395.

37. de Almeida PE, Meyer EH, Kooreman NG, et al. 2014; Trans-planted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat Commun. 5:3903. DOI: 10.1038/ncomms4903. PMID: 24875164. PMCID: PMC4075468.

38. Zhao T, Zhang ZN, Westenskow PD, et al. 2015; Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 17:353–359. DOI: 10.1016/j.stem.2015.07.021. PMID: 26299572. PMCID: PMC9721102.

Fig. 1
Pluripotent marker expressions of human pluripotent stem cells (hPSCs). (A) alkaline phosphatases staining is the result of using conventional dyes as substrates for measuring the activity of elevated alkaline phosphatase in hPSCs. The characteristics of the fluorescently labeled cells are observed in the general hPSCs morphologies. (B) Reverse transcription polymerase chain reaction analysis reveals the expression of three germ layer markers (PAX6, LMO2, GSC) in embryoid bodies. Scale bar=500 μm. hESC: human embryonic stem cell, hiPSC: human induced pluripotent stem cell, Homo-SCNT-hESC: homozygous somatic cell nuclear transfer-hESC.
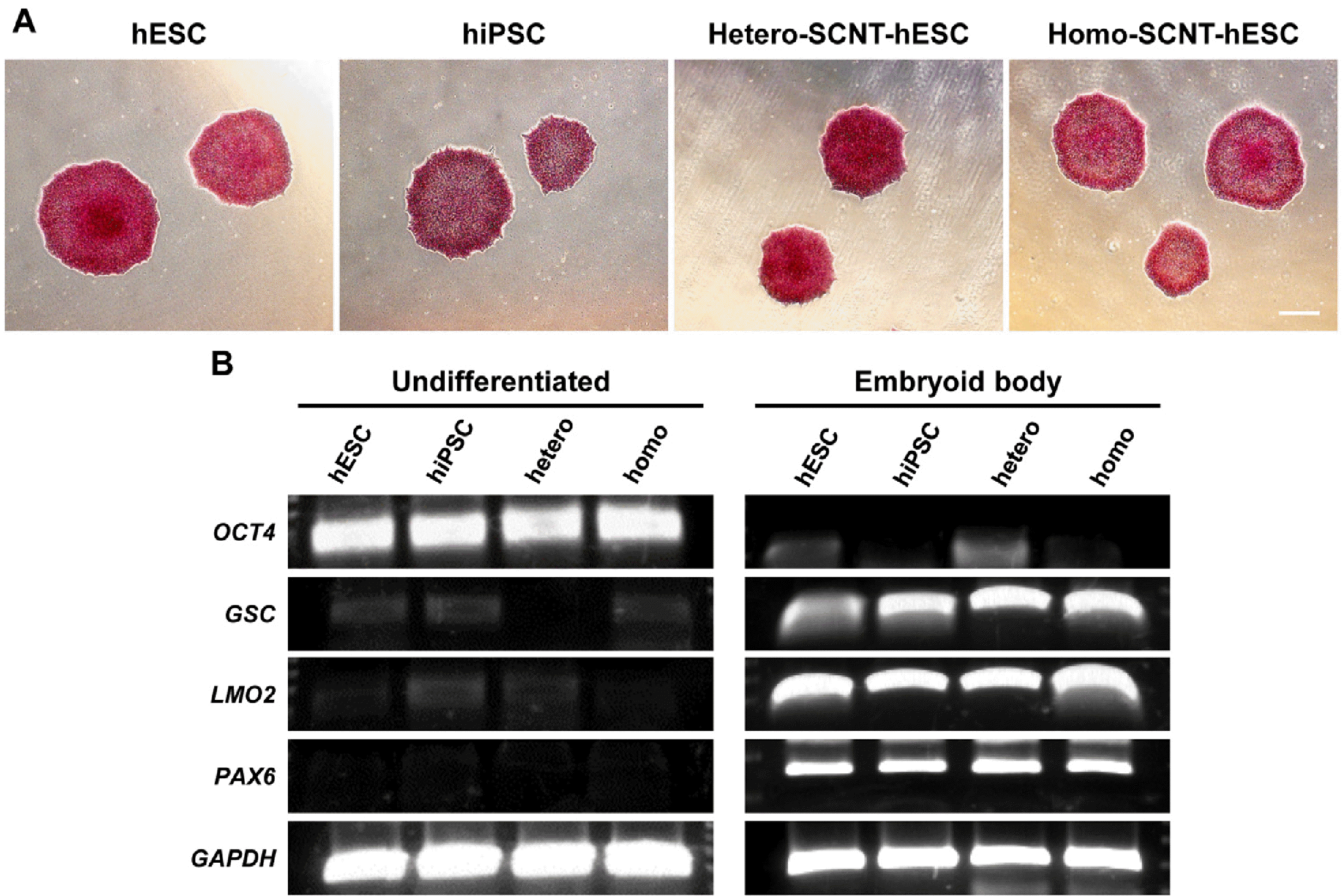
Fig. 2
Generation of neural precu-rsor cells (NPCs) from human pluripotent stem cells (hPSCs). (A) Sche-matic describing the differentiation protocol of NPCs via spherical neural mass (SNM) from hPSCs. hPSCs transitioned through stages of embryoid body (EB), neural rosette, SNMs, and NPCs. (B-E) Typical morphology of the hPSC colony. (F-I) hPSCs were indu-ced for 5 days with suspended EB. (J-M) After EB attachment, neural rosettes were induced for 5∼10 days. (N-Q) After the selection of the SNMs, they were passaged 3 times for purification. (R-U) After 3∼4 passages, the SNMs were dissociated into single cells. Scale bar=100 μm. hESC: human embryonic stem cell, hiPSC: human indu-ced pluripotent stem cell, Homo-SCNT-hESC: homozygous somatic cell nuclear transfer-hESC.
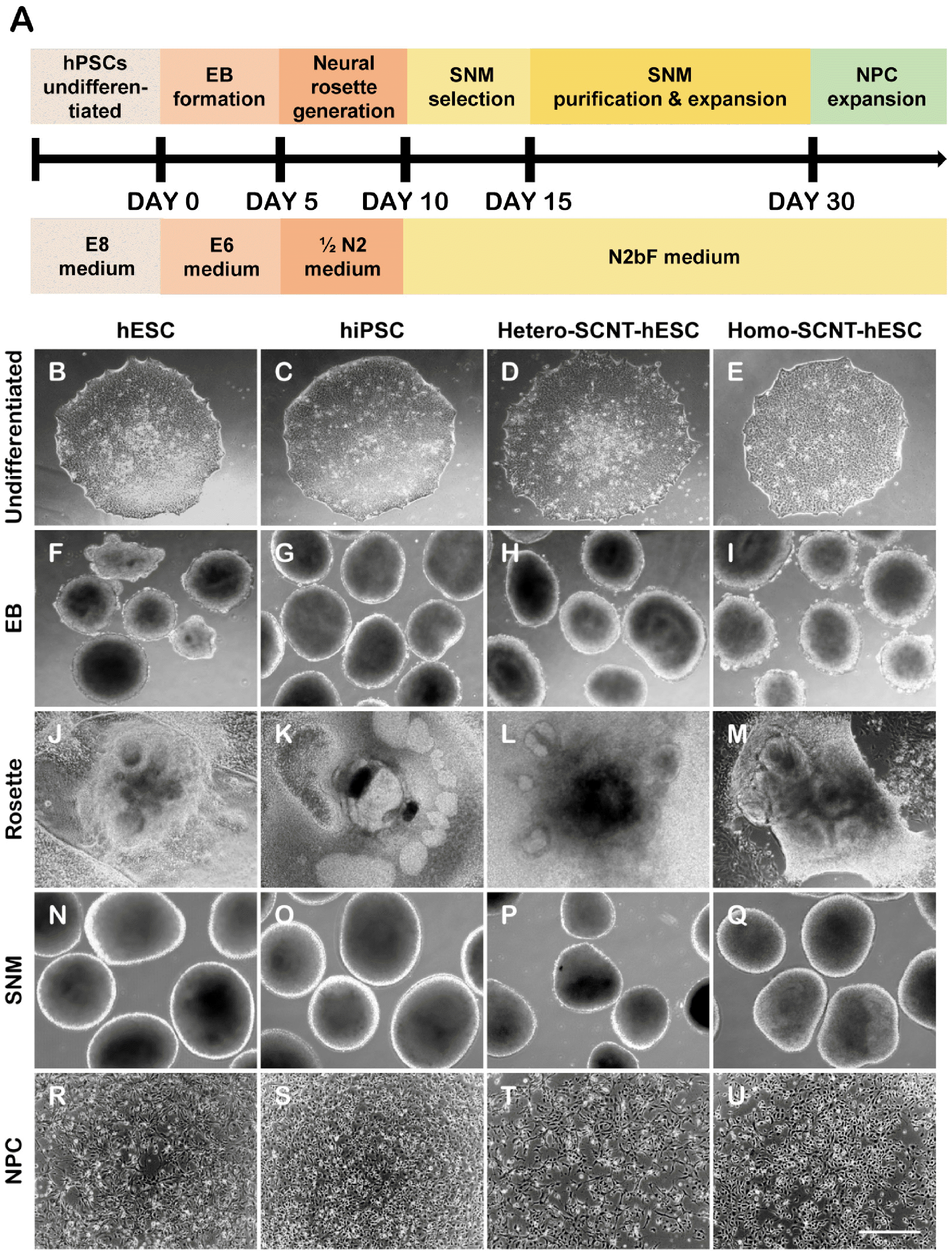
Fig. 3
Neural precursor cell specifications are similarly induced in various human pluripotent stem cells (hPSCs). (A) Neural rosettes were ide-ntified according to rosette markers such as PAX6 and ZO-1. (B) Repre-sentative images indicating the spherical neural mass markers NESTIN and SOX2. (C) After single cell dissocia-tion of neural precursor cells, the left panel also reveals the neural precu-rsor cell markers NESTIN and SOX2. The right panel presents the quantified data from the left panel. Scale bar=50 μm. hESC: human embryonic stem cell, hiPSC: human induced pluripotent stem cell, Homo-SCNT-hESC: homozygous somatic cell nuclear transfer-hESC.
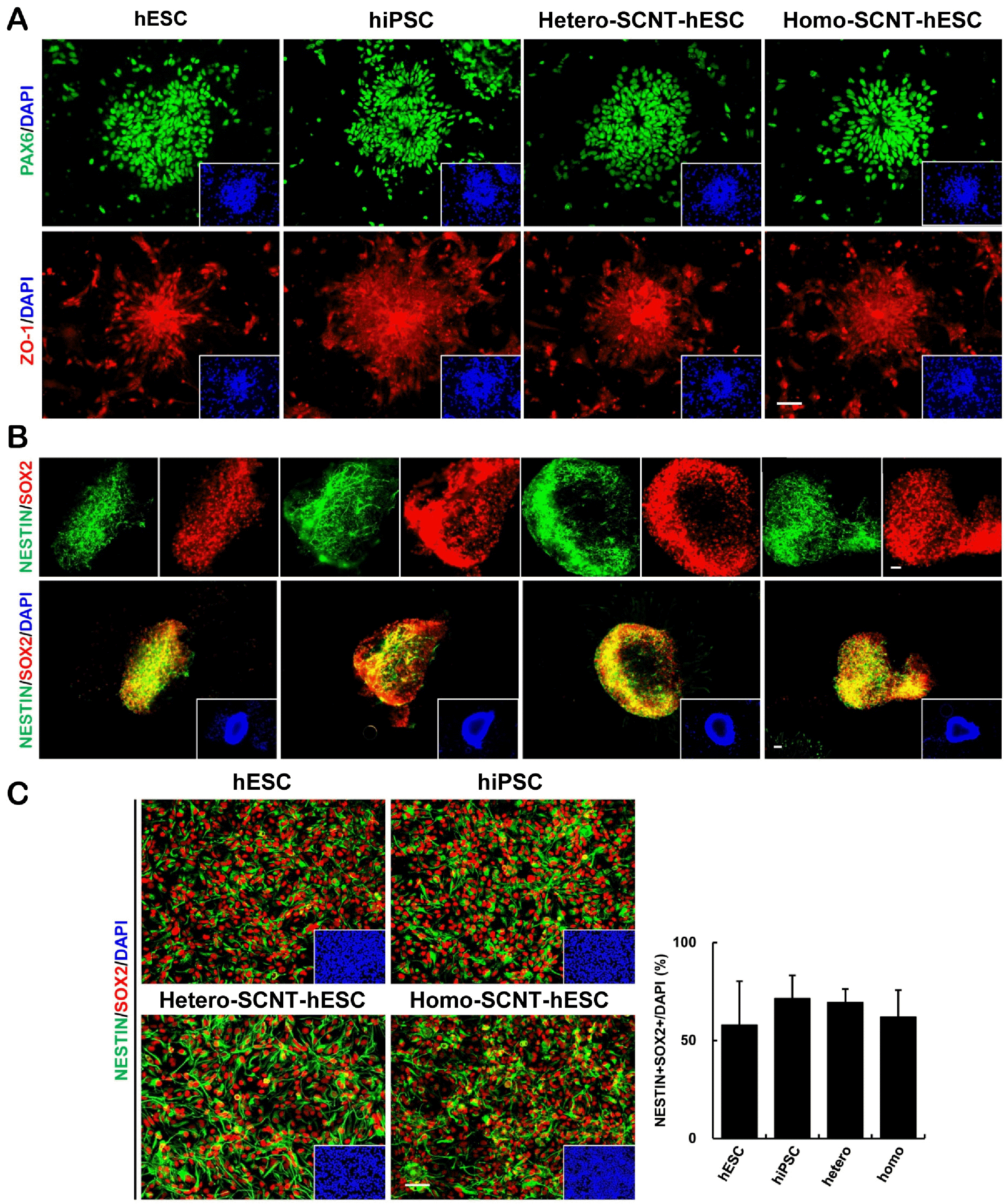
Fig. 4
In vitro differentiation potentials of human pluripotent stem cells (hPSCs)-derived neural precursor cells are similar in various hPSCs. (A) Dif-ferentiation states into typical neurons, astrocytes, oligodendrocytes were indicated by the expression markers TUJ1, GFAP, and OLIG2, respectively. (B) Representative images and quantification of individually induced marker positive cells after differentiation for 14 days. Scale bar=50 μm. hESC: human embryonic stem cell, hiPSC: human induced pluripotent stem cell, Homo-SCNT-hESC: homozygous somatic cell nuclear transfer-hESC.
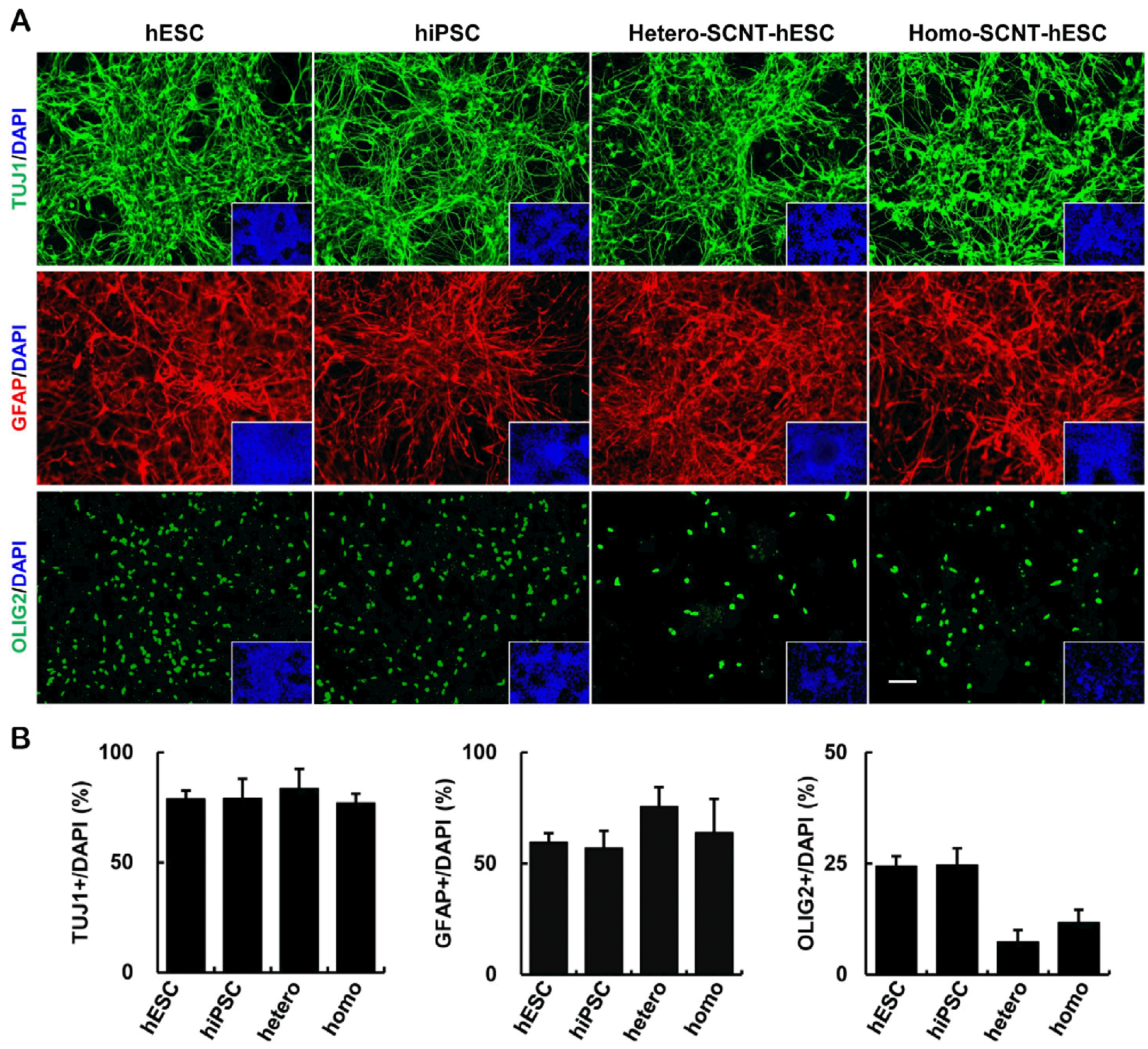
Fig. 5
Differentiation of human pluripotent stem cells-neural precursor cells (hPSCs-NPCs) into dopaminer-gic (DA) neurons. (A) Schematic procedures for the production of DA neurons. (B) Approximately one month after hPSCs-NPCs differentiation, TUJ1- and TH- positive neurons were expressed by immunocytochemical cha-racterization. (C) Quantification of the stereological count of TH-positive and TUJ1-positive cells. Scale bar=50 μm. hESC: human embryonic stem cell, hiPSC: human indu-ced pluripotent stem cell, homo-SCNT-hESC: homozygous somatic cell nuclear transfer-hESC.
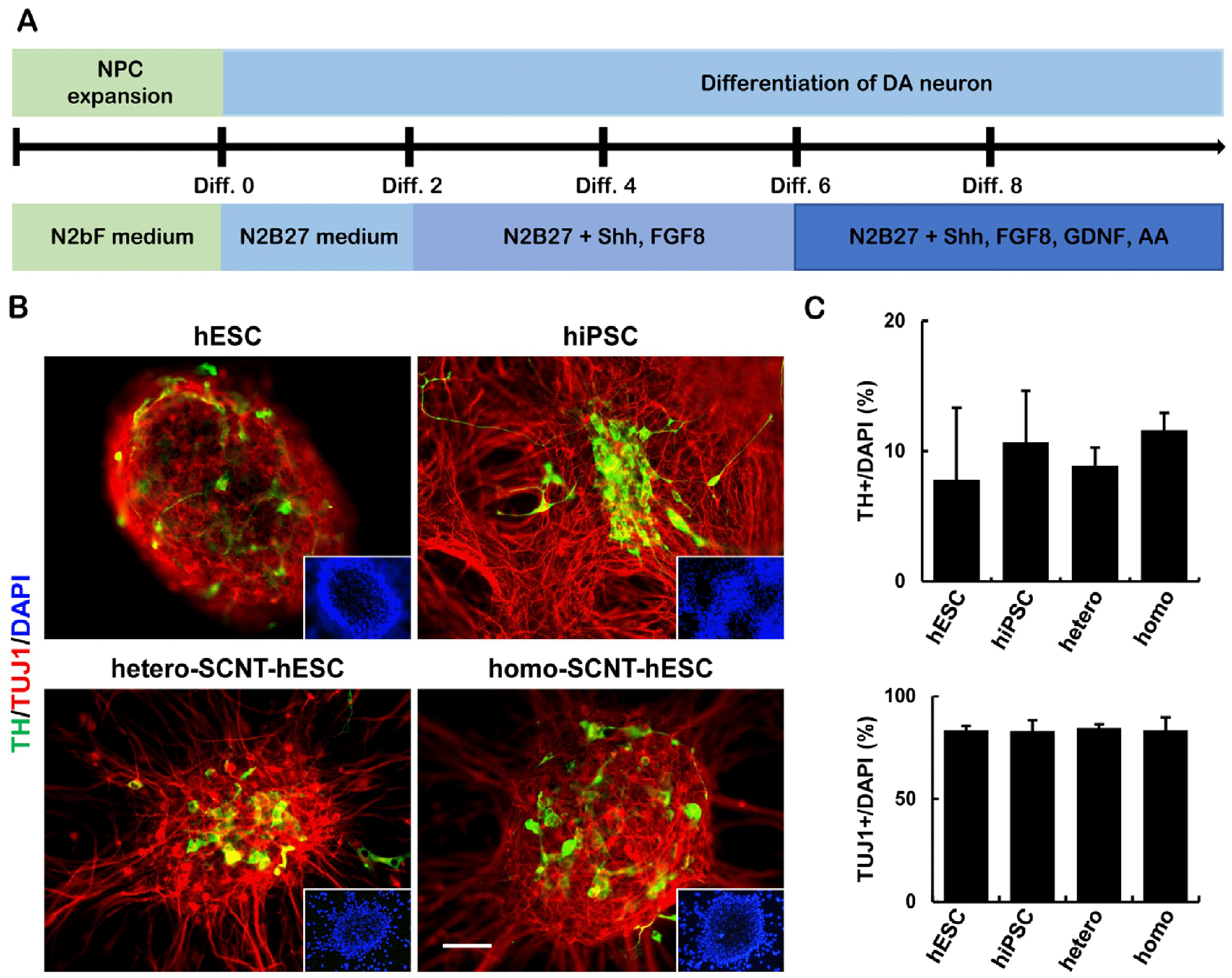
Fig. 6
Major histocompatibility complex (MHC) Class I and II expression increase as neuronal differentiation progresses. MHC Class I and II expression was not detected in undi-fferentiated human pluripotent stem cells. However, MHC Class I and II expression was induced during embryoid body (EB) formation and maintained until the completion of neuronal differentiation. And there were no differences in MHC Class I and II expression patterns between human embryonic stem cell (hESC) and somatic cell nuclear transfer (SCNT)-hESC. Scale bars=200 μm. Homo: homozygous, SNM: spherical neural mass.
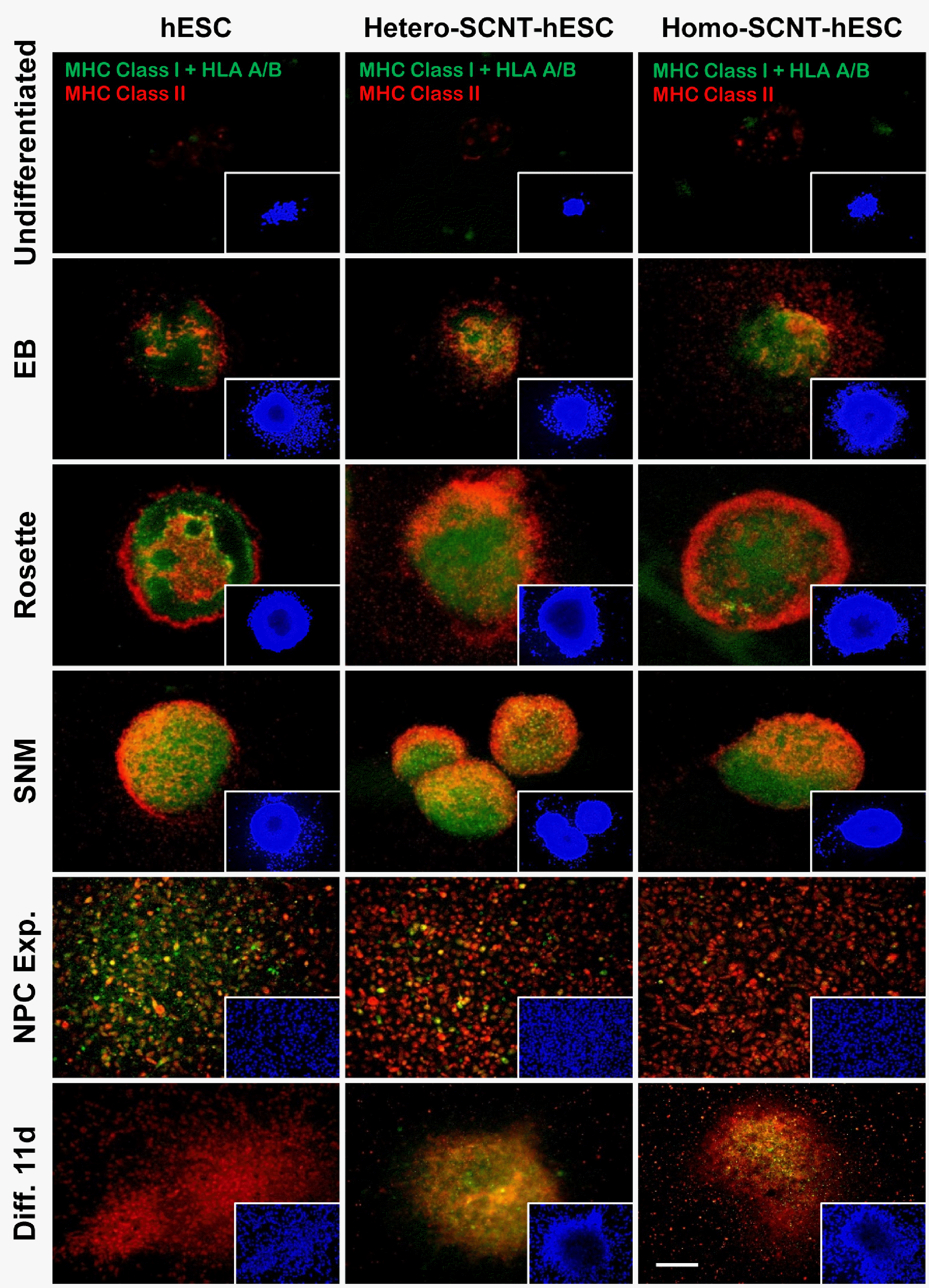
Table 1
Primer information for RT-PCR




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download