Abstract
Hepatic hydrothorax is a pleural effusion (typically ≥500 mL) that develops in patients with cirrhosis and/or portal hypertension in the absence of other causes. In most cases, hepatic hydrothorax is seen in patients with ascites. However, ascites is not always found at diagnosis and is not clinically detected in 20% of patients with hepatic hydrothorax. Some patients have no symptoms and incidental findings on radiologic examination lead to the diagnosis of the condition. In the majority of cases, the patients present with symptoms such as dyspnea at rest, cough, nausea, and pleuritic chest pain. The diagnosis of hepatic hydrothorax is based on clinical manifestations, radiological features, and thoracocentesis to exclude other etiologies such as infection (parapneumonic effusion, tuberculosis), malignancy (lymphoma, adenocarcinoma) and chylothorax. The management strategy involves a stepwise approach of one or more of the following: Reducing ascitic fluid production, preventing fluid transfer to the pleural space, fluid drainage from the pleural cavity, pleurodesis (obliteration of the pleural cavity), and liver transplantation. The complications of hepatic hydrothorax are associated with significant morbidity and mortality. The complication that causes the highest morbidity and mortality is spontaneous bacterial empyema (also called spontaneous bacterial pleuritis).
Hepatic hydrothorax is defined as the accumulation of transudative pleural effusion (generally ≥500 mL) in patients with chronic liver disease and portal hypertension, after excluding other etiologies such as cardiac, pulmonary, renal disorders and so on.1,2
Hepatic hydrothorax is an important pulmonary complication in decompensated hepatic cirrhosis, in addition to hepatopulmonary syndrome and portopulmonary hypertension. The prevalence of hepatic hydrothorax is 5-15% in patients with decompensated hepatic cirrhosis and it contributes to 2-3% of the incidence of pleural effusion overall. Hepatic cirrhosis, a chronic liver disease, is the 11th leading cause of death worldwide, contributing to 2.4% of the total deaths worldwide. The data from the Global Burden of Disease Study (GBD 2017) showed that there were 10.6 million cases of decompensated hepatic cirrhosis, with an estimated 1.5 million cases of hepatic hydrothorax worldwide.3
Pleural effusion associated with liver disease was first described by Laennec in the nineteenth century, and Morrow et al.4 in 1958 were the first to describe a massive right pleural accumulation following cirrhosis. In the majority of cases, hepatic hydrothorax occurs in patients with ascites. However, ascites is not always present at diagnosis and is not clinically detected in 20% of patients with hepatic hydrothorax.4,5 The management of hepatic hydrothorax is complex and requires a multidisciplinary approach. In this article, we aim to comprehensively review the management of hepatic hydrothorax based on recent research.
The pleural cavity is the space between the internal wall of the thorax and the lung. It is lined by a mesothelial fibrous membrane consisting of parietal and visceral layers. Under normal conditions, the pleural cavity contains a small amount of fluid that serves as a lubricant between the parietal and visceral pleural surfaces. The pleural fluid also plays a role in maintaining a negative pressure between the lung and the lung cavity, allowing effective inhalation and preventing lung collapse. The pleural fluid originates from capillaries on the surface of the parietal pleura that move based on Starling's force and is reabsorbed by the lymphatic stomata on the surface of the parietal pleura, especially in the mediastinal region and diaphragm to maintain pleural fluid volume within the normal range of 0.1-0.3 mL/kg.6 Fluid can also enter the pleural cavity from the pulmonary interstitium through the visceral pleura or from the peritoneal cavity through small holes in the diaphragm at the rate of 0.5 mL/hour.7
Excessive accumulation of fluid in the pleural cavum may occur due to increased production or decreased absorption, or both. In pleural effusions that result from increased hydrostatic pressure or decreased oncotic pressure in the capillaries (e.g., in hypoalbuminemia), transudative pleural fluid is usually present, while increased capillary permeability (generally due to inflammation) or impaired lymphatic drainage generally results in an exudate. Reabsorption rates may increase up to 40-fold as a physiologic response to additional pleural fluid before fluid begins to accumulate in the pleural cavity.7,8
Hepatic hydrothorax occurs due to the direct transfer of fluid from the peritoneal cavity to the pleural cavity through a diaphragmatic defect. These diaphragmatic defects are usually <1 cm in size and commonly occur on the right side (85%), with 13% occurring on the left side and 2% occurring bilaterally. During embryological development, the left side of the diaphragm is more muscular, and the right side is more tendinous due to its proximity to the liver. Hence, embryological defects of the diaphragm are generally more common in the right hemidiaphragm. Diaphragmatic defects associated with hepatic hydrothorax can be classified into 4 forms based on their morphology: Type 1, no obvious defect (9.1-31.7%); Type 2, a small blister on the diaphragm (36.4-41.3%); Type 3, a fenestrated defect on the diaphragm (20.6-72.7%); and Type 4, multiple slits on the diaphragm (1.6-9.1%). The presence of these defects can be confirmed by various modalities, including direct visualization during surgery, doppler ultrasonography, magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT)-CT, and scintigraphy.9-11
Diaphragmatic defects occur in up to 20% of the normal population, but in patients with ascites, the increased abdominal pressure and thinning of the diaphragm due to malnutrition may enlarge the defect. Fluid movement from the abdominal cavity to the pleura is typically one-way, driven by a permanent pressure gradient resulting from intrathoracic negative pressure during the respiratory cycle and intra-abdominal positive pressure. While hepatic hydrothorax usually occurs in patients with ascites, in a minority of cases, it can also develop in patients without ascites. This most commonly occurs in patients with large diaphragmatic defects where ascites may go undetected on physical examination despite the presence of a sizeable pleural effusion.12
Increased abdominal pressure due to ascites and a thinning diaphragm as a result of malnutrition increases the risk of rupture, allowing fluid flow into the pleural compartment, further facilitated by intrathoracic negative pressure. Hypoalbumin and lymphatic duct leakage, as well as cardiac disorders such as diastolic dysfunction and left atrial enlargement, may also be factors contributing to pleural fluid accumulation.2,8
The respiratory symptoms of hepatic hydrothorax vary, ranging from cough, pleuritic chest pain, and tightness, to life-threatening respiratory distress. Most effusions are mild to moderate, and only 6% have massive effusions that fill more than half of the hemithorax. Some patients had no symptoms and were incidental findings on radiologic examination, but the majority of cases had symptoms: dyspnea at rest (34%), cough (22%), nausea (11%), and pleuritic chest pain (8%). The severity of symptoms is influenced by the volume of the effusion, the rate of fluid accumulation, and the concomitant presence of other cardiopulmonary disorders. Patients with hepatic hydrothorax will experience dyspnea and/or hypoxia when 1-2 liters of fluid accumulate in the pleural cavity. Hemodynamic disturbances may occur when there is a rapid accumulation of effusion fluid.2,10
Patients with hepatic hydrothorax usually also present with other signs of cirrhosis and portal hypertension, such as ascites, spider naevi, asterixis, hepatosplenomegaly, caput medusa, and hepatic encephalopathy. Under certain conditions, patients with hepatic hydrothorax may also develop complications such as acute tension hydrothorax, which cause respiratory distress that can be life-threatening. This could occur when the defective diaphragm ruptures suddenly as a result of increased intra-abdominal pressure, cardiac tamponade, which may occur due to massive effusion. In patients with hepatic hydrothorax with signs and symptoms of fever, pleuritic chest pain, or encephalopathy, it is necessary to consider the possibility of a cavum pleura infection, such as spontaneous bacterial empyema (SBEM) (also called spontaneous bacterial pleuritis [SBPL]). SBEM or SBPL is a complication that can occur due to the migration of a bacterial infection in the peritoneum to the cavum pleura or a bacterial invasion through pleural chest tubes, catheters, or other instruments.9
Hepatic hydrothorax is a diagnosis of exclusion. Therefore, other etiologies of pleural effusion, such as cardiac, pulmonary, pleural disorders, and malignancy, must be ruled out first. Supportive examinations, such as echocardiography and brain natriuretic peptide (BNP) blood tests, can be performed to evaluate cardiac function. CT of the thorax can exclude other causes, such as mediastinal malignancy, and lung or pleural lesions, and abdominal ultrasound can be performed to evaluate the hepatic mass, ascites, and hepatic and portal venous flow. In patients with massive pleural effusions, radiologic examinations should be repeated after diuretic therapy or therapeutic thoracocentesis to assess for pulmonary or pleural disorders covered by the effusion.4,9
The diagnosis of hepatic hydrothorax is based on clinical manifestations, radiologic features, and thoracocentesis to exclude other etiologies such as infection (spontaneous bacterial peritonitis [SBP], tuberculosis), malignancy (lymphoma, adenocarcinoma) and chylothorax.2 Chylothorax can be easily distinguished from hepatic hydrothorax by its milky white appearance and high serum triglyceride content (>110 mg/dL).13
Pleural fluid obtained through thoracocentesis needs to be evaluated for differential cell count, Gram staining, bacterial culture, presence of protein, albumin, lactate dehydrogenase (LDH), fluid pH, and bilirubin concentration.9 The results of the pleural fluid analysis usually show a transudative effusion with Light's criteria. However, in some cases, there can be misclassification in the analysis wherein the results are termed exudative in patients with hepatic hydrothorax. Such cases can occur when the patient is on diuretic therapy, where there is a contraction of extracellular fluid volume and an increase in the pleural fluid protein and LDH concentrations. In such cases, it is necessary to calculate the serum-pleural fluid albumin gradient (SPAG) which is analogous to the serum- ascites albumin gradient (SAAG), with values >1.1 g/dL indicating a transudative process. In addition to using SPAG, ratio need not unite such as <0.6 g/dL may also be a more accurate method to determine transudative effusion.13,14
Other findings usually observed in hepatic hydrothorax include alkaline pH, glucose levels similar to serum glucose, polymorphonuclear (PMN) cells <250 cells/mm3, and total protein <2.5 g/dL. SBPL is generally diagnosed if the pleural PMN cells are >500 cells/mm3, or >250 cells/mm3 with a positive culture, after excluding parapneumonic effusion or empyema. Hepatic hydrothorax is diagnosed using international criteria as follows: 1) Gradient of serum albumin to pleural fluid >1.1; 2) Total protein in the pleural fluid <2.5 g/dL or pleural fluid/serum total protein ratio <0.5; 3) Pleural fluid/serum LDH ratio <0.6; and 4) Polymorphonuclear cell count <250 cells/mm3 (Table 1).15
While the etiology of hepatic hydrothorax remains uncertain, other tests to analyze the pleural fluid such as PCR for mycobacterium, amylase for pancreatic pleural effusion, cytology to rule out malignancy, and evaluation of triglyceride levels to assess chylothorax may be considered. Hepatic hydrothorax typically presents as a right-sided pleural effusion and diagnosis is easy in most cases.16 When left-sided pleural effusion or hepatic hydrothorax without ascites occurs, scintigraphy may be an option to confirm the relationship between the pleural and peritoneal cavities. Hepatic hydrothorax can also be diagnosed through direct visualization of the diaphragmatic defect with video-associated thoracic surgery (VATS), although this procedure is invasive and should only be performed if the diagnosis remains unclear or if there is a plan to repair the defect.9
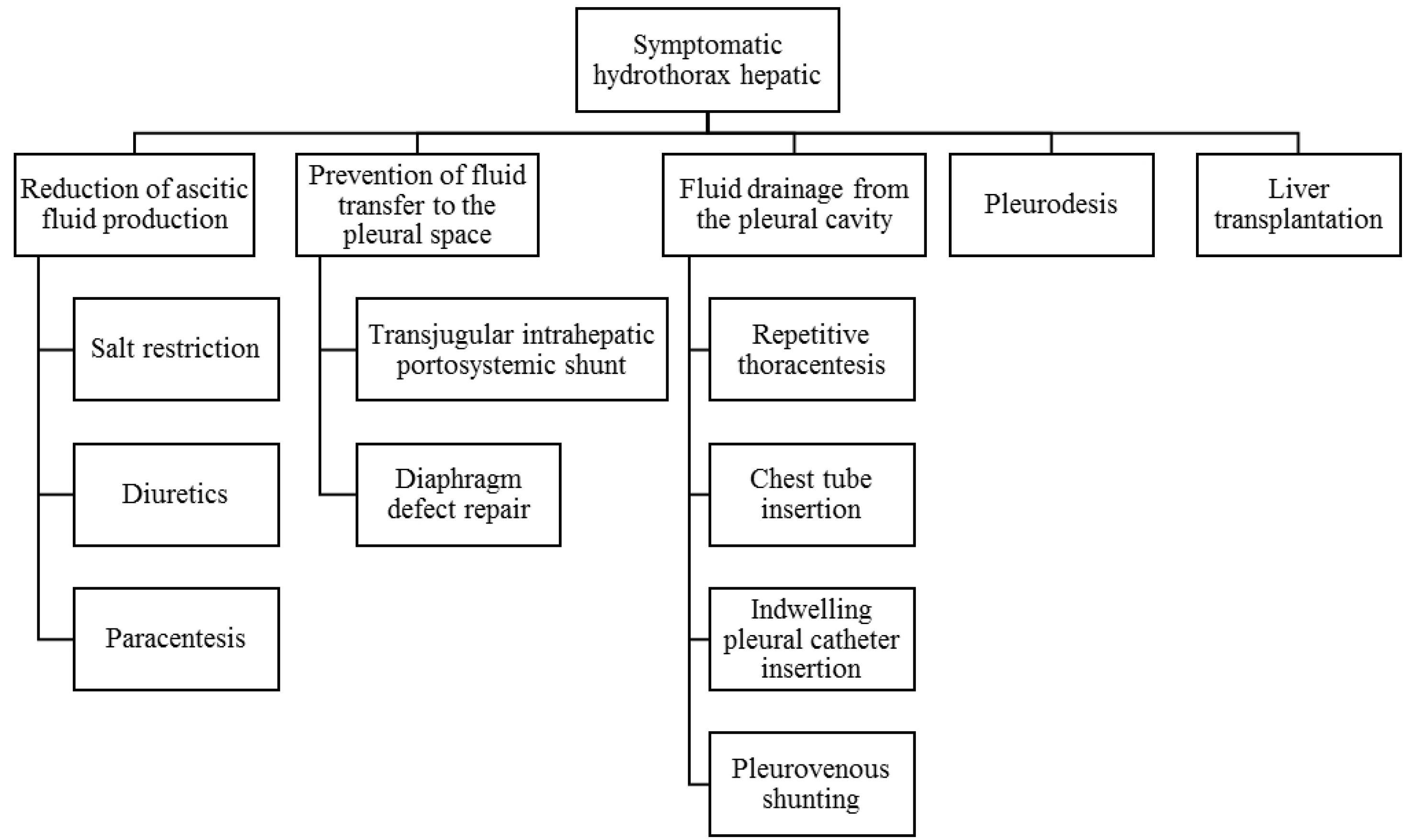
The management of hepatic hydrothorax is complex, involving a multidisciplinary approach. The management strategy includes a stepwise approach of one or more of the following five methods: 1) Reducing ascitic fluid production; 2) Preventing fluid transfer to the pleural space; 3) Fluid drainage from the pleural cavity; 4) Pleurodesis (obliteration of the pleural cavity); 5) Liver transplantation.9
The first line of treatment for hepatic hydrothorax is a salt-restricted diet.17 Salt consumption in patients with hepatic hydrothorax should be limited to less than 2 g/day (<88 mEq). Reduction of salt intake by 10-20%, especially in first-time ascites patients, may lead to a reduction in ascites.18 Stricter salt restriction may speed up ascites healing but is not recommended as it may lead to malnutrition.19 Salt restriction should be customized according to each patient's condition.20
A low-salt diet is generally not sufficient to reduce hepatic hydrothorax and prevent fluid reaccumulation. Therefore, a combination of loop diuretics and aldosterone receptor antagonists is required. Administration of furosemide and spironolactone can be considered as an option with a maximum weight loss target of 0.5 kg/day in ascites patients without peripheral edema and 1 kg/day in patients with peripheral edema. Diuretic therapy is said to be effective if there is a weight loss of at least 2 kg/week. Spironolactone and furosemide can be started at a dose of 100:40 mg/day and titrated as needed.
Body weight, blood pressure, orthostatic symptoms, serum electrolytes, and renal function need to be evaluated in patients on diuretic therapy. If weight loss is inadequate, the urinary sodium/potassium ratio can be assessed, where a ratio of >=1 indicates that the patient has received adequate diuretic treatment but may consume salt beyond the recommended level. In such cases, the level of salt restriction (<2 g/day) needs to be improved. In patients, an inadequate weight loss and urine sodium/potassium ratio <1 indicate inadequate diuretic therapy. The dose of the diuretics needs to be increased with a maximum of 160 mg furosemide and 400 mg spironolactone given daily with a titration interval of 1-2 weeks until a stable and effective regimen can be achieved.2,9,21
Approximately 20-30% of cirrhotic patients with hepatic hydrothorax develop persistent or recurrent pleural effusions despite salt restrictions and diuretic administration. This condition is referred to as diuretic-resistant hepatic hydrothorax. In certain cases, aggressive diuresis is not feasible due to electrolyte disturbances, renal insufficiency, hemodynamic instability, or precipitation of hepatic encephalopathy. This condition is known as diuretic-intractable hepatic hydrothorax. Both diuretic-resistant and diuretic-intractable hepatic hydrothorax require the consideration of other therapeutic modalities. There is also the term “refractory hepatic hydrothorax” which is defined as pleural effusion with the following criteria: 1) Inadequate response to salt restriction and the maximal dose of diuretics (spironolactone 400 mg/day and furosemide 160 mg/day), and serial thoracocentesis >2 times; or 2) Rapid recurrence after therapeutic thoracocentesis.15
Other medical therapies that can be given are splanchnic and peripheral vasoconstrictors. The vasoconstrictors of choice include octreotide, midodrine, and terlipressin, which increase arterial volume and decrease the activation of the renin-angiotensin-aldosterone system (RAAS), thereby increasing renal sodium excretion. Octreotide is a somatostatin analog that directly inhibits the RAAS axis, increasing renal plasma flow and natriuresis. In patients with renal failure, a single dose of octreotide may cause adverse renal effects. Therefore, a combination with the alpha-adrenergic agonist midodrine is recommended. In addition, octreotide administration may cause pulmonary and cardiac side effects and also increase the risk of tachyphylaxis.9
Terlipressin is a vasopressin analog that is also used in the management of hepatorenal syndrome (HRS). In patients with hepatitis B- and D-related cirrhosis with hepatic hydrothorax and HRS refractory to thoracentesis and octreotide, terlipressin and albumin may show improvement in signs and symptoms of HRS and hepatic hydrothorax.9
In hepatic hydrothorax patients with permagna ascites, the large-volume paracentesis (LVP) procedure and albumin infusion to prevent associated circulatory disorders (8 g for every liter of ascites fluid removed) showed significant improvement in lung capacity. The ratio of partial pressure of oxygen in arterial blood (PaO2) to the fraction of inspiratory oxygen concentration (FiO2), (P/F) ratio and symptoms of hepatic hydrothorax improved within 2 hours after LVP. The LVP procedure can also alleviate respiratory distress in hepatic hydrothorax patients with ascites and is performed before thoracocentesis. There was a significant increase in total lung capacity and improvement in symptoms within 2 hours after an average of 3.5 liters of ascites fluid was removed by paracentesis.21,22 Paracentesis should be performed in every patient with new-onset ascites as it helps determine the etiology and allows early diagnosis of complications such as SBP.23
Fluid accumulation in portal hypertension occurs when the portosystemic gradient (the difference in pressure between the portal vein and the right hepatic vein or inferior vena cava) is increased, generally greater than 12 mmHg (normal ≤5 mmHg). The TIPS reduces portal hypertension by lowering the portosystemic gradient. Although TIPS does not address the cause of portal hypertension, decreasing the portosystemic gradient can address various complications of portal hypertension, including acute and recurrent variceal bleeding, refractory ascites, and refractory hepatic hydrothorax.2
Prior to performing TIPS, comprehensive investigations need to be conducted. This includes laboratory investigations (hepatic and renal profile, electrolytes, coagulation), and calculation of the Model for End-stage Liver Disease (MELD) score, to quantify the severity of the liver disease. The MELD score predicts the 3-month mortality risk in patients with hepatic hydrothorax and is also used in patients requiring hepatic transplantation. It is also necessary to evaluate the cardiac function with an echocardiogram and radiological examination to assess hepatic parenchyma and vascularization. Contraindications for TIPS include the following: Bacteremia, heart failure, severe tricuspid regurgitation, pulmonary hypertension, unresolved biliary obstruction, uncontrolled HE, uncorrected coagulopathy, portal vein thrombosis, hepatic mass, and also patients with a MELD score >18. Complications of TIPS include hepatic encephalopathy, worsening of hepatic function, hepatic ischemia, and shunt stenosis.2
Surgical closure of the diaphragmatic defect prevents fluid flow into the pleural cavity from the abdomen but has a high mortality rate in patients with decompensated cirrhosis (Child-Turcotte-Pugh [CTP] class C). Surgery may be considered in patients with refractory hepatic hydrothorax who are not TIPS candidates but have low MELD scores. This can be done via video-assisted thoracoscopy (VATS) or open thoracotomy with or without a mesh, as well as laparoscopic surgery. The type of diaphragm defect influences the type of surgery chosen, e.g., type 1 defects are mesh-only, while types 2, 3, 4 are mesh-only with or without suturing.9
When medical therapy has not been able to control symptoms, or when renal complications occur due to such therapy, then therapeutic thoracocentesis needs to be performed. Thoracocentesis is performed to relieve respiratory distress or hypoxia. It is recommended that thoracocentesis should not exceed 2 liters in one collection because there is a risk of re-expansion pulmonary edema due to the removal of large amounts of fluid. The intravenous injection of albumin to prevent circulatory dysfunction is generally not required due to the relatively small volume of fluid withdrawn.22
In approximately 27% of cases, the management of hepatic hydrothorax becomes more complex as repeated thoracocentesis is required. In addition, in refractory hepatic hydrothorax, patients who are not candidates for TIPS or transplantation, or in those awaiting transplantation, repeat thoracentesis is the management of choice to manage symptoms. Complications of hemothorax may occur especially in patients with coagulopathic disorders with a PT or aPTT more than double the normal limit, thrombocytopenia (<50,000/mm3), serum creatinine >6 mg/dL, and a high MELD score.2,4,9
Chest tube insertion has a very high complication rate of 88%, including infection, renal failure, and electrolyte disturbances with a 33% mortality rate due to empyema and sepsis. Patients who have a chest tube inserted have twice the risk of an increased length of stay and mortality compared to patients who undergo thoracocentesis. Therefore, chest tube insertion is generally contraindicated in patients with hepatic hydrothorax. However, chest tube insertion can be performed in the management of infected pleural fluid.9 Chest tube placement should be avoided while other invasive procedures may be considered on a case-by-case basis.9
IPC insertion allows intermittent drainage and facilitates pleurodesis. This procedure can be used as a management option for symptomatic malignant pleural effusions and can be a bridging therapy for liver transplantation. Insertion of an IPC allows intermittent drainage at home and can be considered in recurrent hepatic hydrothorax. It can also be chosen as a bridge to transplantation or as a palliative procedure option in patients who cannot undergo liver transplantation. However, the IPC has a risk of complications such as pleural infection, empyema, pneumothorax, and catheter malfunction.9,24
A pleurovenous shunt is an indwelling catheter that drains fluid directly from the pleural cavity to the systemic venous circulation. PVS became attractive for treating hepatic hydrothorax as none of the patients required further treatment during a mean follow-up of 13.3 months. However, complications such as shunt failure, pulmonary edema, post-shunt coagulopathy, deep vein thrombosis, infection, and catheter insertion site leakage have been reported and long-term outcomes are not well known. In addition, PVS cannot be performed in cases complicated by infection such as empyema or recurrent SBP.25
A peritoneovesical shunt procedure can also be performed where the device is placed subcutaneously and works to pump excess peritoneal fluid to the bladder where it is eliminated through the urine. The removal of ascites is expected to reduce the formation of hepatic hydrothorax. Both the pleurovenous shunt and peritoneovesical shunt are still not widely used because they are still at the research stage.6,9
Pleurodesis is a procedure that aims to eliminate the space between the parietal and visceral pleura by using chemical irritants or mechanical abrasion to induce fibrosis to prevent recurrent effusion. Pleurodesis can be considered in patients who are not candidates for TIPS or in hepatic hydrothorax patients who are refractory to TIPS and can be a bridging therapy for liver transplantation. Chemical pleurodesis agents that can be used include talcum, tetracycline, doxycycline, bleomycin, povidone-iodine, and pisibanil (OK-432) with or without minocycline.21
The primary therapy for hepatic hydrothorax is sodium restriction and diuretics, but when medical therapy fails, the only definitive treatment is liver transplantation.10 Liver transplantation is the definitive therapy for refractory hepatic hydrothorax. Patients with diuretic-resistant, and diuretic-intractable hepatic hydrothorax, history of SBPL, or a high MELD score (>15) need to be referred for evaluation for liver transplantation.9 Mortality from hepatic hydrothorax is independent of age, gender, MELD-sodium (Na) score, renal function, hepatocellular carcinoma (HCC) status, and the occurrence of bleeding varices. Therefore, early liver transplantation should be considered.26 However, according to Ma et al.27 in 2022, in a group of hepatic hydrothorax patients not given liver transplantation, those of the male sex and having hepatic encephalopathy had a higher risk of death.27
While liver transplantation is the best treatment option, most patients are not eligible, and a large proportion of those who are eligible die while waiting for a transplant.6 In difficult cases, the only recourse is surgical treatment targeting the primary closure of the diaphragmatic defect (Fig. 1).25
SBEM or SBPL is a complication of hepatic hydrothorax associated with high morbidity and mortality, with mortality rates estimated at 20%.28 SBEM is an infection of the pleural effusion in patients with cirrhosis in the absence of underlying pneumonia. SBEM and empyema secondary to pneumonia need to be distinguished because the pathogenesis and treatment for the two conditions are very different, where SBEM can be compared to SBP. However, in a study, as many as 40% of SBEM cases were not associated with SBP and although rare, SBEM can occur in patients with hepatic hydrothorax without clinically detectable ascites.4
SBEM should be suspected in patients with hepatic hydrothorax who present with fever, pleuritic pain, encephalopathy, and/or decreased renal function. Although the mechanism of SBEM is still unclear, it is believed that the process is like SBP, which results from the direct spread of bacteria from the peritoneal cavity. However, transient bacteremia infecting the pleural cavity is also thought to be the mechanism of SBEM, especially in hepatic hydrothorax patients who do not have SBP. The bacteria causing SBEM are usually similar to those causing SBP, namely Escherichia coli, Streptococcus spp, Enterococcus, and Klebsiella, although in certain conditions it can also be caused by uncommon organisms such as Aeromonas veronii.
The risk factors for SBEM include high Child-Pugh and MELD scores, SBP, decreased serum albumin, and low pleural fluid total protein values (<1.5 g/dL). The diagnosis of SBEM is based on Lazaridis et al.'s (2004)29 diagnostic criteria, namely, 1) Positive pleural fluid culture with PMN cells >250 cells/mm3 or PMN cells >500 cells/mm3 regardless of culture results; 2) Pleural effusion with a SPAG value >1.1 g/dL; 3) Absence of pneumonia or infectious process seen on radiologic examination.4
The treatment for SBEM is similar to that for SBP. Third-generation cephalosporins (e.g., ceftriaxone and cefotaxime) are the antibiotics of choice in SBEM. Cefotaxime 2 g every 8 hours intravenously has shown good results in patients with cirrhosis. Carbapenems can be used in patients with nosocomial infections and those at high risk of infections by extended- spectrum beta-lactamase (ESBL) producing pathogens, diabetes, and patients who have received cephalosporin therapy in the last 3 months. Therapy should be given as soon as the diagnosis is made and continued for 7-10 days. In patients with slow clinical improvement, repeat thoracocentesis may be performed to assess the response to antibiotics.
Chest tube insertion should be avoided in SBEM, except in cases where there is pus in the pleural cavity. A worse prognosis can be expected in patients with SBEM characterized by a high MELD score, requiring treatment in the intensive care unit (ICU), and failure to respond to initial antibiotic therapy. Repeated episodes of SBEM are one of the indications for liver transplant evaluation in hepatic cirrhosis patients with hepatic hydrothorax.4,30
Acute tension hydrothorax is a life-threatening complication of hepatic hydrothorax. In this condition, mediastinal compression and cardiopulmonary collapse occur, which can cause shock in patients. The presence of massive quantities of pleural fluids triggers an increase in intra-thoracic pressure and causes a decrease in diastolic filling and cardiac output. Although rare, spontaneous diaphragm rupture can occur in patients with hepatic cirrhosis, causing acute tension hepatic hydrothorax. Spontaneous diaphragm rupture can occur due to increased abdominal pressure triggered by various factors, such as prolonged cough or certain body positions. Diaphragm muscle weakness due to malnutrition or certain genetic conditions can also be a predisposing factor for diaphragm rupture which can trigger acute tension hydrothorax.31
Hepatic hydrothorax is a complication of liver cirrhosis that requires special attention. Hepatic hydrothorax can present with symptoms that vary from being asymptomatic to cough, chest tightness, pleuritic chest pain, and life-threatening respiratory failure. In addition to clinical manifestations and radiological examination, pleural fluid analysis is required to determine the diagnosis and assess the complications of hepatic hydrothorax. The management of hepatic hydrothorax is quite complex and requires a multidisciplinary approach considering the clinical condition of the patient, the risk of complications that may occur, as well as the availability of health personnel and facilities. Proper diagnosis and therapy are the keys to the successful management of hepatic hydrothorax.
ACKNOWLEDGEMENTS
The author thanks the Faculty of Medicine, Universitas Airlangga and Dr. Soetomo General Academic Hospital.
REFERENCES
1. Badillo R, Rockey DC. 2014; Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore). 93:135–142. DOI: 10.1097/MD.0000000000000025. PMID: 24797168. PMCID: PMC4632908.
2. Chaaban T, Kanj N, Bou Akl I. 2019; Hepatic hydrothorax: an updated review on a challenging disease. Lung. 197:399–405. DOI: 10.1007/s00408-019-00231-6. PMID: 31129701.

3. GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:245–266. DOI: 10.1016/S2468-1253(19)30349-8. PMID: 31981519.
4. Al-Zoubi RK, Abu Ghanimeh M, Gohar A, Salzman GA, Yousef O. 2016; Hepatic hydrothorax: clinical review and update on consensus guidelines. Hosp Pract (1995). 44:213–223. DOI: 10.1080/21548331.2016.1227685. PMID: 27580053.

5. Morrow CS, Kantor M, Armen RN. 1958; Hepatic hydrothorax. Ann Intern Med. 49:193–203. DOI: 10.7326/0003-4819-49-1-193. PMID: 13545745.

6. Singh A, Bajwa A, Shujaat A. 2013; Evidence-based review of the management of hepatic hydrothorax. Respiration. 86:155–173. DOI: 10.1159/000346996. PMID: 23571767.

7. Lechner AJ, Matuschak GM, Brink DS. 2012. Respiratory: an integrated approach to disease. McGraw Hill;New York:
8. Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. 2018. Principles of Internal Medicine. 20th ed. McGraw Hill;New York:
9. Banini BA, Alwatari Y, Stovall M, et al. Multidisciplinary management of hepatic hydrothorax in 2020: an evidence-based review and guidance. Hepatology. 2020; 72:1851–1863. DOI: 10.1002/hep.31434. PMID: 32585037.

10. Lv Y, Han G, Fan D. 2018; Hepatic Hydrothorax. Ann Hepatol. 17:33–46. DOI: 10.5604/01.3001.0010.7533. PMID: 29311408.

11. Tsao GG. Dooley JS, Lok AS, Garcia-Tsao G, Pinzani M, editors. 2018. Chapter 9 Ascites Mechanisms of ascites formation. Sherlock's Diseases of the Liver and Biliary System. 13th ed. Wiley-Blackwell;New York: p. 127–150. DOI: 10.1002/9781119237662.ch9.
12. Feldmen M, Friedman LS, Brandt LJ. 2010. Sleisenger and Fordtran's Gastrointestinal and Liver Disease- 2 Volume Set: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier;New York:
13. Pippard B, Bhatnagar M, McNeill L, Donnelly M, Frew K, Aujayeb A. 2022; Hepatic hydrothorax: a narrative review. Pulm Ther. 8:241–254. DOI: 10.1007/s41030-022-00195-8. PMID: 35751800. PMCID: PMC9458779.

14. Bielsa S, Porcel JM, Castellote J, Mas E, Esquerda A, Light RW. 2012; Solving the Light's criteria misclassification rate of cardiac and hepatic transudates. Respirology. 17:721–726. DOI: 10.1111/j.1440-1843.2012.02155.x. PMID: 22372660.

15. Yoon JH, Kim HJ, Jun CH, Cho SB, Jung Y, Choi SK. 2019; Various treatment modalities in hepatic hydrothorax: what is safe and effective? Yonsei Med J. 60:944–951. DOI: 10.3349/ymj.2019.60.10.944. PMID: 31538429. PMCID: PMC6753336.

16. Adams SR. Hepatic hydrothorax. Proceedings of UCLA Health. 2020; 4.
17. Maghfirah DM, Abubakar A, Yusuf F. 2018; Management of ascites in hepatic cirrhosis. Nangroe Med J. 1:47–58.
18. Runyon BA. AASLD Practice Guidelines Committee. 2009; Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 49:2087–2106. DOI: 10.1002/hep.22853. PMID: 19475696.

19. European Association for the Study of the Liver. 2010; EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 53:397–417. DOI: 10.1016/j.jhep.2010.05.004. PMID: 20633946.
20. Kandarini Y. 2015. Nutritional management in prialysis and dialysis CKD patients. USDI;Available from: https://simdos.unud.ac.id/uploads/file_penelitian_dir/c79f978ea9cf8074706ebd6237fae79d.pdf.
21. Garbuzenko DV, Arefyev NO. 2017; Hepatic hydrothorax: an update and review of the literature. World J Hepatol. 9:1197–1204. DOI: 10.4254/wjh.v9.i31.1197. PMID: 29152039. PMCID: PMC5680207.

22. Krok KL. 2014; Hepatic hydrothorax: Current concepts. Clin Liver Dis (Hoboken). 4:35–37. DOI: 10.1002/cld.375. PMID: 30992917. PMCID: PMC6448729.

23. Koul A, Sood J. Vohra V, Gupta N, Jolly AS, Bhalotra S, editors. 2023. Preoperative Assessment and Optimization of Liver Transplant Patient: Ascites and Hydrothorax. Peri-operative Anesthetic Management in Liver Transplantation. Springer Nature Singapore;Singapore: p. 115–126. DOI: 10.1007/978-981-19-6045-1_9.

24. Gilbert CR, Shojaee S, Maldonado F, et al. 2022; Pleural interventions in the management of hepatic hydrothorax. Chest. 161:276–283. DOI: 10.1016/j.chest.2021.08.043. PMID: 34390708.

25. Jung Y, Song SY, Na KJ, Chon SH, Jun CH, Choi SK. 2020; Minimally invasive surgical strategy for refractory hepatic hydrothorax. Eur J Cardiothorac Surg. 57:881–887. DOI: 10.1093/ejcts/ezz342. PMID: 31958113.

26. Osman KT, Abdelfattah AM, Mahmood SK, et al. 2022; Refractory hepatic hydrothorax is an independent predictor of mortality when compared to refractory ascites. Dig Dis Sci. 67:4929–4938. DOI: 10.1007/s10620-022-07522-8. PMID: 35534742.

27. Ma B, Shang T, Huang J, et al. 2022; Analysis of clinical features and prognostic factors in patients with hepatic hydrothorax: a single-center study from China. BMC Gastroenterol. 22:333. DOI: 10.1186/s12876-022-02412-9. PMID: 35799114. PMCID: PMC9264701.

28. Mirza M, Ahmed R, Ayaz A, Manzoor K. 2023; Spontaneous bacterial empyema (SPEM) with concurrent spontaneous bacterial peritonitis (SBP) in liver cirrhosis. Am J Respir Crit Care Med. 207:A5023. DOI: 10.1164/ajrccm-conference.2023.207.1_MeetingAbstracts.A5023.

29. Lazaridis KN, Frank JW, Krowka MJ, Kamath PS. 2004; Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med. 107:262–267. DOI: 10.1016/S0002-9343(99)00217-X. PMID: 10492320.

30. Uddin MM, Regmi N, Usama M, et al. 2020; Spontaneous bacterial empyema-an underdiagnosed complication of hepatic hydrothorax. Arch Clin Med Case Rep. 5:64–69. DOI: 10.26502/acmcr.96550327.
31. Tharappel KJ, Bolanthakodi N, Vidyasagar S, Varma M. 2019; Acute tension hydrothorax in chronic liver disease secondary to spontaneous diaphragmatic rupture. BMJ Case Rep. 12:e231604. DOI: 10.1136/bcr-2019-231604. PMID: 31678925. PMCID: PMC6827727.

Table 1
Hepatic Hydrothorax and SBPL Diagnosis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download