Abstract
Pancreatic resections, depending on the location of the tumor, usually require division of the vasculature of either the distal or proximal part of the stomach. In certain situations, such as total pancreatectomy and/or with splenic vein occlusion, viability of the stomach may be threatened due to inadequate venous drainage. We discuss three cases of complex pancreatic surgeries performed for carcinoma of the pancreas at a tertiary care center in India, wherein the stomach was salvaged by reimplanting the veins in two patients and preserving the only draining collateral in one case after the gastric venous drainage was compromised. The perioperative and postoperative course in these patients and the complications were analyzed. None of these 3 patients developed any complication related to gastric venous congestion, and additional gastrectomy was avoided in all these patients. Re-establishment of the Gastric venous outflow after extensive pancreatic resections helps to avoid additional gastric resection secondary to venous congestive changes.
Venous drainage of the stomach occurs mainly through the coronary vein (left gastric vein [LGV]), right and left gastroepiploic vein (RGEV and LGEV), and short gastric veins, which drain into the Porto-splenic circulation, and via phrenic veins, which may drain into the inferior vena cava or supra-renal vein [1]. Gastric venous drainage is closely related to the venous drainage of the pancreas. Total pancreatectomy (TP) and splenectomy may entail the division of all main gastric veins, and the remaining drainage through the inferior phrenic vein might be inadequate, especially for the distal part of the stomach; thus, making gastric resection obligatory. Splenic vein (SV) occlusion can result from pancreatic adenocarcinoma, especially when the tumour involves the confluence of the main portal vein (MPV), superior mesenteric vein (SMV), and SV. The resultant collaterals formed due to chronic SV occlusion are an important route for gastric venous drainage. We present three cases wherein gastric salvage was possible with due attention to preservation and/or restoration of gastric venous drainage.
A 52-year-old lady, a known diabetic, presented with steatorrhea, weight loss, and upper abdominal pain for 6 months. A triphasic computed tomography (CT) scan of the abdomen and magnetic resonance cholangiopancreatography showed a lesion with diffuse pancreatic involvement suggestive of intraductal papillary mucinous neoplasm (IPMN)-associated pathology. The serum CA 19-9 level at presentation was 89 IU/mL. She underwent a TP with splenectomy. Intraoperatively, the lesion involved the head and body of the pancreas. Dense peripancreatic inflammation due to prior episodes of pancreatitis made dissection of the splenic vessels and LGV difficult. LGV (draining into the SV in this case) had to be ligated because of dense adhesions around this area. A pylorus-resecting procedure was performed. At the end of resection, gastric congestion was evident. Therefore, a decision was taken to reimplant the LGV into the inferior mesenteric vein (IMV), as the SV was also ligated and the RGEV was reimplanted into a colic vein (Fig. 1). Help of the microvascular surgical team was sought in view of the narrow calibre of both the veins, and both anastomoses were performed under an operating microscope with a 9-0 Prolene suture. The operative time was 660 minutes, and the intraoperative blood loss was 2,000 mL. Post anastomosis, the congestion and colour of the stomach improved immediately. The remaining reconstruction was performed in a regular fashion. Precautionary upper GI endoscopy on the second postoperative day revealed a healthy pink gastric mucosa. Patient developed only mild (grade A) delayed gastric emptying in the post-operative period and recovered uneventfully. Histopathology revealed a moderately differentiated adenocarcinoma in the setting of IPMN (pT2N0). The patient is alive and is doing well 64 months after surgery with no evidence of recurrence.
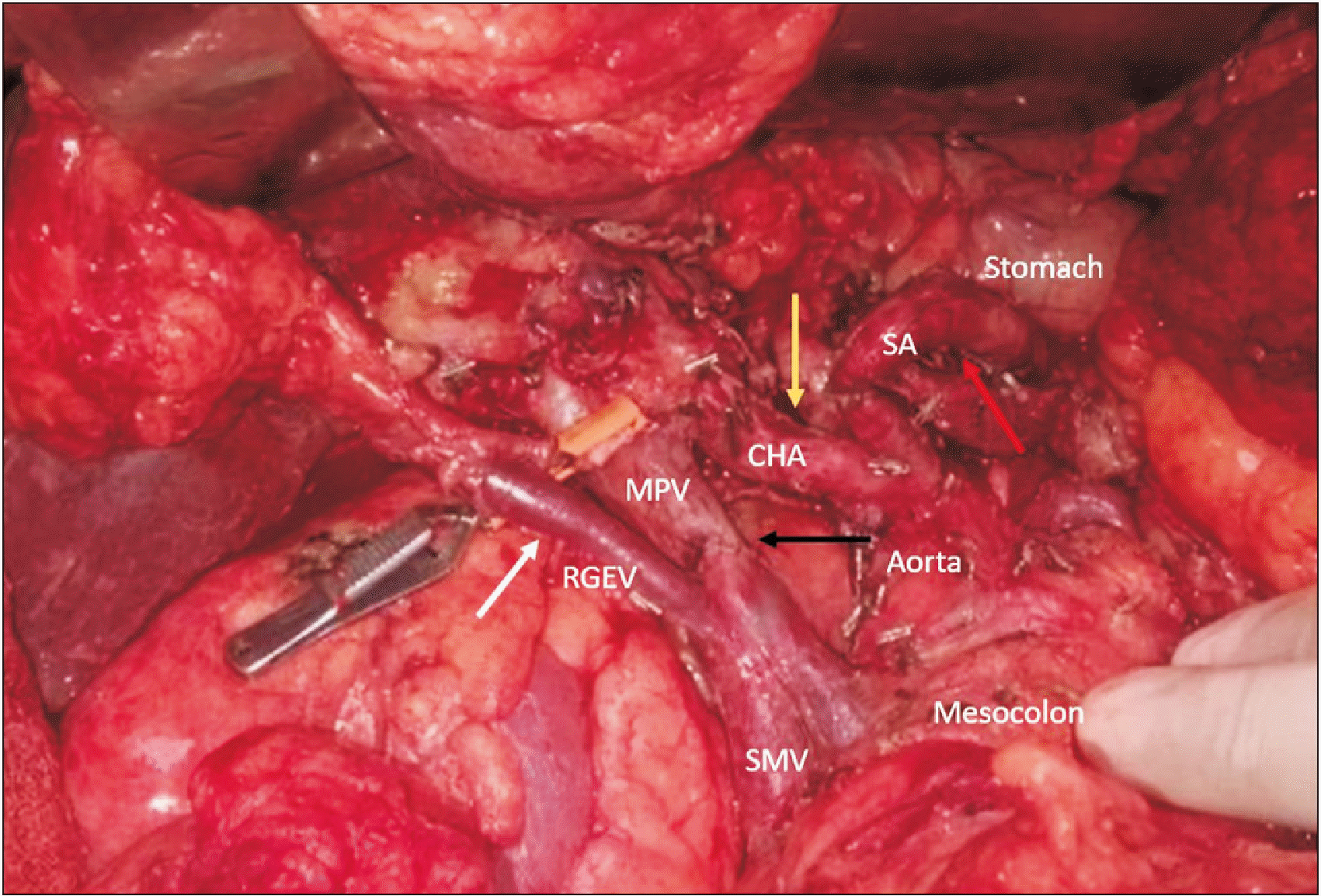
A 66-year-old gentleman presented with a complaint of epigastric pain for 20 days. A triphasic CT scan (Fig. 2A) showed a borderline resectable pancreatic head tumour with deformed spleno-portal confluence and a luminal filling defect in the portal vein. The tumour was abutting the common hepatic artery and encasing the gastroduodenal artery (GDA). He received two cycles of chemotherapy (Gemcitabine and Paclitaxel) followed by concurrent Capecitabine-based radiotherapy at the previous treatment centre. A repeat CT scan at our centre showed stable disease. He was administered additional 2 cycles of FOLFIRINOX, and a reassessment CT scan was again suggestive of stable disease with 1 cm encasement of the GDA and a persistent deformity at the splenoportal confluence of approximately 2 cm in length. Occlusion of the confluence had resulted in omental venous collateral formation with significantly dilated RGEV carrying splenic drainage into the portal circulation. Intraoperatively, the splenoportal confluence and GDA were involved, but the latter was free at its origin. The RGEV was dilated to around 8 mm in diameter. The RGEV, which is usually sacrificed during pancreaticoduodenectomy, was preserved as it was the sole venous drainage for both the stomach and spleen in this case (Fig. 2B and 3), as SV reconstruction was not feasible due to the long length of SV involvement and coronary vein had to be sacrificed due to involvement by the tumour. The short gastric veins were patent; however, the entire gastric venous drainage was via the RGEV alone, as the SV had to be ligated and was unreconstructible due to long length involvement. Hence, SV ligation was performed after resection and the decision to preserve the RGEV was made intraoperatively after noticing gastric congestion after performing a bull-dog clamp test on this dilated vein. Pylorus-preserving pancreaticoduodenectomy was performed with 3 cm segmental portal vein resection and type III reconstruction (primary reconstruction with end-to-end anastomosis). The operative time was 540 minutes, and blood loss was 2,300 mL. The intraoperative course and postoperative recovery were uneventful. Postoperative CT showed normal gastric enhancement and mural thickness, indicating adequate venous outflow. Histopathology revealed moderately differentiated adenocarcinoma of the pancreatic head (pT4N1). On follow-up, the patient developed liver metastases 7 months after the surgery and he died 10 months after surgery.
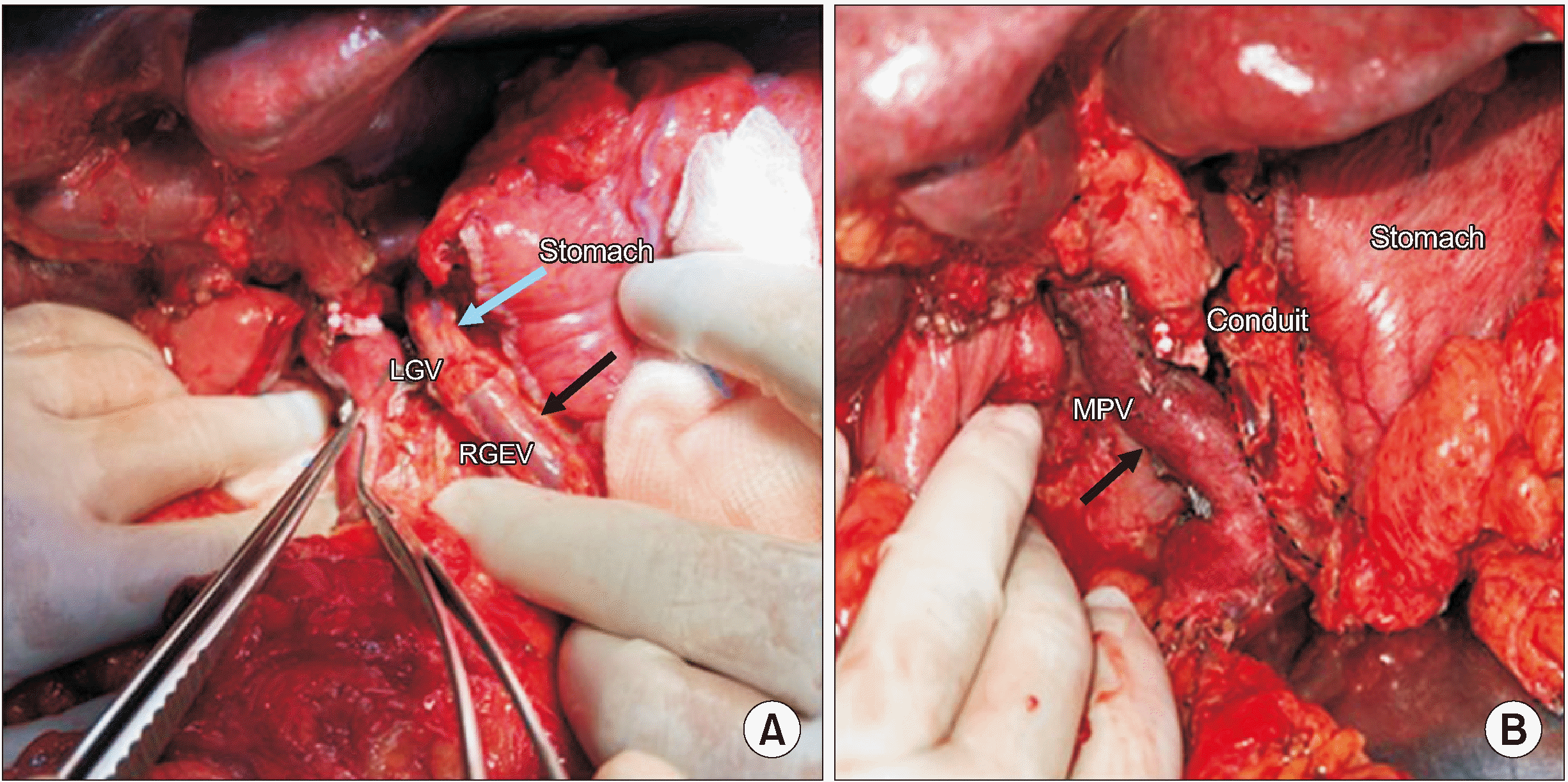
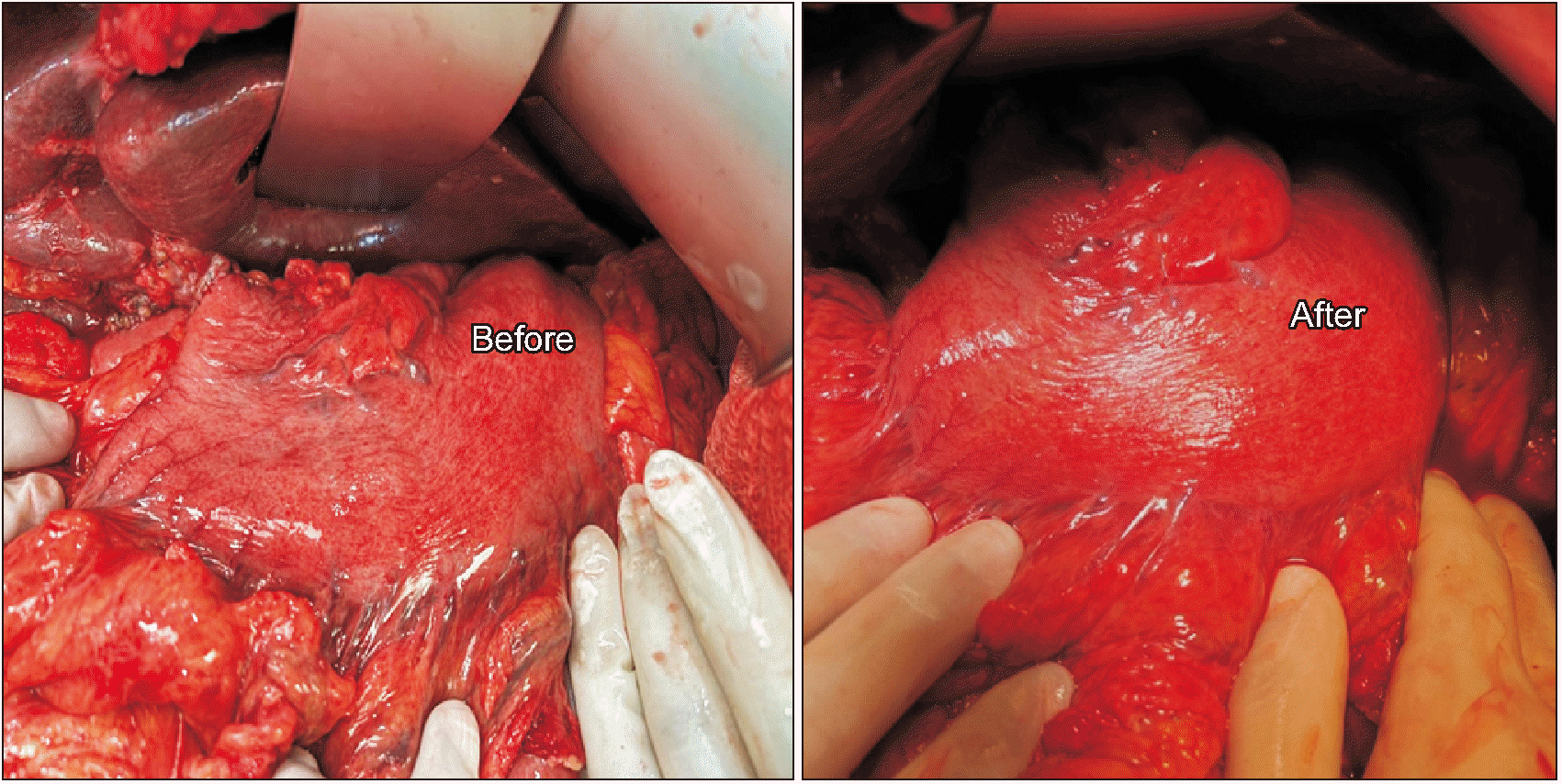
A 49-year-old gentleman presented with a history of left-sided abdominal pain radiating to the back for 20 days. CECT of the abdomen and pelvis showed a hypodense lesion in the uncinate process and another hypodense lesion in the body extending to the pancreatic tail. The lesion was encasing the splenic artery and vein leading to formation of collaterals draining the spleen and stomach. Involvement of LGV insertion into the SV by the tumour was noted on the imaging along with dilated RGEV and LGEV. His CA-19.9 level was 16,742 U/mL and endoscopic ultrasound-guided fine needle aspiration was suggestive of adenocarcinoma. After receiving 5 cycles of FOLFIRINOX, reassessment CT showed a stable lesion with no evidence of disease spread and a declining CA-19.9 level (1,833 U/mL). In view of the multifocality of the tumour and stable disease on neoadjuvant chemotherapy, the decision to proceed with TP was taken. Intraoperatively, similar findings were confirmed as noted on the preoperative CT scan. The RGEV and LGEV were ligated, while keeping both the cut ends long with the intent of venous reconstruction, in case if gastric congestion would develop after LGV division. As the LGV was clamped with a bulldog, gastric congestion became evident. The LGV was divided with the decision to reconstruct the venous outflow. Gastric venous stasis was managed by first constructing an end-to-end LGV-RGEV anastomosis using a 7-0 prolene suture (Fig. 4A). This venous conduit, in turn, was drained into the MPV by means of a side-to-side anastomosis (Fig. 4B). Gastric congestion reverted soon after the release of vascular clamps and confirmed patency of the venous anastomosis (Fig. 5). Postoperatively, gastric function recovered well with the patient tolerating gradual diet escalation and nasogastric tube removal by POD2. CECT performed on POD6 showed that the venous conduit drained into the MPV with good opacification and no gastric wall oedema. The patient was discharged on POD14. The final histopathology showed a viable residual tumour (Evans grade 4) with two tumour epicenters, one in the head and another in the body of the pancreas extending into the tail. All margins were free. The final stage was ypT3ypN1, with 3 out of 38 lymph nodes being positive. On follow-up, the patient developed liver metastases six months post-surgery and is currently on palliative chemotherapy.
TP with splenectomy may require distal or total gastrectomy in view of ischemic changes in the stomach due to decreased blood supply [2]. Harao et al. [2] have described a technique of gastric salvage by preserving the spleen in case of pancreatic head cancer where a TP was required to achieve a negative margin. In another case report, the authors salvaged the stomach in a case of TP, by performing a segmental duodenectomy and preserving the GDA, right gastroepiploic artery, gastrocolic trunk, and RGEV to prevent gastric venous congestion [3]. Tanaka et al. [4] have noted that gastric venous reconstruction to relieve venous stasis improves patients’ quality of life, reduces postoperative morbidity and mortality, as well as rates of gastrectomy and complications. In this article, we described novel methods of dual-vein reconstruction, RGEV-preserving pancreaticoduodenectomy, and combined LGV-RGEV venous conduit anastomosis to the portal vein.
In the first case of this case series, TP with splenectomy was important to ensure the radicality of the procedure. The SV had to be divided at the confluence with the SMV due to disease status. As the LGV was draining proximal to the confluence of the SV and SMV, all gastric veins were severed resulting in gastric congestion. This was circumvented by reimplanting the coronary vein into the IMV and the right gastro-epiploic vein into the middle colic vein. To the best of our knowledge, this is the first description of this dual venous reconstruction technique for gastric salvage.
In the second case, a borderline resectable pancreatic tumour resulted in chronic spleno-portal venous occlusion and collateral formation. On preoperative imaging, a significantly dilated RGEV along with SV occlusion and resultant collaterals were observed. Venous drainage of the stomach was mainly through the short gastric veins and LGEV into the SV and then through the RGEV into the SMV. Therefore, preservation of the latter was important for gastric salvage.
In the third case, as a multifocal pancreatic adenocarcinoma was infiltrating the splenic vessels and insertion of the LGV into the SV, all gastric veins had to be divided to achieve the R0 status during TP. Gastric venous drainage was re-established by anastomosing the LGV-RGEV venous conduit to the MPV. Dual venous reconstruction and venous conduit reconstruction were performed as a precautionary measure in Case 1 and Case 3, keeping in mind the complications of thrombosis of the only draining small caliber gastric vein to provide additional safety.
In their series of 38 patients who underwent TP, Nakao et al. [5] advocated that at least one draining vein should be preserved or reconstructed during TP to avoid a distal gastrectomy. They observed postoperative gastric bleeding evident from a nasogastric tube in two patients (5.3%) after TP, with one needing RGEV-Left ovarian vein anastomosis. The LGV-IMV anastomosis has been described by Sandroussi and McGilvray [6]. In such cases, distal stomach drainage is more often affected than proximal stomach because of a well-developed gastroesophageal junction submucosal venous plexus and para-oesophagal veins draining into the azygous vein. Accelerated return of gastric function with LGV preservation and significantly increased delayed gastric emptying with LGV ligation were observed by Kurosaki and Hatakeyama [7] The importance of LGV preservation has been emphasized in many other prior reports [8,9]. Another large retrospective observational study performed by Loos et al. [10] underscored the importance of LGV preservation during TP. They noted that splenectomy and LGV resection were independently associated with gastric venous congestion and showed increased mortality in patients with gastric congestion (7.4% vs. 2.8%). The GENDER study, a prospective non-randomized observational study which has begun recently, studying the effects of gastric venous reconstruction after TP should hopefully enlighten us more on the issue [11]. In our institutional experience of 17 TPs over a period of 12 years, ten were total pancreato-splenectomies and seven were without splenectomy. In two cases of TP, gastric veins were reconstructed, as described in this article, and in the remaining case, at least one of the draining veins of stomach was preserved. A sound operative plan based on the preoperative review of cross-sectional imaging is paramount to avoid inadvertent venous damage, and, thereby, a gastrectomy [12].
Gastric congestion secondary to compromised venous return can have deleterious consequences after TP. Detailed study of preoperative imaging is essential to plan the preservation of collaterals and/or the reconstruction of gastric veins to prevent an additional gastric resection during a major pancreatectomy.
REFERENCES
1. Skandalakis LJ, Colborn GL, Skandalakis JE, Skandalakis PN, Loukas M, Mitilas P. Fischer JE, Bland KI, Callery MP, Clagett GP, Jones DB, LoGerfo FW, editors. 2007. Chapter 68: Anatomic Considerations in Gastroduodenal Surgery. Mastery of Surgery. 5th ed. Lippincott Williams and Wilkins;p. 831–833.
2. Harao M, Hishinuma S, Tomihawa M, Baba H, Ogata Y. 2009; Whole stomach and spleen preserving total pancreatectomy: a new surgical technique for pancreatic cancer. Hepatogastroenterology. 56:1549–1551.
3. Hishida M, Nakao A, Hatsuno T, Yano H, Tanaka T, Takano N, et al. 2011; Total pancreatectomy with segmental duodenectomy preserving right gastroepiploic vein. Hepatogastroenterology. 58:198–201.
4. Tanaka M, Ito H, Ono Y, Matsueda K, Mise Y, Ishizawa T, et al. 2019; Impact of portal vein resection with splenic vein reconstruction after pancreatoduodenectomy on sinistral portal hypertension: who needs reconstruction? Surgery. 165:291–297. DOI: 10.1016/j.surg.2018.08.025. PMID: 30268375.

5. Nakao A, Yamada S, Fujii T, Tanaka H, Oshima K, Oshima Y, et al. 2018; Gastric venous congestion and bleeding in association with total pancreatectomy. J Hepatobiliary Pancreat Sci. 25:150–154. DOI: 10.1002/jhbp.523. PMID: 29143477. PMCID: PMC5814835.

6. Sandroussi C, McGilvray ID. 2010; Gastric venous reconstruction after radical pancreatic surgery: case report and review of the literature. J Gastrointest Surg. 14:1027–1030. DOI: 10.1007/s11605-010-1192-0. PMID: 20387128.

7. Kurosaki I, Hatakeyama K. 2005; Preservation of the left gastric vein in delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. J Gastrointest Surg. 9:846–852. DOI: 10.1016/j.gassur.2005.02.009. PMID: 15985243.

8. Kulu Y, Schmied BM, Werner J, Muselli P, Büchler MW, Schmidt J. 2009; Total pancreatectomy for pancreatic cancer: indications and operative technique. HPB (Oxford). 11:469–475. DOI: 10.1111/j.1477-2574.2009.00085.x. PMID: 19816610. PMCID: PMC2756633.

9. Sugiyama M, Atomi Y. 2000; Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg. 24:66–70. DOI: 10.1007/s002689910013. PMID: 10594206.

10. Loos M, Mehrabi A, Ramouz A, Contin P, Strobel O, Müller-Stich BP, et al. 2022; Gastric venous congestion after total pancreatectomy is frequent and dangerous. Ann Surg. 276:e896–e904. DOI: 10.1097/SLA.0000000000004847. PMID: 33914472.

11. Mehrabi A, Loos M, Ramouz A, Dooghaie Moghadam A, Probst P, Nickel F, et al. 2021; Gastric venous reconstruction to reduce gastric venous congestion after total pancreatectomy: study protocol of a single-centre prospective non-randomised observational study (IDEAL Phase 2A) - GENDER study (Gastric vENous DrainagE Reconstruction). BMJ Open. 11:e052745. DOI: 10.1136/bmjopen-2021-052745. PMID: 34675020. PMCID: PMC8532556.
12. Kawasaki K, Kanaji S, Kobayashi I, Fujita T, Kominami H, Ueno K, et al. 2010; Multidetector computed tomography for preoperative identification of left gastric vein location in patients with gastric cancer. Gastric Cancer. 13:25–29. DOI: 10.1007/s10120-009-0530-y. PMID: 20373072.

Fig. 1
(A) End-to-side microvascular anastomosis between the CV (white arrow) and IMV (blue arrow). (B) End-to-end microvascular anastomosis between the RGEV (white arrow) and colic vein (blue arrow). CV, coronary vein; IMV, inferior mesenteric vein; RGEV, right gastroepiploic vein.
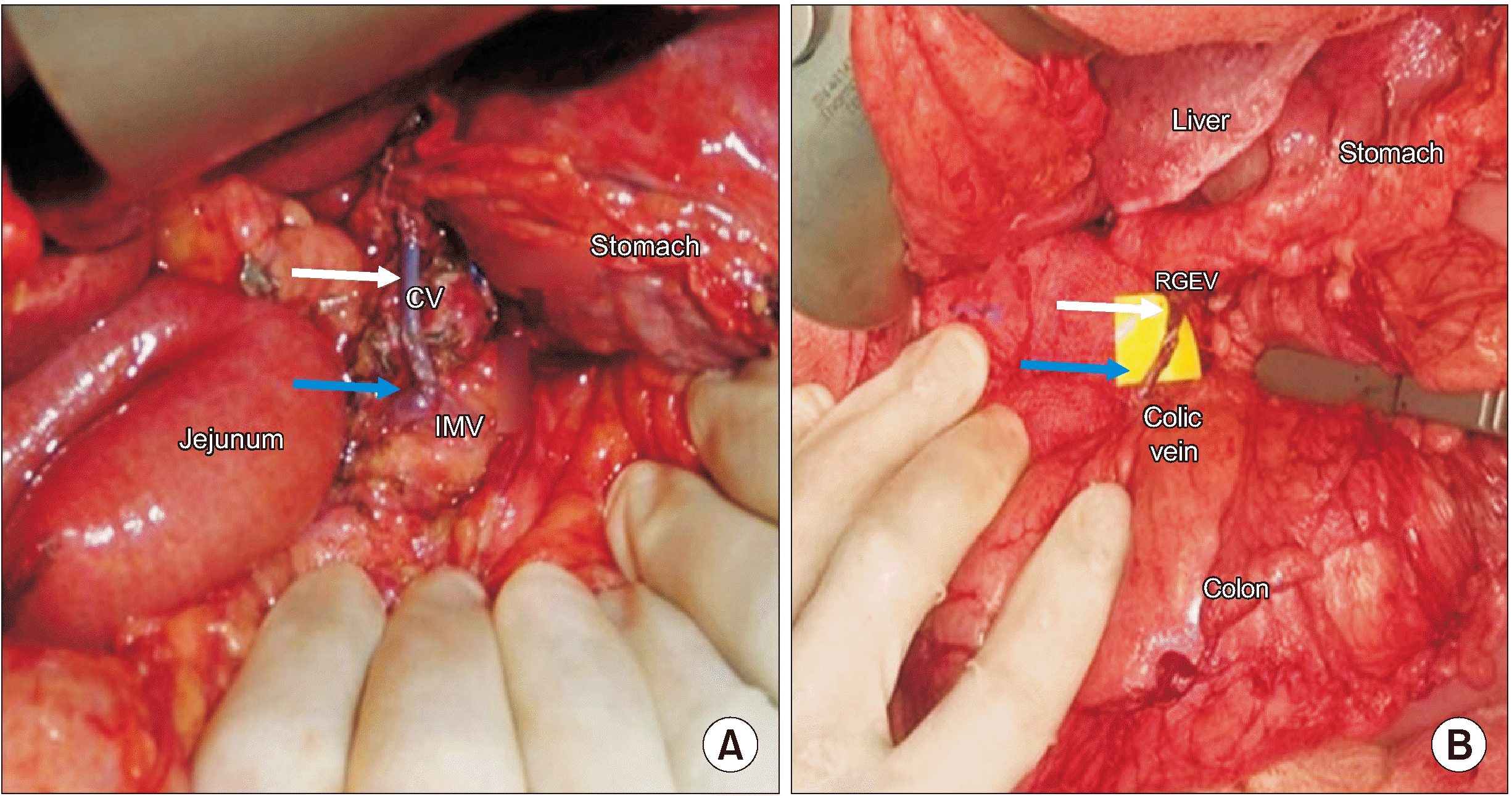
Fig. 2
(A) CT showing a borderline resectable tumour with spleno-portal confluence involvement: a red dotted circle showing the area of involvement. (B) A pancreatic mass (yellow arrow) invading the portal vein and SMV confluence. The preserved RGEV (white arrow) is seen draining into the SMV (Looped by a blue vessel tape and marked by a black arrowhead). SA, splenic artery; CHA, common hepatic artery; RGEV, right gastroepiploic vein; SMV, superior mesenteric vein.
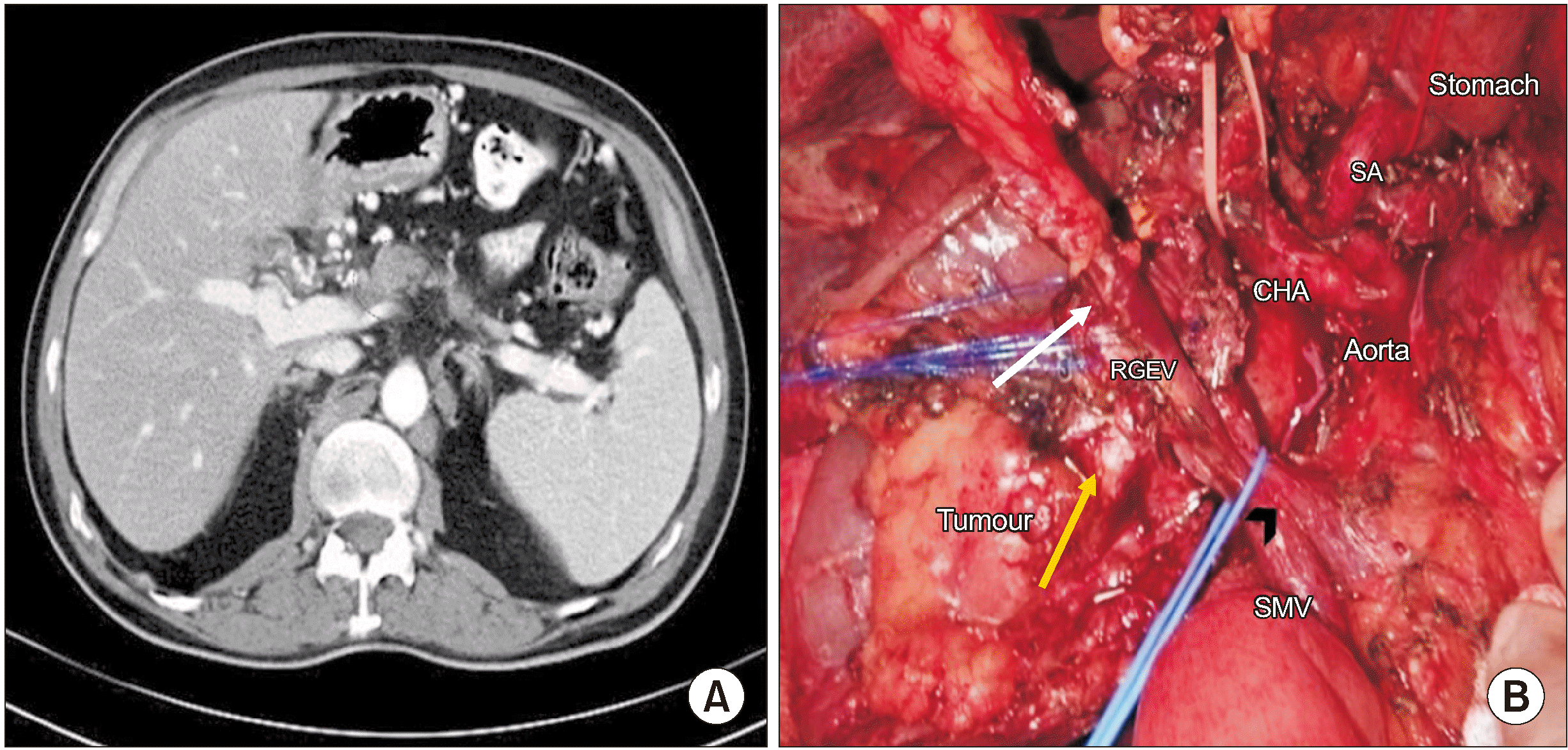
Fig. 3
Post-resection photograph showing a dilated RGEV (white arrow) draining into the SMV. The CHA (yellow arrow) and SA (red arrow), and end-to-end venous anastomosis (black arrow) are seen. RGEV, right gastroepiploic vein; MPV, main portal vein; CHA, common hepatic artery; SA, splenic artery; SMV, superior mesenteric vein.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download