This article has been
cited by other articles in ScienceCentral.
Abstract
Backgrounds/Aims
Optimal intravenous fluid management during the perioperative period for patients undergoing pancreaticoduodenectomy (PD) within the framework of enhanced recovery after surgery (ERAS) is unclear. Studies have indicated that excessive total body salt and water can contribute to the development of oedema, leading to increased morbidity and extended hospital stays. This study aimed to assess the effects of an intravenous therapy regimen during postoperative day (POD) 0 to 2 in PD patients within ERAS.
Methods
A retrospective interventional cohort study was conducted, and it involved all PD patients before and after implementation of ERAS (2009–2017). In the ERAS group, a targeted maintenance fluid regimen of 20 mL/kg/day with a sodium requirement of 0.5 mmoL/kg/day was administered. Outcome measures included the mmol of sodium and chloride administered, length of stay, and morbidity (postoperative pancreatic fistula, POPF; acute kidney injury, AKI; ileus).
Results
The study included 169 patients, with a mean age of 64 ± 11.3 years. Following implementation of the intravenous fluid therapy protocol, there was a significant reduction in chloride and sodium loading. However, in the multivariable analysis, chloride administered (mmoL/kg) did not independently influence the length of stay; or rates of POPF, ileus, or AKI (p > 0.05).
Conclusions
The findings suggested that a postoperative intravenous fluid therapy regimen did not significantly impact morbidity. Notably, there was a trend towards reduced length of stay within an increasingly comorbid patient cohort. This targeted fluid regimen appears to be safe for PD patients within the ERAS program. Further prospective research is needed to explore this area.
Go to :

Keywords: Fluid therapy, Pancreatic cancer, Whipple procedure, Intravenous, Acute kidney injury
INTRODUCTION
Pancreatic cancer is the 8th most commonly diagnosed cancer in Australia [
1]. Typically, malignancies of the pancreatic head and periampullary region are managed via pancreaticoduodenectomy (PD), a procedure that has historically been associated with high postoperative morbidity and mortality. Advancement in surgical and anaesthetic techniques in perioperative care has led to the evolution of PD into a procedure with a significantly low mortality of less than 2% [
2] but morbidity ranging from 30% to 60% [
3]. Common postoperative complications include postoperative pancreatic fistula (POPF), ileus, delayed gastric emptying, and wound infections. To improve patient outcomes, in recent times, research has been directed towards optimal perioperative care known as ‘enhanced recovery after surgery’ (ERAS). ERAS is a single program incorporating evidence-based multimodal interventions during the perioperative period in order to attenuate the loss of, and improve the restoration of, functional capacity after surgery [
4].
Perioperative fluid management is a core domain of ERAS pathways that has undergone significant developments; however, it still remains highly contested. Many pathways have moved away from the traditional liberal fluid therapy. Current evidence has highlighted the association of liberal fluid management with the most harm-causing increased complications and length of stay (LOS) [
5,
6]. On the other hand, a recent large trial comparing a restrictive versus liberal fluid regimen concluded that overall, a restrictive regimen led to increased rates of acute kidney injury (AKI) [
7]. Hyperchloraemia is thought to occur secondary to salt and water excess (particularly hyperchloraemic acidosis) causing multisystemic effects, including interstitial oedema with implications for pulmonary function, renal function, gastrointestinal motility, impaired wound healing, and anastomotic breakdown [
8,
9]. Recent therapies have favoured a more restrictive fluid administration, which conversely can be complicated by imposing a state of hypovolaemia leading to impaired oxygen delivery, haemodynamic compromise, and subsequent organ dysfunction [
10]. Both insufficient or excess fluid delivery has been shown to have a direct link with perioperative morbidity, leading to increased postoperative complications [
11]; therefore, a number of studies have suggested using a goal-directed therapy approach to perioperative fluid management in order to achieve a net zero balance [
12]. Until recently, most studies have assessed IV fluid regimens in the context of colorectal surgery, and it remains unclear whether these findings can be translated to PD patients. In a recent single center retrospective study, Weinberg et al. [
5] found that those with complications post PD had a higher median volume of IV therapy, greater cumulative positive fluid balance, and longer median LOS. This finding was also echoed by Kulemann et al. [
13].
To date, data on multiple interventions of enhanced recovery programs after PD remain sparse and literature assessing the benefits of targeted IV therapy in this arena is very rare. In this pre-post study, we aim to assess the clinical impact of our postoperative IV fluid therapy protocol between postoperative day (POD) 0 to 2 within the enhanced recovery program for PD.
Go to :

MATERIALS AND METHODS
Ethics approval for this study was provided by the South Metropolitan Health Service Human Research Ethics Committee (HREC ref: 15-040-1). A retrospective pre-post study was performed in a prospectively maintained database of 169 consecutive patients who underwent PD at the South Metropolitan Health Service in Western Australia (WA). In alignment with the guidelines released by the ERAS Society, we formulated our initial ERAS protocol (
Table 1) and implemented it in a cohort of 140 patients between 2011–2017, which was divided into a transition phase (n = 14) and an ERAS (n = 126) phase. In the transition phase, patients received total parenteral nutrition rather than enteral nutrition, which was introduced in the later ERAS phase. The ERAS group was compared to a reference cohort of patients who underwent PD prior to the implementation of ERAS from 2009 to 2011 (n = 29).
Table 1
Summary of the ERAS protocol implemented
|
Day |
Intervention |
|
Day of surgery |
Oral PPI |
|
Admission |
Octreotide 200 mcg
Fasted minimum 6 hours for food, 2 hours for clear fluids
preOp® drinks complete (not for IDDM)
No bowel preparation |
|
Day of surgery |
Antimicrobial prophylaxis
Insertion of NJ tube, urinary catheter, surgically placed intrabdominal drains
Normothermia maintained ≥ 37℃
Anti-embolic stockings or calf compression pumps in situ
PCA analgesia and wound catheters
Subcutaneous Octreotide
Subcutaneous Heparin
Sips of clear fluid
IV therapy–0.5 mmoL sodium/kg via CSL and 5% dextrose
Jejunostomy feeds commenced with 4 hourly flush
Respiratory exercises
Monitored in HDU overnight |
|
Day 1 |
Water 90 mL/h
IV therapy as per regime
Jejunostomy feeds increased as per regime
Mobilise 5 metres with assistance
Stepdown to ward level care |
|
Day 2 |
Cessation of IV antibiotics
Commence clear fluids
Jejunostomy (enteral) feeds increased as per regime to target rate
Out of bed minimum 1 hour BD, with assistance on ambulation
Indwelling catheter removed |
|
Day 3 |
Commence nourishing fluidsa)
Jejunostomy feeds at target rate
Nasogastric tube spigotteda)
Cease PCA
Drain bottles changed and sent for amylase and lipase |
|
Day 4 |
Wound catheters removed
APS oral protocol
Jejunostomy feeds ceased
Removed NJT/NGT |
|
Day 5 |
Drain bottles changed and sent for amylase and lipase
Removal of intra-abdominal drains if no evidence of POPF |
|
Day 6+ |
Once tolerating nourishing fluids, diet progressed to 1-week pureed diet and then 1-week soft diet |

Targeted IV therapy was delivered with the aim to maintain central euvolaemia whilst avoiding salt and water excess. As part of our ERAS protocol, a postoperative maintenance IV fluid regimen of concurrent administration of compound sodium lactate (CSL) and 5% dextrose, amounting to a total fluid requirement of 20 mL/kg/day fluid and sodium administration of 0.5 mmoL/kg/day was prescribed (Appendix 1). Additionally, patients received nasojejunal feeds at 40 mL/h from day 0. Intraoperatively, we aimed for euvolaemia with the aid of non-invasive techniques, such as Oesphageal Doppler, LiDCO, or pulse pressure variation alongside observations, strict input/output measures, arterial blood gas analysis, and central venous pressure. Fluid deficits, including electrolyte and blood loss, were corrected with appropriate blood products with a target of Hb ≥ 70 and electrolytes within normal limits. The regimen was tapered once enteral intake was established (typically beyond POD 2). Preferred maintenance regimen of dextrose and CSL plus further total IV fluid intake (colloid/crystalloid) was recorded from POD 0 to 2. Then, the total sodium and chloride in mmol per kilogram from POD 0 to 2 was calculated. Patients in the pre-ERAS period did not have a prescribed fluid protocol and management was at the discretion of the treating surgeon and anaesthetist.
All operations were performed by a consistent team of three consultant surgeons specialised in hepatobiliary surgery. Uniform surgical techniques, including pancreaticojejunostomy reconstruction, were employed for all patients.
To compile the necessary information, a manual review of health records was conducted. Histopathology and biochemical results were obtained from a designated application system utilised by the Department of Health, WA. The data were collected and organised using REDCap electronic data capture tools hosted at The University of Western Australia [
14].
Clinical variables and surgical outcomes were assessed. Clinical variables consisted of sex, age, body mass index (BMI), Charlson score, American Society of Anaesthesiologists (ASA) score, and histopathology. Postoperative morbidity included any post-surgical complications up to the day of discharge, and they were assessed according to the Clavien-Dindo classification [
15]. Specific morbidity outcomes measured included POPF, AKI, and ileus. POPF was defined and classified according to the guidelines put forth by the International Study Group of Pancreatic Fistula [
16]. Postoperative ileus was defined as sustained non-mechanical obstruction for more than 4 days after the operation, and it was confirmed by plain abdominal radiography. AKI was defined according to the criteria of the Kidney Disease: Improving Global Outcomes Group [
17].
IBM SPSS Statistics for Mac, version 23 (IBM Corp.) was utilised for statistical analysis. Categorical variables were compared using chi-square tests, while continuous variables were assessed using one-way analysis of variance. Descriptive data were presented as medians with ranges or as the number of patients and percentage. General linear modelling, specifically analysis of covariance, was employed to determine if any predictors significantly influenced the LOS. Logistic regression models were employed to assess the association between predictors and the occurrence of POPF, ileus, and AKI. All statistical tests were two-tailed, and a significance level of p < 0.05 was considered statistically significant.
Go to :

RESULTS
In this study, a cohort of 169 patients with a mean age of 64 ± 11.3 years was included. Among these patients, 98 (58.0%) were male. The demographic and clinical characteristics of the cohort are presented in
Table 2. Importantly, demographics and pathology were not significantly different between groups except for the Charlson score, which demonstrated that the ERAS group had a three-fold increase in the comorbidity burden (
p = 0.03). The total mmol per kg of sodium and chloride administered from POD 0 to 2 was significantly reduced at all measured timepoints in the ERAS group compared to the pre-ERAS group (
Fig. 1).
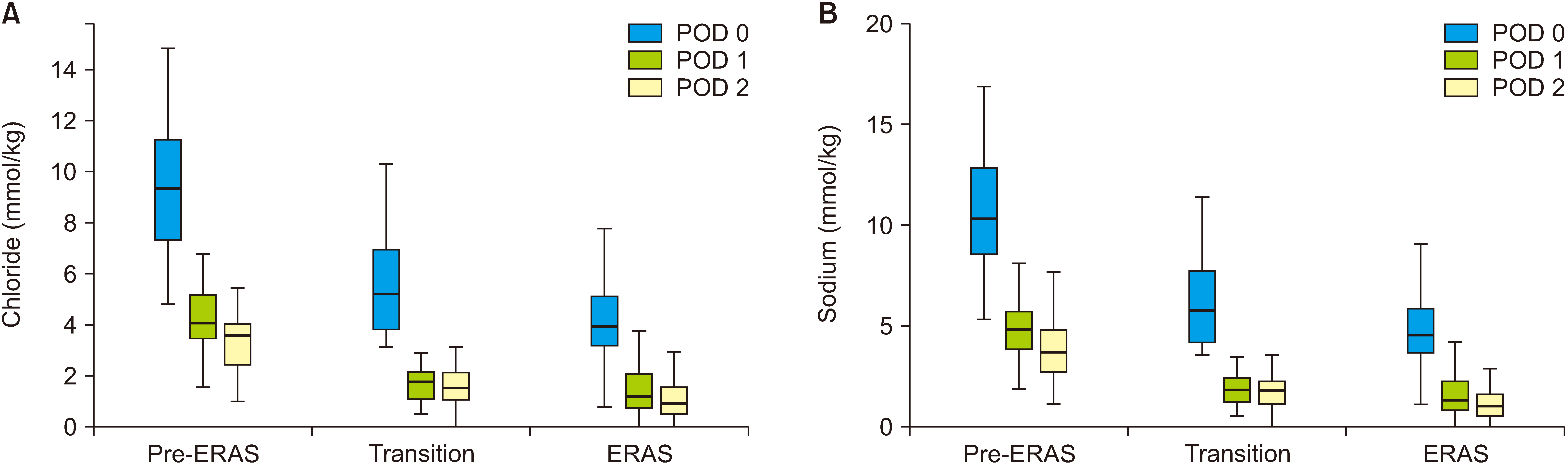 | Fig. 1Total intravenous fluid administration of (A) chloride and (B) sodium. Values are expressed as mmol per kg on POD 0, 1, and 2. POD, postoperative day; ERAS, enhanced recovery after surgery. 
|
Table 2
Patient characteristics, pathology, and preoperative variables
|
Pre-ERAS (n = 29) |
Transition (n = 14) |
ERAS (n = 126) |
p
|
|
Age (yr) |
62.1 ± 10.9 |
61.3 ± 10.3 |
65.1 ± 11.5 |
0.27 |
|
Sex (M/F) |
14/15 |
7/7 |
77/49 |
0.37 |
|
BMI (kg/m2) |
26.1 (19.8–36.4) |
27.5 (18.8–40.7) |
26.3 (18.7–43.1) |
0.63 |
|
ASA grade |
|
|
|
0.10 |
|
I |
3 (10.3) |
0 (0) |
4 (3.2) |
|
|
II |
20 (69.0) |
10 (71.4) |
64 (50.8) |
|
|
III |
6 (20.7) |
4 (28.6) |
53 (42.1) |
|
|
IV |
0 (0) |
0 (0) |
5 (4.0) |
|
|
Charlson grade |
|
|
|
0.03*
|
|
Mild (< 3) |
6 (20.7) |
4 (28.6) |
16 (12.7) |
|
|
Moderate (3–4) |
20 (69.0) |
8 (57.1) |
64 (50.8) |
|
|
Severe (≥ 5) |
3 (10.3) |
2 (14.3) |
46 (36.5) |
|
|
Biliary drainage |
20 (69.0) |
9 (64.3) |
50 (40.0) |
0.58 |
|
Neoadjuvant therapy |
0 (0) |
0 (0) |
11 (8.7) |
0.13 |
|
Pathology |
|
|
|
0.19 |
|
Benign |
3 (10.3) |
3 (21.4) |
9 (7.1) |
|
|
Malignant |
26 (89.7) |
11 (78.6) |
117 (92.9) |
|
|
Malignant lesion classification |
|
|
|
|
|
Pancreatic adenocarcinoma |
19 (73.1) |
5 (45.5) |
51 (43.5) |
|
|
Cholangiocarcinoma |
2 (7.7) |
3 (27.2) |
16 (13.7) |
|
|
Duodenal adenocarcinoma |
1 (3.8) |
0 (0) |
10 (8.5) |
|
|
Ampullary adenocarcinoma |
3 (11.5) |
2 (18.2) |
21 (17.9) |
|
|
Other |
1 (3.8) |
1 (9.1) |
19 (16.2) |
0.47 |

The ERAS cohort had median LOS of 12 days (IQR 8–19 days) compared to the pre-ERAS group with a median LOS of 15 days (IQR 14–23 days) (
p = 0.53) (
Table 3). With respect to the morbidity outcomes in the ERAS group, the percentage of overall morbidity was 31.7% (n = 38), ileus was 19% (n = 23), clinically significant POPF was 9.5% (n = 12), respectively, out of which all patients had a grade B fistula (
Table 3). These values were not statistically significantly different when compared to those in the pre-ERAS group (
Table 3). There was no significant difference in the percentage of AKI observed in the ERAS group in comparison to the pre-ERAS group, which was 15.1% (n = 19) and 13.8% (n = 4), respectively. With respect to the severity of AKI noted in the ERAS group, 63.2% (n = 12) of cases were classified into stage 1, 15.8% (n = 3) into stage 2, and 21.1% (n = 4) into stage 3.
Table 3
|
Pre-ERAS (n = 29) |
Transition (n = 14) |
ERAS (n = 126) |
p
|
|
Length of stay (day) |
15 (14–23) |
14 (12–24) |
12 (8–19) |
0.53 |
|
Ileus |
4 (13.8) |
0 (0) |
23 (18.3) |
0.22 |
|
POPF |
4 (13.8) |
2 (14.3) |
12 (9.5) |
0.78 |
|
AKI |
4 (13.8) |
1 (7.1) |
19 (15.1) |
0.07 |
|
Overall morbidity (CD ≥ 3) |
7 (24.1) |
5 (35.7) |
38 (30.2) |
0.71 |

On further analysis of the entire database cohort, sodium and chloride were highly correlated (Pearson R = 0.986); therefore, only chloride (mmoL/kg) was applied to logistic regression and general linear modelling. On general linear modelling of LOS against various predetermined variables, total chloride load (mmoL/kg) was not found to be a significant factor (Appendix 2). On further multivariable regression analysis, the level of chloride administered did not significantly impact POPF, ileus, or AKI (Appendix 3–5). Interestingly, the development of AKI was independently affected by male gender, higher BMI, higher ASA, and a longer time to indwelling catheter removal (p < 0.05) (Appendix 5).
Go to :

DISCUSSION
Within the context of enhanced recovery programs, optimal perioperative IV fluid therapy remains contentious. Due to complications, such as organ dysfunction and interstitial oedema linked to liberal and restrictive IV therapies, there has been a shift to targeted IV therapy.
Targeted IV therapy is delivered with the aim to maintain central euvolaemia whilst avoiding salt and water excess. Intraoperatively, we aimed for euvolaemia with the aid of non-invasive techniques alongside observations, strict input/output measures, arterial blood gas analysis, and central venous pressure. Postoperatively, a maintenance fluid prescription was administered in addition to further fluid to maintain euvolaemia. The value of 0.5 mmoL sodium/kg/day was used in this study, as we recognised that there is a significant amount of additional sodium load routinely included with administration of other fluids in the immediate postoperative period (e.g., electrolyte replacement, antibiotics, and other medications). Together with the normal physiological response to surgical stress and consequential sodium retention, opting for a lower target would allow room for a safety net to account for these factors. In our centre, our IV fluid regimen led to a significant reduction in the total IV fluid volume administered on POD 0 and 2 and a significant reduction in the total sodium and chloride IV load administered between POD 0 and 2 between the pre-ERAS and ERAS phases. There was also a notable reduction in interpatient variability during the ERAS phase. Nevertheless, in this current study, we found that our postoperative regimen did not significantly reduce the LOS, nor did it grossly impact the incidence of ileus, POPF, AKI, or overall morbidity. Notably, there was a non-significant downward trend in the LOS and POPF.
Several studies have demonstrated that implementation of targeted therapy in isolation has the potential to reduce both LOS and complications. Unfortunately, as demonstrated in our study, this has not been translated to studies within the context of ERAS [
18]. Consistent with our study findings, a meta-analysis by Huang et al. [
19] did not establish a correlation between the perioperative volume of IV fluid administered and postoperative complications in patients undergoing PD. Furthermore, in one of the largest multicentre trials, the OPTIMISE study, a non-significant trend towards decreased complications and 180-day mortality was observed in the targeted therapy group [
12]. A recent retrospective study by Sulzer et al. [
20] found that restrictive goal-directed therapy was associated with lower rates of delayed gastric emptying and pancreatic leak, a finding that was not observed in our study. It is important to note that this study employed a restrictive regimen only in the intraoperative period.
The lack of translation of positive benefits of targeted IV therapy into the context of ERAS may highlight that a targeted regimen within the context of multiple interventions that take place in enhanced recovery programs, may play a smaller role, or rather work in conjunction with other interventions in improving the overall morbidity. It is important to acknowledge that although we did not identify an added benefit or harm in our study, the ERAS phase comprised a markedly more complex cohort, which may have masked the potential statistical significance.
As noted earlier, one of the most recent large trials comparing a restrictive versus liberal fluid regimen was the RELIEF study, which noted an overall increase in rates of AKI with a restrictive fluid regimen [
7]. Although we did not observe this finding in our study, it is important to note that the conclusions drawn by Myles et al. [
7] examined all types of abdominal surgeries out of context of ERAS. Interestingly, we did find that patients who were in the later part of our ERAS study experienced less AKI than those in the earlier part of ERAS as protocols became more established. It is possible that although a restrictive regimen or targeted regimen may not be favourable in some surgical disciplines, it may be more beneficial in other settings, such as in pancreatic surgery. This is reflected in further analysis of the RELIEF study, when a further subgroup analysis of hepatobiliary surgery was assessed, and for operations performed in Australia, generally the rise in AKI was not demonstrated. Thus, our findings may not be conflicting regarding PD specifically.
The chief limitation of this study was its retrospective nature. Clinical details were based on documentation, and therefore, certain biases could not be avoided. Furthermore, oral fluid intake could be a confounding factor and it is difficult to determine the amount absorbed. A further limitation encountered was that additional elements implemented as part of the ERAS phase besides the fluid protocol may have contributed to the results. This is further discussed in our previously published paper [
21]. Additionally, the small number of cases in the pre-ERAS group is reflective of our centre emerging as a higher volume centre for PD procedures in recent years. Our findings could be further improved in a prospective review; however, this would require careful consideration. This is especially challenging, as most elements of ERAS in PD performed in the department are now embedded in daily practice as standard of care (e.g., early mobilization and multimodal analgesia). This was a challenge noted by other retrospective studies on ERAS.
Our findings suggest that our postoperative IV fluid therapy regimen can be delivered without an increase in harm. We believe future studies should make further consideration not only of the volume of IV fluid but also of the type and duration of therapy. In our study, we hypothesised that cumulative sodium and chloride load across formulaic solutions deserved specific consideration above a volume-based approach alone in evaluating morbidity, as done in previous studies. A combination therapy of CSL and dextrose, as used in our study, allows the avoidance of excess salt postoperatively, a time when patients exhibit impaired ability to excrete sodium and chloride [
22]. Therefore, administering low-sodium, low-volume fluids expedite the patient’s return to a zero sodium and fluid balance in the postoperative period. The administration of normal saline has been observed to induce a predictable hyperchloraemic metabolic acidosis, which has been associated with potential adverse effects [
9]. Furthermore, to date, most studies have focused on targeted IV therapy primarily in the intraoperative period. Fluid type was not included in this study given the plethora of fluids employed over the nine-year period, but together with total volume administered, they deserve inclusion as additional variables in future studies. We assessed the effect of a postoperative fluid regimen until the second POD, as beyond this timepoint, patients were typically commenced on enteral hydration. Miller et al. [
23] also agreed that consideration should be given to continue goal-directed therapy in patients (particularly those who are at high risk) in the postoperative period until oral intake is established.
In summary, our study supports the notion that targeted IV therapy can be safely delivered to patients undergoing PD within an ERAS program in the perioperative period, and without increased morbidity. It is possible that with continued research in the form of larger studies, added benefits may be revealed.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download