1. Fernandez-Cruz L. Holzheimer RG, Mannick JA, editors. 2001. Periampullary carcinoma. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt;p. 1–9.
2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. 2014; Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74:2913–2921. DOI:
10.1158/0008-5472.CAN-14-0155. PMID:
24840647.

3. Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. 2016; Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 264:457–463. DOI:
10.1097/SLA.0000000000001850. PMID:
27355262.
4. Ironside N, Barreto SG, Loveday B, Shrikhande SV, Windsor JA, Pandanaboyana S. 2018; Meta-analysis of an artery-first approach versus standard pancreatoduodenectomy on perioperative outcomes and survival. Br J Surg. 105:628–636. DOI:
10.1002/bjs.10832. PMID:
29652079.

5. Jiang X, Yu Z, Ma Z, Deng H, Ren W, Shi W, et al. 2020; Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: a meta-analysis. Int J Surg. 73:14–24. DOI:
10.1016/j.ijsu.2019.11.007. PMID:
31751791.

6. Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. 2009; Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford). 11:18–24. DOI:
10.1111/j.1477-2574.2008.00013.x. PMID:
19590619. PMCID:
PMC2697870.

7. Gockel I, Domeyer M, Wolloscheck T, Konerding MA, Junginger T. 2007; Resection of the mesopancreas (RMP): a new surgical classification of a known anatomical space. World J Surg Oncol. 5:44. DOI:
10.1186/1477-7819-5-44. PMID:
17459163. PMCID:
PMC1865381.

8. Kawabata Y, Hayashi H, Ishikawa N, Tajima Y. 2016; Total meso-pancreatoduodenum excision with pancreaticoduodenectomy in lower biliary tract cancer. Langenbecks Arch Surg. 401:463–469. DOI:
10.1007/s00423-016-1435-y. PMID:
27102325.

9. Adham M, Singhirunnusorn J. 2012; Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur J Surg Oncol. 38:340–345. DOI:
10.1016/j.ejso.2011.12.015. PMID:
22264964.

11. Xu J, Tian X, Chen Y, Ma Y, Liu C, Tian L, et al. 2017; Total mesopancreas excision for the treatment of pancreatic head cancer. J Cancer. 8:3575–3584. DOI:
10.7150/jca.21341. PMID:
29151943. PMCID:
PMC5687173.

12. Ramia JM, De-la-Plaza R, Manuel-Vazquez A, Lopez-Marcano A, Morales R. 2018; Systematic review of the mesopancreas: concept and clinical implications. Clin Transl Oncol. 20:1385–1391. DOI:
10.1007/s12094-018-1869-5. PMID:
29675778.

13. Nehme F, Lee JH. 2022; Preoperative biliary drainage for pancreatic cancer. Dig Endosc. 34:428–438. DOI:
10.1111/den.14081. PMID:
34275165.

14. S , Lahiri RP, Phillips M, Pinn G, Pencavel TD, Kumar R, et al. 2021; Which patients benefit from preoperative biliary drainage in resectable pancreatic cancer? Expert Rev Gastroenterol Hepatol. 15:855–863. DOI:
10.1080/17474124.2021.1915127. PMID:
34036856.

15. MG , van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M, et al. 2017; Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 161:365–372. DOI:
10.1016/j.surg.2016.06.058. PMID:
27692778.

16. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 161:584–591. DOI:
10.1016/j.surg.2016.11.014. PMID:
28040257.

17. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. 2017; The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 67:93–99. DOI:
10.3322/caac.21388. PMID:
28094848.

19. Isaji S, Murata Y, Kishiwada M. Neoptolemos JP, Urrutia R, Abbruzzese JL, Büchler MW, editors. 2018. New Japanese Classification of Pancreatic Cancer. Pancreatic Cancer. Springer New York;p. 1021–1037. DOI:
10.1007/978-1-4939-7193-0_84.

20. Peparini N. 2015; Mesopancreas: a boundless structure, namely the rationale for dissection of the paraaortic area in pancreaticoduodenectomy for pancreatic head carcinoma. World J Gastroenterol. 21:2865–2870. DOI:
10.3748/wjg.v21.i10.2865. PMID:
25780282. PMCID:
PMC4356904.

21. Khiem T, Hoi H, Hiep T, Khue K, Duy V, Inoue Y, et al. 2022; Total laparoscopic pancreaticoduodenectomy with left posterior superior mesenteric artery first-approach and plexus-preserving circumferential lymphadenectomy: step-by-step technique with a surgical case report (with video). World J Surg Oncol. 20:269. DOI:
10.1186/s12957-022-02730-y. PMID:
36028841. PMCID:
PMC9419321.

22. Hackert T, Werner J, Weitz J, Schmidt J, Büchler MW. 2010; Uncinate process first--a novel approach for pancreatic head resection. Langenbecks Arch Surg. 395:1161–1164. DOI:
10.1007/s00423-010-0663-9. PMID:
20582600.

24. Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, et al. 2014; Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 156:591–600. DOI:
10.1016/j.surg.2014.06.016. PMID:
25061003.

25. Coco D, Leanza S, Guerra F. 2019; Total pancreatectomy: indications, advantages and disadvantages - a review. Maedica (Bucur). 14:391–396.
26. Nguyen TK, Nguyen HH, Nguyen CL, Luong TH, Dinh LD, Le VD, et al. 2022; Case report: Candidiasis of gastrojejunostomosis after pancreaticoduodenectomy: preliminary experience from two cases. Front Oncol. 12:888927. DOI:
10.3389/fonc.2022.888927. PMID:
36091142. PMCID:
PMC9449841.

27. Nguyen TK, Luong TH, Nguyen NC, Nguyen HH, Le VK, Trinh HS, et al. 2021; Hepatic lymphorrhea following pancreaticoduodenectomy: preliminary diagnosis and treatment experience from case series of four patients. Ann Med Surg (Lond). 68:102648. DOI:
10.1016/j.amsu.2021.102648. PMID:
34386232. PMCID:
PMC8346360.

28. Pessaux P, Varma D, Arnaud JP. 2006; Pancreaticoduodenectomy: superior mesenteric artery first approach. J Gastrointest Surg. 10:607–611. DOI:
10.1016/j.gassur.2005.05.001. PMID:
16627229.

29. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. 2021; Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 19:439–457. DOI:
10.6004/jnccn.2021.0017. PMID:
33845462.

30. Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA. 2012; 'Artery- first' approaches to pancreatoduodenectomy. Br J Surg. 99:1027–1035. DOI:
10.1002/bjs.8763. PMID:
22569924.
31. Nagakawa Y, Watanabe Y, Kozono S, Boggi U, Palanivelu C, Liu R, et al. 2021; Surgical approaches to the superior mesenteric artery during minimally invasive pancreaticoduodenectomy: a systematic review. J Hepatobiliary Pancreat Sci. 29:114–123. DOI:
10.1002/jhbp.905. PMID:
33523604.

32. Einama T, Takihata Y, Aosasa S, Konno F, Kobayashi K, Yonamine N, et al. 2023; Prognosis of pancreatic cancer based on resectability: a single center experience. Cancers (Basel). 15:1101. DOI:
10.3390/cancers15041101. PMID:
36831444. PMCID:
PMC9954753.

33. Okada K, Murakami Y, Kondo N, Uemura K, Nakagawa N, Seo S, et al. 2019; prognostic significance of lymph node metastasis and micrometastasis along the left side of superior mesenteric artery in pancreatic head cancer. J Gastrointest Surg. 23:2100–2109. DOI:
10.1007/s11605-019-04359-x. PMID:
31410820.

34. Kondo N, Uemura K, Nakagawa N, Okada K, Seo S, Takahashi S, et al. 2020; Superior mesenteric artery plexus-preserving pancreatoduodenectomy with circumferential dissection of lymph nodes. J Gastrointest Surg. 24:1712–1719. DOI:
10.1007/s11605-020-04629-z. PMID:
32410173.

35. Kim JR, Kim H, Kwon W, Jang JY, Kim SW. 2021; Pattern of local recurrence after curative resection in pancreatic ductal adenocarcinoma according to the initial location of the tumor. J Hepatobiliary Pancreat Sci. 28:105–114. DOI:
10.1002/jhbp.854. PMID:
33084211.

37. Gaedcke J, Gunawan B, Grade M, Szöke R, Liersch T, Becker H, et al. 2010; The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg. 395:451–458. DOI:
10.1007/s00423-009-0494-8. PMID:
19418067. PMCID:
PMC2848727.

38. Inoue Y, Saiura A, Yoshioka R, Ono Y, Takahashi M, Arita J, et al. 2015; Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach. Ann Surg. 262:1092–1101. DOI:
10.1097/SLA.0000000000001065. PMID:
25587814.

39. Ramia JM, De-la-Plaza R, Manuel-Vazquez A, Lopez-Marcano A, Morales R. 2018; Systematic review of the mesopancreas: concept and clinical implications. Clin Transl Oncol. 20:1385–1391. DOI:
10.1007/s12094-018-1869-5. PMID:
29675778.

40. Kuroki N, Ono Y, Sato T, Inoue Y, Oba A, Ito H, et al. 2022; Long-term outcome of patients with postoperative refractory diarrhea after tailored nerve plexus dissection around the major visceral arteries during pancreatoduodenectomy for pancreatic cancer. World J Surg. 46:1172–1182. DOI:
10.1007/s00268-022-06457-5. PMID:
35119513.

41. Inoue Y, Saiura A, Oba A, Kawakatsu S, Ono Y, Sato T, et al. 2019; Optimal extent of superior mesenteric artery dissection during pancreaticoduodenectomy for pancreatic cancer: balancing surgical and oncological safety. J Gastrointest Surg. 23:1373–1383. DOI:
10.1007/s11605-018-3995-3. PMID:
30306451.

42. Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Müller-Stich B, et al. 2017; The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford). 19:1001–1007. DOI:
10.1016/j.hpb.2017.07.007. PMID:
28838632.

43. Nguyen LT, Do DH, Van Nguyen H, Nguyen KT, Nguyen CD. 2022; Lymph node characteristics and short-term outcomes for resectable pancreatic ductal adenocarcinoma in vietnam: a retrospective single-center study. Indian J Surg. 84:458–463. DOI:
10.1007/s12262-022-03344-0.

44. Torres SM, Vaz da Silva DG, Ribeiro HSC, Diniz AL, Lobo MM, de Godoy AL, et al. 2020; Short-term outcomes after vascular resection for pancreatic tumors: lessons learned from 72 cases from a single Brazilian Cancer Center. J Surg Oncol. 121:857–862. DOI:
10.1002/jso.25799. PMID:
31808559.

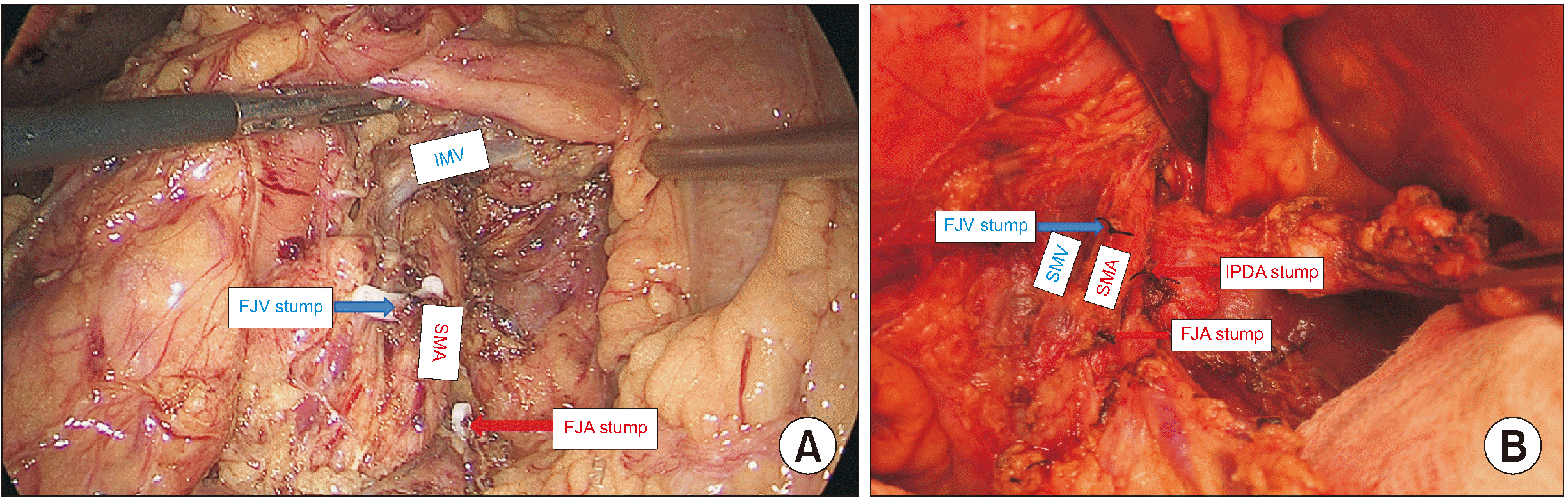
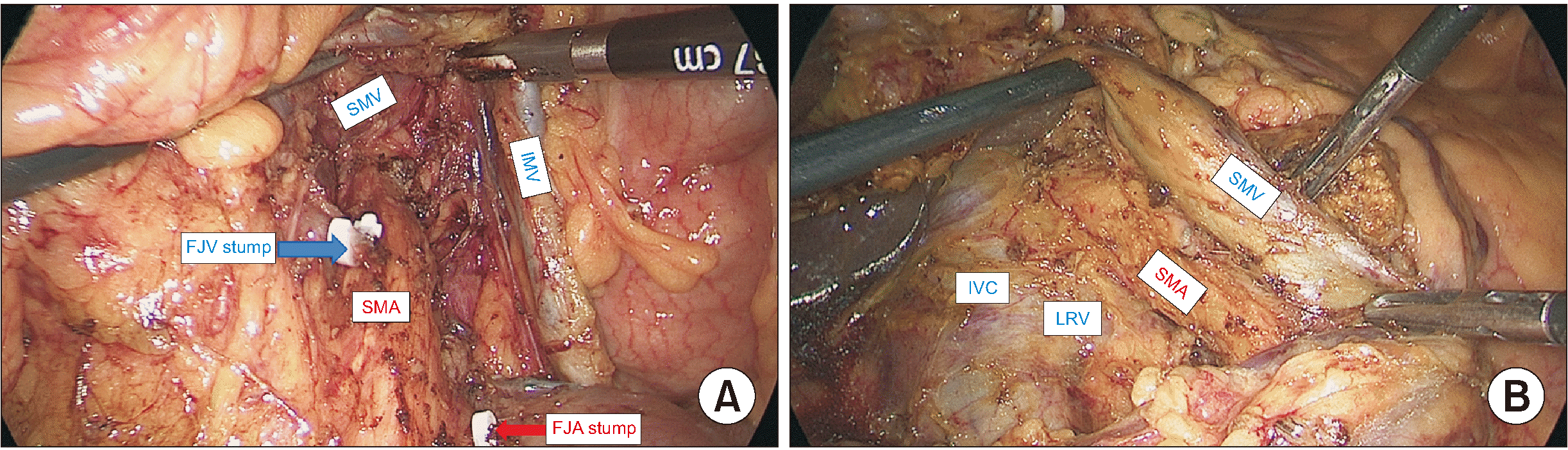
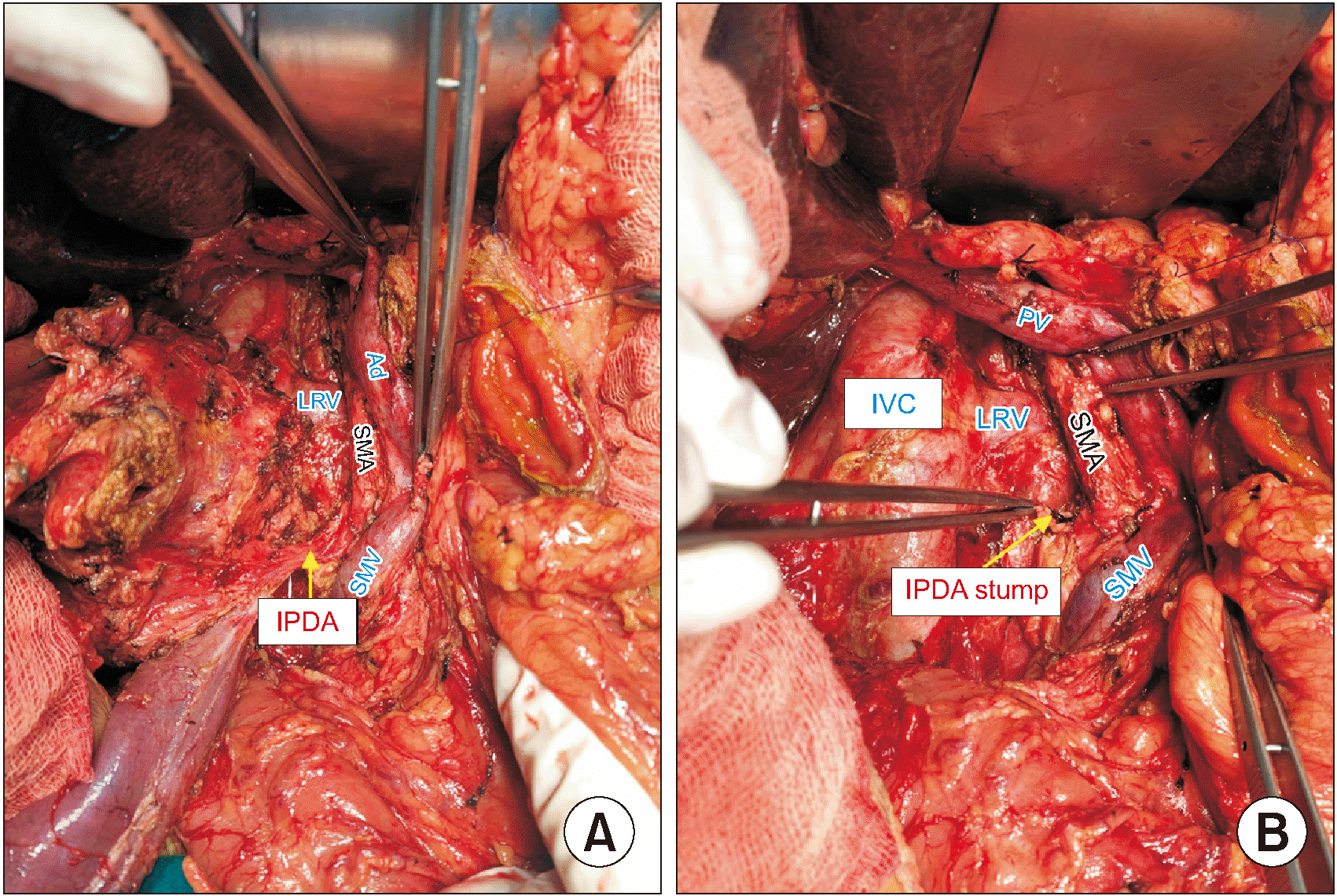
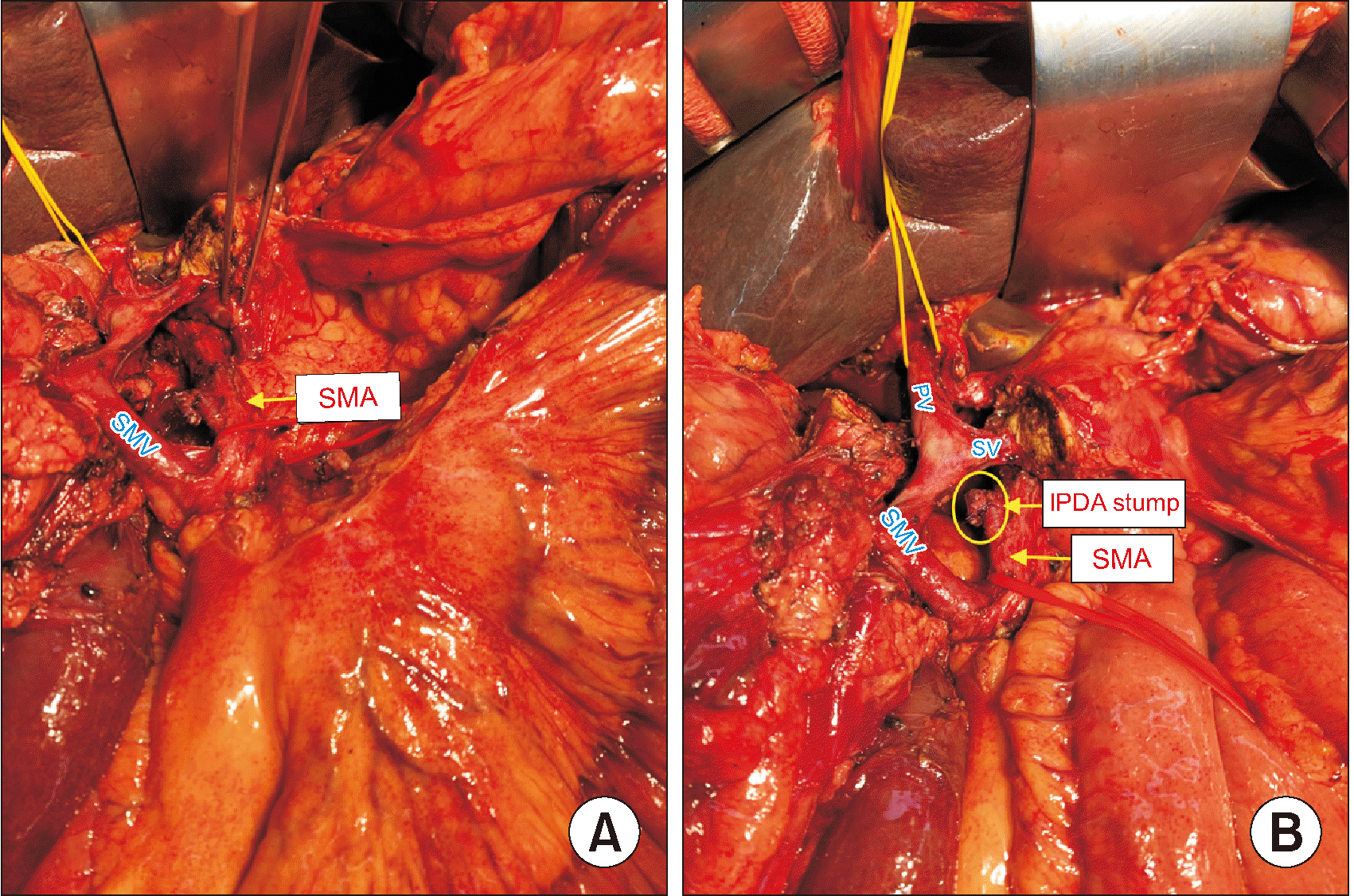
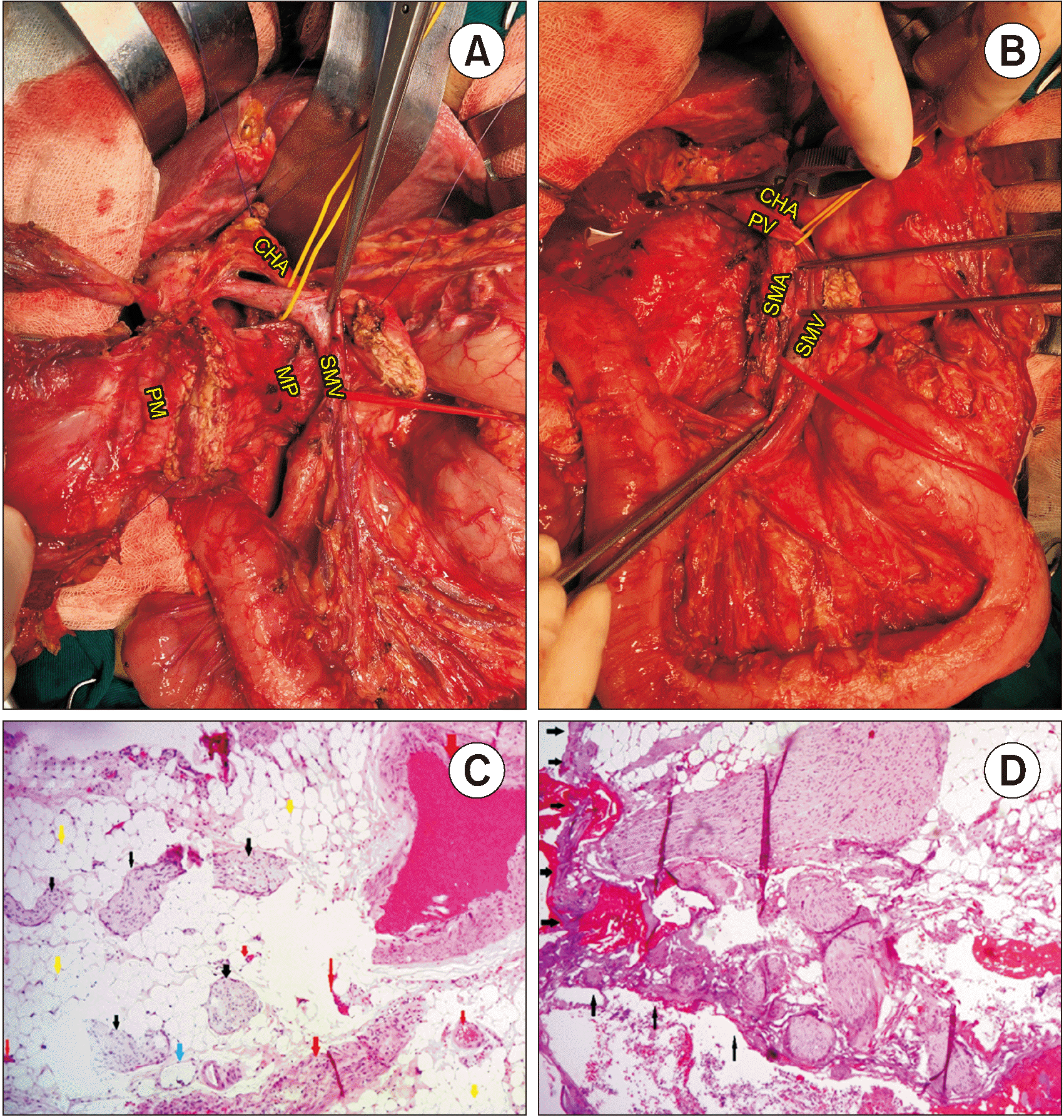
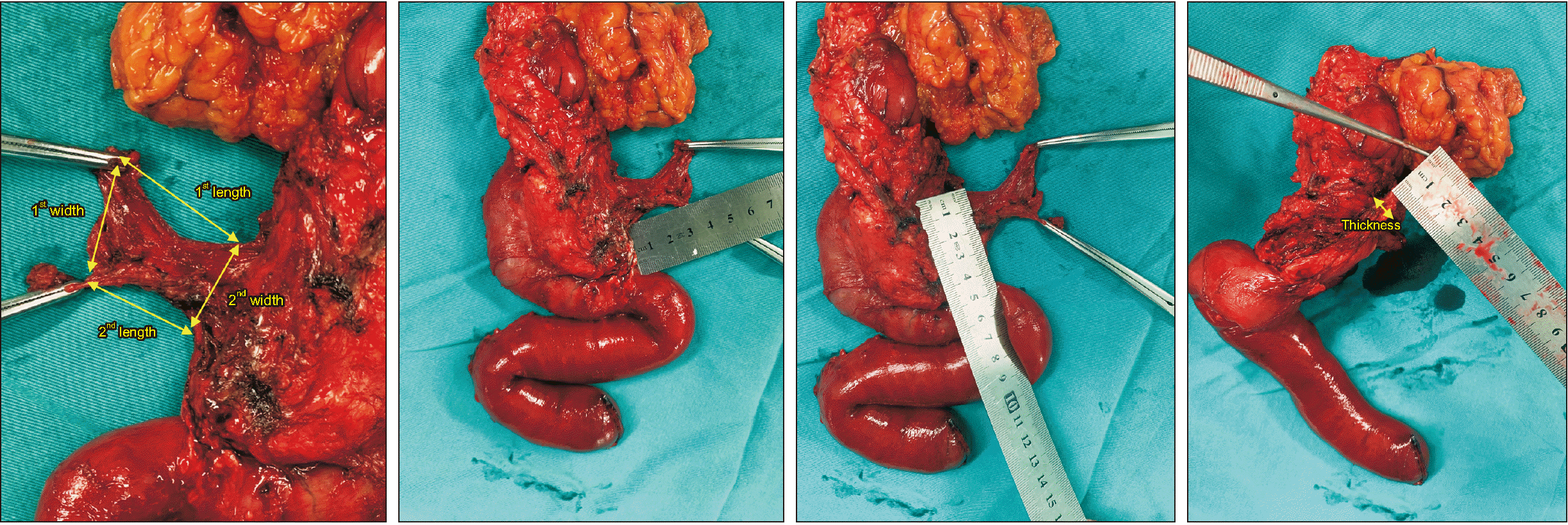
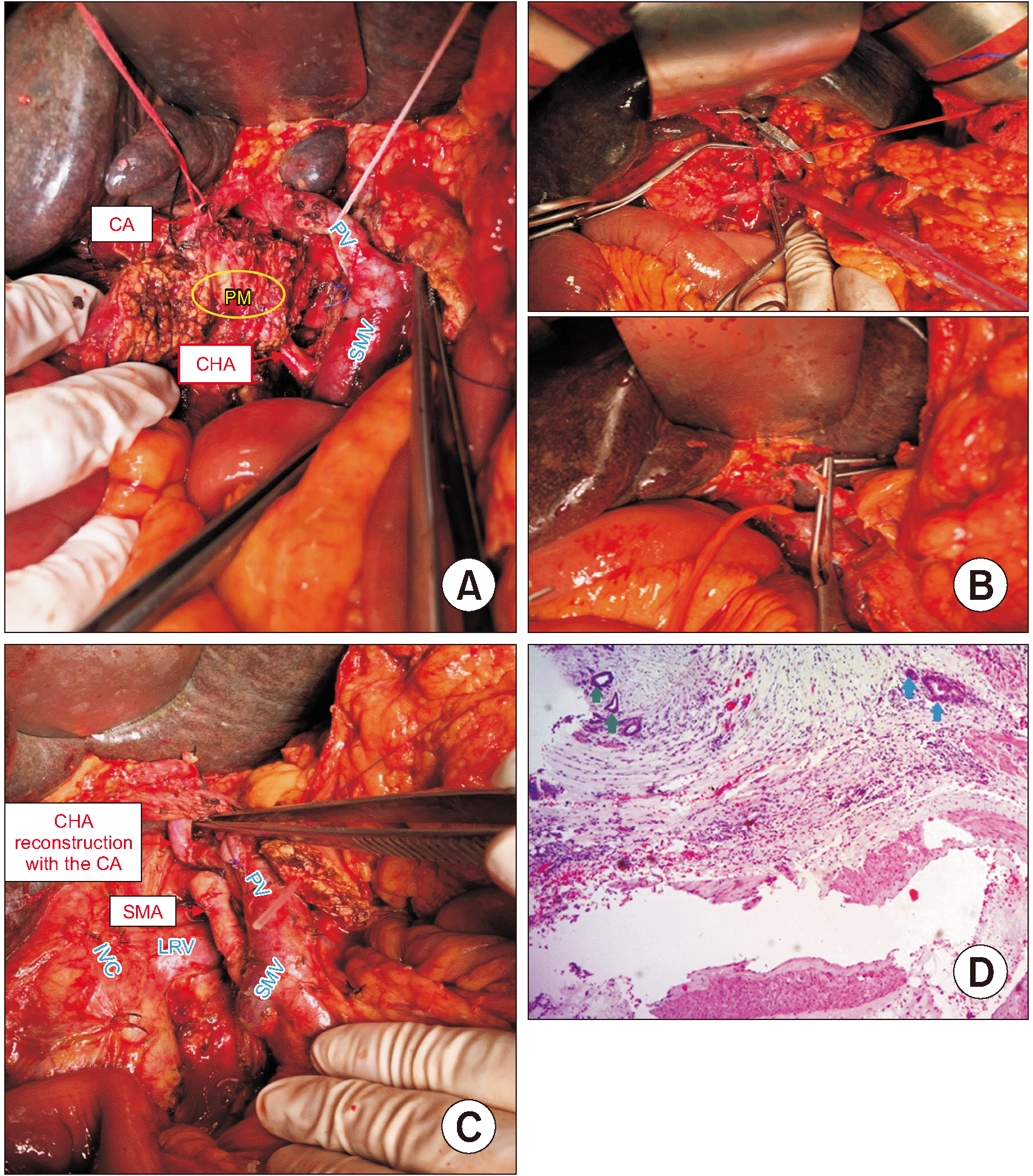
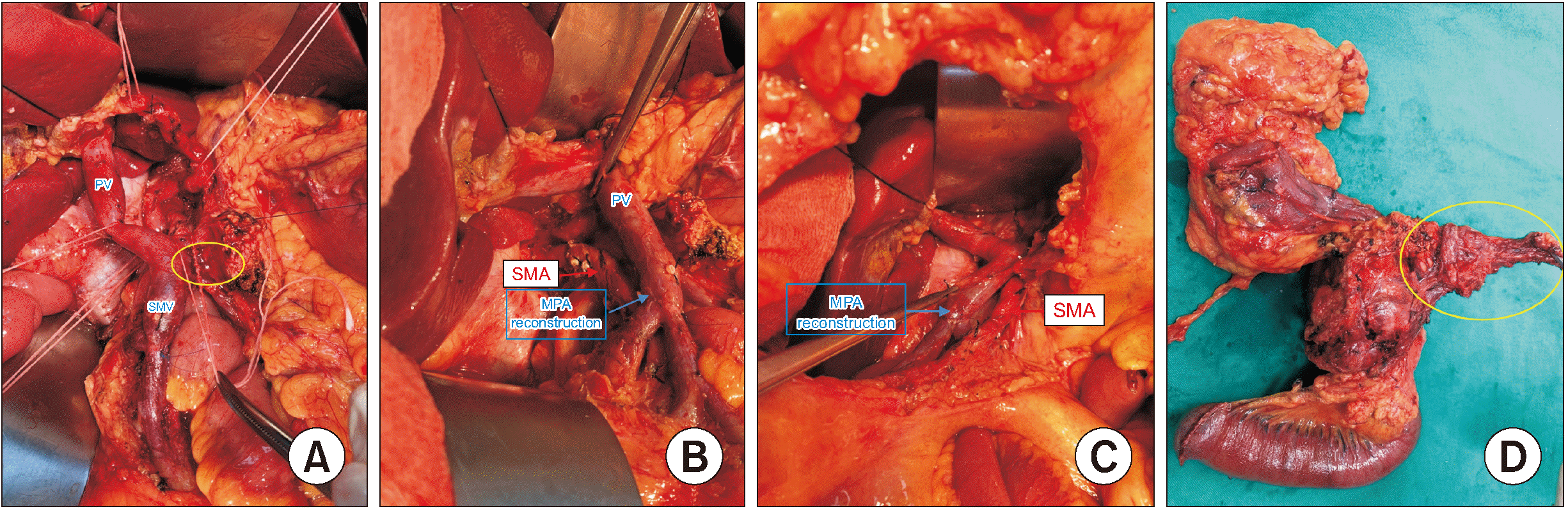




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download