Abstract
Backgrounds/Aims
Double duct sign (DDS) (dilated common bile and pancreatic duct) is synonymous with pancreatic head/peri-ampullary tumor (PHPAT). There is limited evidence on whether incidental DDS (I-DDS) is associated with an increased risk of malignancy. This study aimed to evaluate 5-year outcomes of I-DDS.
Methods
Patients were categorized according to their risk of malignancy. ‘Low-risk’ patients, including those with I-DDS between 2010 and 2015, were analyzed in this study. The primary outcome was incidence of PHPAT within five years of identification of DDS. Histology results from endoscopic ultrasound-guided biopsy were considered diagnostic. Secondary outcomes were incidence of benign causes, extent of follow-up investigations, and clinical indicators of malignancy in patients with DDS.
Results
Among 103 patients with DDS, 20 had I-DDS. Subsequent follow-up of these 20 patients found no patient with PHPAT, two (10%) patients with chronic pancreatitis, and 18 (90%) patients with no cause found. The median follow-up duration for ‘low-risk’ patients was 7.3 years (range, 6–11 years). The mean number of follow-up investigations per patient was two (range, 0–9). Investigations included computed tomography (n = 27), magnetic resonance cholangiopancreatography (n = 23), endoscopy (n = 16), and ultrasound (n = 14). Patients with jaundice were more likely to have malignancy (p < 0.01). Those with abdominal pain were more likely to have a benign cause (p < 0.01). Hyperbilirubinemia and/or deranged liver enzymes and raised CA19-9 were more likely to be associated with PHPAT (p < 0.01).
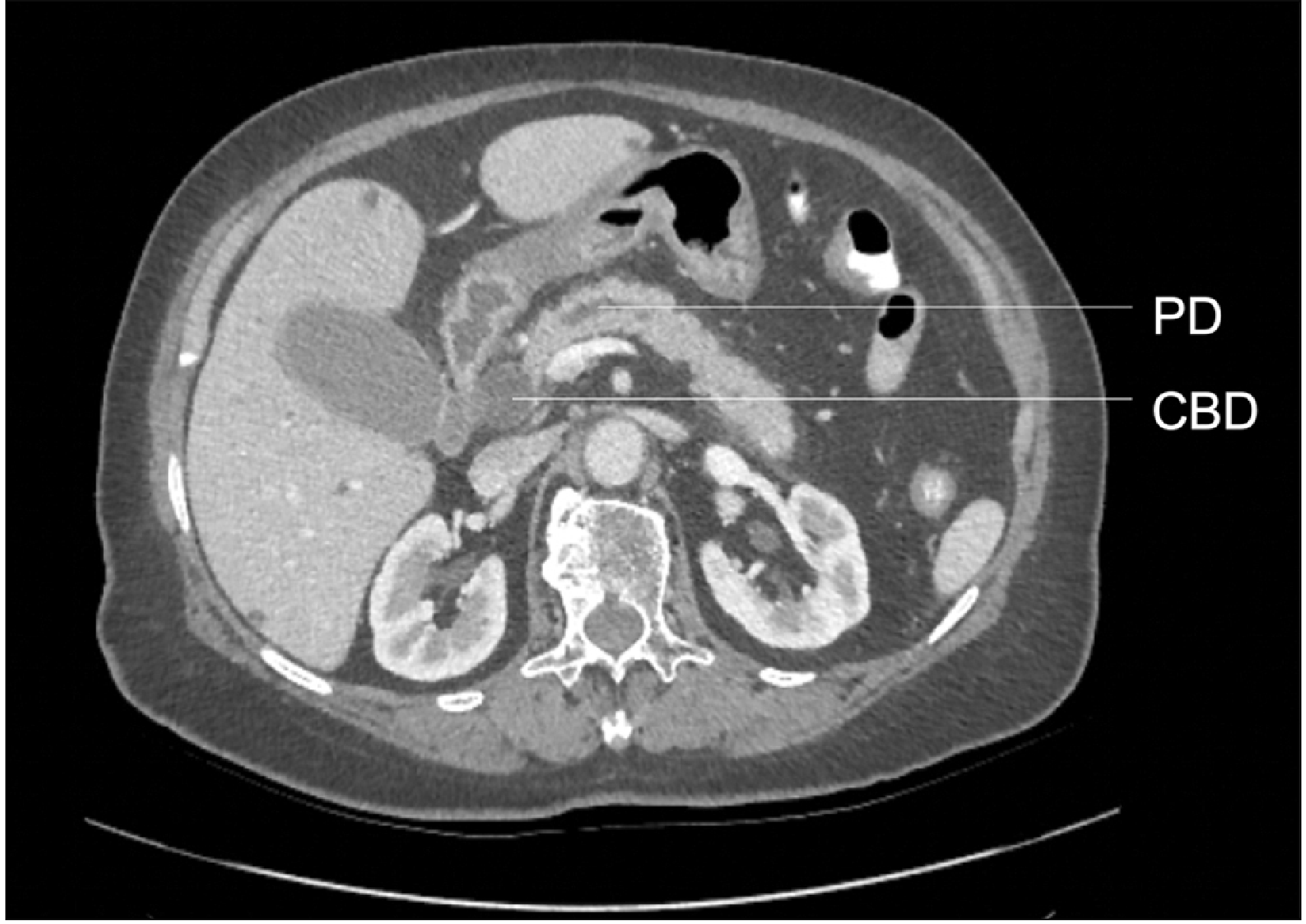
A double duct sign (DDS) is the presence of both a dilated common bile duct (CBD) and pancreatic duct (PD) on imaging [1-3]. Although this sign was first described in endoscopic retrograde cholangiopancreatography (ERCP) [2,4], it is also seen in magnetic resonance cholangiopancreatography (MRCP) (Fig. 1), computed tomography (CT) (Fig. 2), and ultrasound (US) [1,2]. Simultaneous dilation of CBD and PD is most often caused by an intra- or extra-ductal stricture at the junction between the two ducts or further downstream. Obstruction at this level can result in blockage of bile and pancreatic secretions, leading to DDS. This radiological sign is mainly synonymous with pancreatic head/peri-ampullary tumor (PHPAT) [1].
Research has shown that the prevalence of PHPAT ranges from 58% to 85% in patients with DDS [3]. Up to 10% of patients with DDS have a benign cause [4], including chronic pancreatitis and CBD stones. Rarer causes include lymphoma and retroperitoneal fibrosis [1,2]. In a small number of cases, no responsible pathology can be found despite additional investigations. However, there is little evidence regarding the long-term risk of malignancy in these patients [3,5-9].
Some studies advocate investigation with endoscopic ultrasound (EUS) due to its diagnostic accuracy for malignancy [9-12]. However, there are no explicit guidelines on the timing or extent of investigative processes for unexplained DDS [3]. In our hospital, these patients are currently investigated with cross-sectional imaging and blood tests, including liver function tests (LFTs–alkaline phosphatase, alanine transaminase, and bilirubin levels are routinely performed) and haemoglobin levels. All biochemical and cross-sectional results are then reviewed by a multidisciplinary team through discussion to decide if EUS is indicated to identify any occult malignancy and plan for further management.
This study aimed to evaluate 5-year risk of malignancy in patients with unexplained DDS according to their clinical presentation and assess methods and extent of follow-up used.
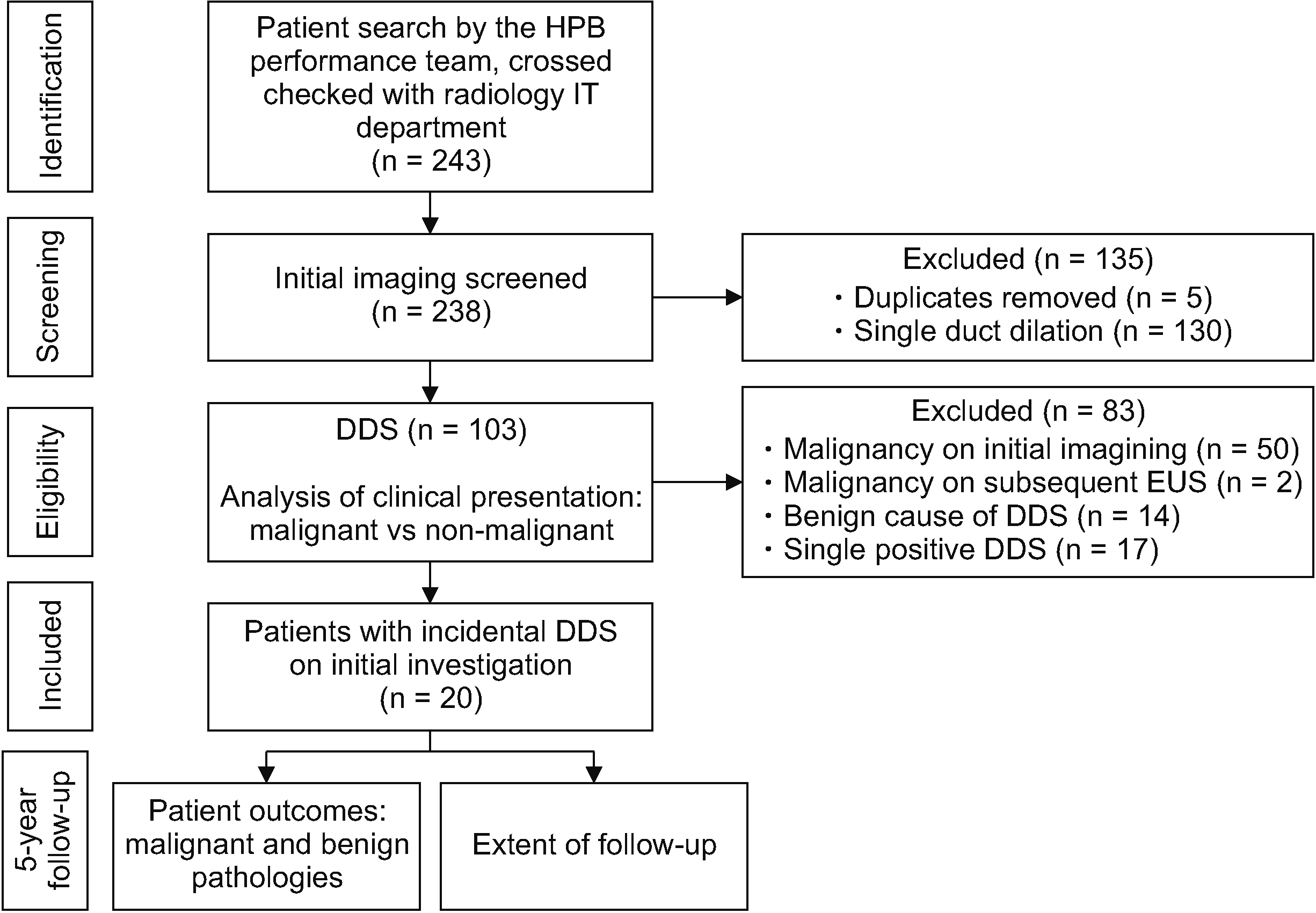
This retrospective cohort study was conducted at a single tertiary center in the United Kingdom. It was approved by the hospital audit department (CA_2020-21-335). Patients with radiologically detected DDS between January 2010 and December 2015 were identified using data from Hepatobiliary-Pancreatic Surgery and Radiology databases (Fig. 3). In our hospital, all cross-sectional images (including CT and MRI) were reviewed and reported by one of six experienced consultant radiologists specializing in Gastrointestinal Radiology. Patients were classified into four categories regarding risk stratification for malignancy according to blood results and features on imaging (Table 1). The likelihood of cancer increased with each level of ‘positivity.’ ‘Triple negative’ was considered for patients with isolated DDS and normal blood/imaging/histological results and ‘triple positive’ was considered for patients with histologically confirmed malignancy. Blood results considered were haemoglobin, bilirubin, liver enzymes, and tumor markers. These tests were chosen for their associations with malignancy. Elevated bilirubin and liver enzymes have been shown to be associated with an increased risk of malignant biliary strictures [13]. Low haemoglobin is well known to be associated with malignancy as a result of chronic inflammation and occult bleeding [14]. Normal imaging results were considered for those without any evidence of malignancy, whereas descriptive terms such as ‘mass’ or ‘lesion’ were deemed suspicious and abnormal. Histological results were based on tissue biopsy reports. Objective categorization of patients according to their clinical risk of cancer might aid in planning further investigations and management. For this study, only triple-negative patients were regarded as having incidental DDS (I-DDS). Triple-negative and single-positive patients were both considered as ‘low-risk.’
The incidence of PHPAT within five years of identification of DDS in ‘low-risk’ patients was noted. Endoscopic ultrasound-guided biopsy (EUSB) confirming malignancy was considered diagnostic. The primary outcome was subsequent diagnosis of a benign or malignant cause of DDS during five years of follow-up in those categorized as triple-negative (I-DDS) and single-positive after initial investigations. ‘Initial investigation’ was defined as the first scan identifying the presence of DDS. The extent of follow-up investigations (including frequency and type) was also analyzed for ‘low-risk’ patients.
Once final diagnoses were determined during the five-year follow-up period, patients were re-categorized into ‘malignant’ and ‘non-malignant’ groups. Further analysis of differences in clinical presentation between both groups was performed, including the presenting complaint, presence of anaemia, hyperbilirubinaemia, and/or deranged LFTs and tumor markers. Tumor markers were CA19-9, carcinoembryonic antigen (CEA), and CA125 with analysis performed within three months before or after imaging diagnosis of DDS. These groups were compared using Wilcoxon rank-sum, chi-squared, and Fisher’s exact tests. All statistical analyses were performed using RStudio (version 1.4.1106).
Amongst 103 patients with DDS, 20 (19%) were found to have incidental (triple-negative) double-duct sign (Fig. 3). These patients had the following initial diagnostic investigations: US abdomen (n = 6), CT of abdomen (n = 12), and MRCP (n = 2). Demographic and biochemical data of ‘low-risk’ patients are described in Table 2. Of those with I-DDS, 90% were females. The median age of ‘low-risk’ patients was 69 years (range, 46–86 years). None of these triple-negative patients was diagnosed with PHPAT within five years of follow-up. Two (10%) patients were diagnosed with benign pathologies, of which both were chronic pancreatitis. In the remaining 18 (90%) patients, the cause of DDS was not identified during follow-up.
Other pathologies not known to cause DDS were also reviewed. Malignancies found during follow-up included lung cancer (n = 2) and liver cancer (n = 1). Benign pathologies during follow-up included extra-hepatic ductal gallstones (n = 1), oesophageal stricture (n = 1), and Barrett’s oesophagus (n = 1).
Nineteen (18%) cases were found to be single-positive. In these patients, 9, 5, and 5 had DDS detected using US, CT, and MRCP, respectively. Females accounted for 68%. The median age of single-positive patients was 70 years (range, 26–94 years). Of these patients, two (11%) were diagnosed with periampullary malignancy on EUSB within five years of follow-up. These patients were diagnosed with cholangiocarcinoma and ampullary cancer at 51 and 28 days, respectively, after DDS was identified. Both patients presented with jaundice and deranged LFTs. For the remaining 17 (89%) patients, no cause of DDS was identified during follow-up despite having abnormal blood tests. Other pathologies detected included lung cancer (n = 1), pulmonary metastasis of unknown origin (n = 1), prostate cancer (n = 1), and extra-hepatic ductal gallstones (n = 1).
Overall, the median follow-up for ‘low-risk’ cases where no malignancy was detected was 7.5 (range, 6–11) years and the median follow-up for benign pathologies (excluding extra-pancreatic or biliary malignancies such as lung cancer) was 7.1 (range, 6–11) years. The mean number of follow-up investigations performed per patient was two (range, 0–9). The most common investigation conducted was CT (n = 27), followed by MRCP (n = 23), endoscopy (n = 16), and US (n = 14).
This study further analyzed early indicators of malignancy. All 103 patients were re-categorized into ‘malignant’ and ‘non-malignant’ groups to explore the likelihood of malignancy based on clinical presentation, including presenting complaint, biochemical tests, and tumor markers (Table 3). This is because clinicians should assess the whole clinical picture of patients rather than isolated imaging results to determine if further investigations are needed and the extent of invasiveness of such investigations. Those with incidental and benign causes (e.g., pancreatitis) of DDS were categorized into the ‘non-malignant’ group. Those with PHPAT were categorized into the ‘malignant’ group. Patients with I-DDS on initial investigations but were subsequently found to have malignant or benign pathologies during follow-up were categorized into appropriate groups according to their follow-up diagnosis.
Of 103 patients with DDS, 52 (50.5%) had malignant disease and 51 (49.5%) had non-malignant causes. There was no significant difference in median age (71 years vs. 69 years, p = 0.26) or sex (62% vs. 73% female, p = 0.32) between both groups. The malignant group was significantly more likely to present with jaundice (63%; p < 0.01), whereas the non-malignant group was more likely to present with abdominal pain (59%; p < 0.01). Nineteen percent of patients in the malignant group also complained of abdominal pain, which was significantly less than that in the non-malignant group. Interestingly, those who presented with weight loss were less likely to have malignancy (malignant, 4%; non-malignant, 18%), although this did not reach statistical significance (p = 0.05). Anaemia appeared to be a poor indicator of malignancy. However, hyperbilirubinemia and/or deranged liver enzymes (p < 0.01) and raised CA19-9 (p < 0.01) were likely to be associated with malignancy.
A double-duct sign is frequently associated with PHPAT. However, research has shown that there are exceptions [1,2]. Most clinicians will investigate I-DDS due to the clinical significance of missing potential cancer diagnosis [11,12]. Evidence suggests that abnormalities on imaging can precede a diagnosis of pancreatic cancer by several months [9,12]. Pancreatic cancer is known to progress rapidly with a high mortality rate compared to ampullary tumors, which are often detected earlier [15,16]. However, it is not easy to differentiate between them without formal histology following surgical resection [16]. Therefore, thorough pre-operative investigation is needed.
In our study, no triple-negative patients and only two of 19 single-positive patients had PHPAT detected during follow-up, suggesting that we might have over-investigated these patients. In contrast, patients with specific clinical features, such as jaundice, hyperbilirubinaemia and deranged LFTs, were more likely to have a higher diagnostic yield for malignancy, compared to patients with normal LFTs and tumor markers. One study has stated that the risk of malignancy in patients with DDS without clinical signs, particularly obstructive jaundice, is as low as 5.9%–7.3% [9]. Other studies have also shown that malignancy is more likely for those with DDS and obstructive jaundice [3,17]. Therefore, it might be more rational to investigate I-DDS depending on clinical presentation, including biochemical and radiological features.
Biochemical evidence in the form of tumor markers might support further investigation of I-DDS. Our results showed that CA19-9 and CA125 levels were more likely to be increased in patients with malignancy, although the increase was only statistically significant for CA19-9. Although those with increased CA19-9 levels are highly sensitive to pancreatic carcinoma, the literature has reported that CA19-9 levels can also be elevated in 14% of patients with benign diseases, namely pancreatitis and other gastrointestinal cancers [18]. Another study has found that combined testing of CA19-9 with CEA and carbohydrate antigen 242 can increase the specificity for pancreatic and peri-ampullary malignancy [19].
Previous research studies have also shown that certain radiological features are more likely to indicate malignancy. For example, ductal dilatation with abrupt tapering rather than diffuse dilatation on cross-sectional imaging such as CT and MRI is more likely to indicate a malignant cause [8,12]. However, EUS is known to have a higher diagnostic yield for malignancy than CT and MRCP [7] (negative predictive value of 100% for pancreatic cancer compared to 28% for CT [12]), which suggests that EUS should be the investigation of choice.
Furthermore, the benefit of investigating patients with DDS should be balanced against the risk of performing investigations. Although complications including haemorrhage, perforation, infection, and acute pancreatitis are rare, their clinical impact can be devastating [20]. Invasive testing with EUSB should be considered carefully, especially for older and co-morbid patients. For patients who cannot tolerate endoscopic, surgical, or oncological management of malignancy, clinicians might argue that risks of undergoing EUSB outweigh its benefits.
It has been estimated that a standard ERCP in the UK costs £794 [21]. Investigating single- or double-positive patients with a higher risk of malignancy rather than triple-negative patients would be a safer and more cost-effective option. We propose the following algorithm for patients with DDS identified on imaging with no apparent cause found (Fig. 4). Our algorithm presents a single follow-up method (with blood tests and cross-sectional imaging) for triple-negative patients with inconclusive results and EUS to investigate single-positive patients. Patients with inconclusive follow-up investigations can be discharged. We suggest a follow-up period of six months given that only two single-positive patients were found to have malignancy within 3 months of follow-up in our study.
This study has some limitations given its small sample size in a single center. Results of this study might be different from other settings with varying population demographics and availability of resources. Although results from this study suggest that it is safe to discharge triple-negative and single-positive patients from follow-up, larger-scale research across multiple centers is needed to confirm our findings.
In conclusion, patients with DDS with no initial worrying clinical, radiological, or biochemical features have a low risk of developing PHPAT within five years. Investigation of DDS should be tailored to those with abnormal clinical features depending on their fitness for investigation and treatment. Patients with DDS and no apparent cause identified in a short-term do not require long-term follow-up.
ACKNOWLEDGEMENTS
The authors thank Plymouth University Hospital Hepato-Pancreatico-Biliary performance team for obtaining the patient list.
REFERENCES
1. Ahualli J. 2007; The double duct sign. Radiology. 244:314–315. DOI: 10.1148/radiol.2441041978. PMID: 17581912.

2. Yadav P, Lal H. 2017; Double duct sign. Abdom Radiol (NY). 42:1283–1284. DOI: 10.1007/s00261-016-0979-1. PMID: 27837241.

3. Sinha R, Gardner T, Padala K, Greenaway JR, Joy D. 2015; Double-duct sign in the clinical context. Pancreas. 44:967–970. DOI: 10.1097/MPA.0000000000000372. PMID: 26087354.

4. Baillie J, Paulson EK, Vitellas KM. 2003; Biliary imaging: a review. Gastroenterology. 124:1686–1699. DOI: 10.1016/S0016-5085(03)00390-1. PMID: 12761726.
5. Cohen J, Sawhney MS, Pleskow DK, Chuttani R, Patel NJ, Sheridan J, et al. 2014; Double-duct sign in the era of endoscopic ultrasound: the prevalence of occult pancreaticobiliary malignancy. Dig Dis Sci. 59:2280–2285. DOI: 10.1007/s10620-014-3133-3. PMID: 24705640.

6. Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. 2019; Chronic pancreatitis or pancreatic tumor? a problem- solving approach. Radiographics. 39:1965–1982. DOI: 10.1148/rg.2019190011. PMID: 31584860.

7. Menges M, Lerch MM, Zeitz M. 2000; The double duct sign in patients with malignant and benign pancreatic lesions. Gastrointest Endosc. 52:74–77. DOI: 10.1067/mge.2000.105775. PMID: 10882966.

8. Gangi S, Fletcher JG, Nathan MA, Christensen JA, Harmsen WS, Crownhart BS, et al. 2004; Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 182:897–903. DOI: 10.2214/ajr.182.4.1820897. PMID: 15039161.

9. Krishna N, Tummala P, Reddy AV, Mehra M, Agarwal B. 2012; Dilation of both pancreatic duct and the common bile duct on computed tomography and magnetic resonance imaging scans in patients with or without obstructive jaundice. Pancreas. 41:767–772. DOI: 10.1097/MPA.0b013e31823ba536. PMID: 22450366.

10. Malik S, Kaushik N, Khalid A, Bauer K, Brody D, Slivka A, et al. 2007; EUS yield in evaluating biliary dilatation in patients with normal serum liver enzymes. Dig Dis Sci. 52:508–512. DOI: 10.1007/s10620-006-9582-6. PMID: 17211694.

11. Coss A, Enns R. 2009; The investigation of unexplained biliary dilatation. Curr Gastroenterol Rep. 11:155–159. DOI: 10.1007/s11894-009-0024-4. PMID: 19281704.

12. Agarwal B, Krishna NB, Labundy J, Safdar R, Akduman EI. 2008; EUS and/or EUS-guided FNA in patients with CT and/or magnetic resonance imaging findings of enlarged pancreatic head or dilated pancreatic duct with or without a dilated common bile duct. Gastrointest Endosc. 68:237–242. DOI: 10.1016/j.gie.2008.01.026. PMID: 18423464.

13. Thomasset SC, Saunders D, Holland A, Dennison AR, Garcea G. 2015; Malignant biliary strictures in patients with a normal bilirubin and/or normal liver enzymes. HPB (Oxford). 17:969–974. DOI: 10.1111/hpb.12468. PMID: 26256123. PMCID: PMC4605334.

14. Natalucci V, Virgili E, Calcagnoli F, Valli G, Agostini D, Zeppa SD, et al. 2021; Cancer related anemia: an integrated multitarget approach and lifestyle interventions. Nutrients. 13:482. DOI: 10.3390/nu13020482. PMID: 33535496. PMCID: PMC7912724.

15. McGuigan A, Kelly P, Turkington R, Jones C, Coleman HG, McCain RS. 2018; Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 24:4846–4861. DOI: 10.3748/wjg.v24.i43.4846. PMID: 30487695. PMCID: PMC6250924.

16. Paluri RK, Kasi A. 2022. Ampullary Cancer [Internet]. StatPearls Publishing;Available from: https://www.ncbi.nlm.nih.gov/books/NBK555958/. cited 2022 Dec 22.
17. Gardner T, Padala K, Sinha R, Greenaway J, Joy D. 2014; PTH-105 how ominous is the "double-duct" sign? a single centre experience. Gut. 63:A257. DOI: 10.1136/gutjnl-2014-307263.551.
18. Safi F, Roscher R, Bittner R, Schenkluhn B, Dopfer HP, Beger HG. 1987; High sensitivity and specificity of CA 19-9 for pancreatic carcinoma in comparison to chronic pancreatitis. Serological and immunohistochemical findings. Pancreas. 2:398–403. DOI: 10.1097/00006676-198707000-00006. PMID: 3306667.

19. Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, et al. 2005; The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 31:164–169. DOI: 10.1016/j.ejso.2004.09.007. PMID: 15698733.

20. Mizuide M, Ryozawa S, Fujita A, Ogawa T, Katsuda H, Suzuki M, et al. 2020; Complications of endoscopic ultrasound-guided fine needle aspiration: a narrative review. Diagnostics (Basel). 10:964. DOI: 10.3390/diagnostics10110964. PMID: 33213103. PMCID: PMC7698484.

21. National Institute for Health and Care Excellence (NICE). 2015. The SpyGlass direct visualisation system for diagnostic and therapeutic procedures during endoscopy of the biliary system [MIB21] [Internet]. NICE;Available from: https://www.nice.org.uk/advice/mib21. cited 2022 Dec 22.
Fig. 1
Double duct sign on magnetic resonance cholangiopancreatography imaging. CBD, common bile duct; PD, pancreatic duct.
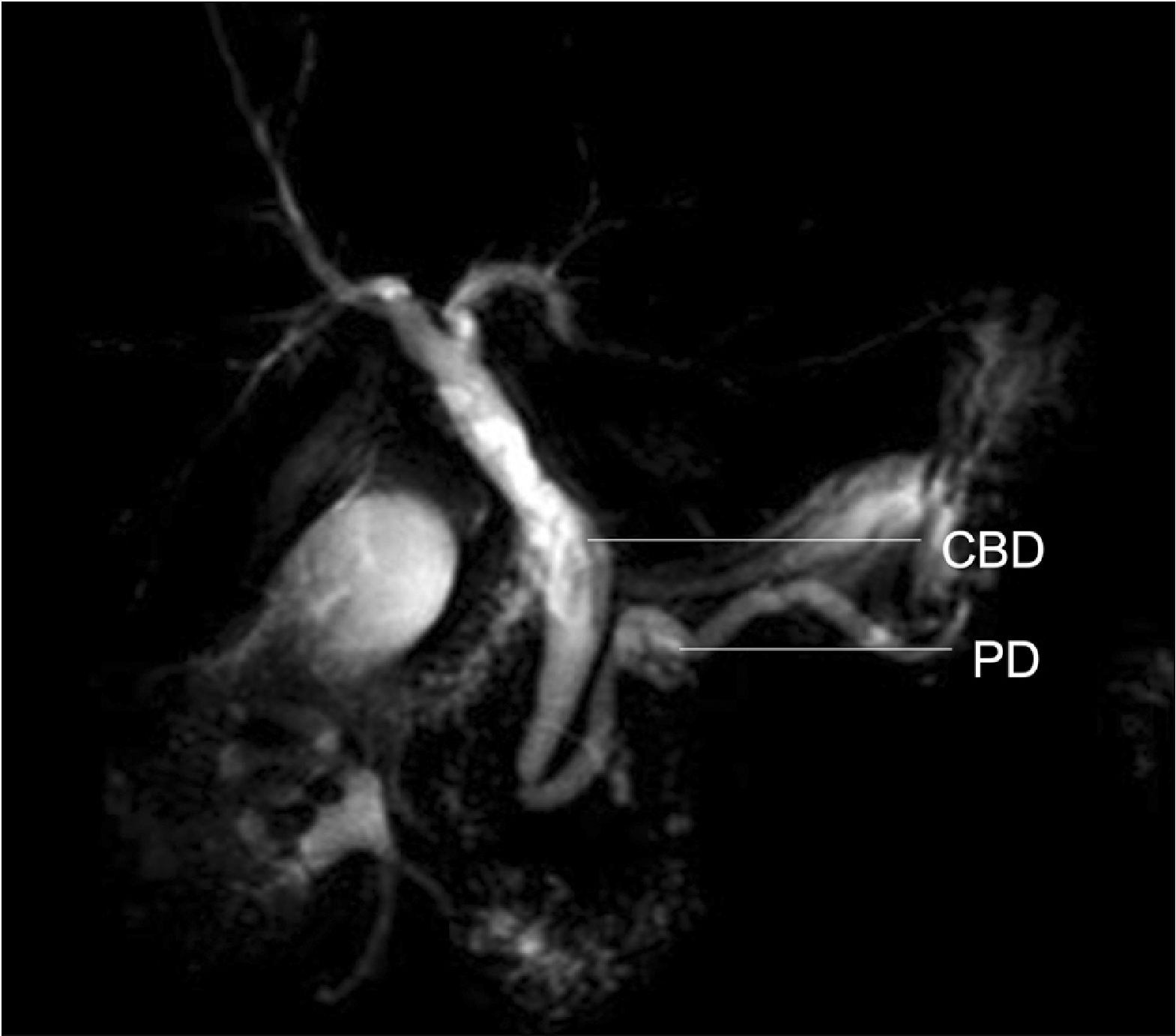
Fig. 4
Proposed investigation pathway for patients with incidental DDS. DDS, double duct sign; LFT, liver function test; MRCP, magnetic resonance cholangiopancreatography; MDT, multidisciplinary team; CEA, carcinoembryonic antigen; EUS, endoscopic ultrasound.
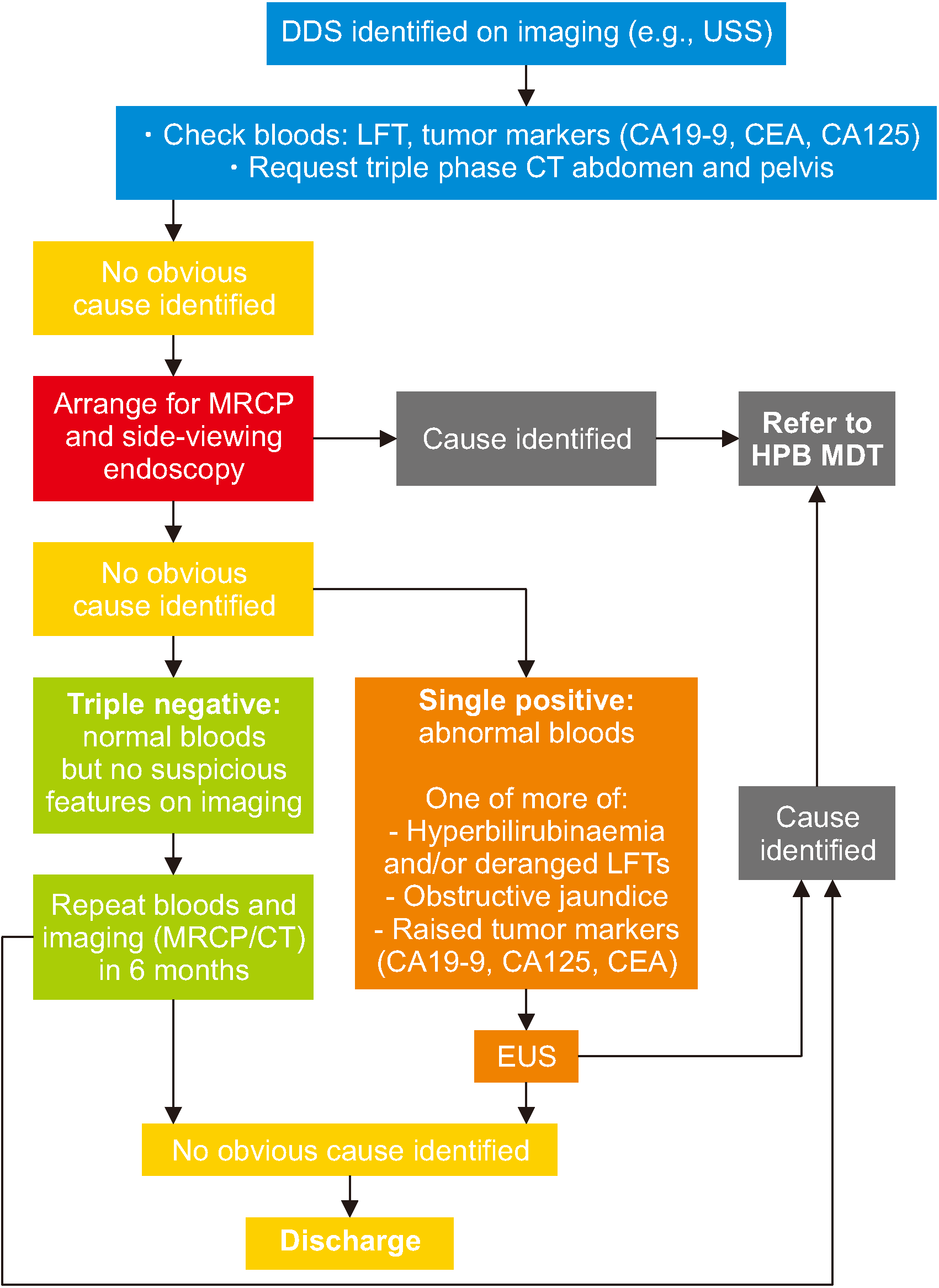
Table 1
Classification of the likelihood of malignancy
| Classification | Description |
|---|---|
| Triple-negativea) | Patients with DDS but no other suspicious findings. |
| Single-positive |
Abnormal biochemistry results such as: • Anaemia • Hyperbilirubinaemia and/or deranged liver enzymes • Elevated tumor markers (CA19-9 or CA125) |
| Double-positive |
Single positive AND suspicious non-invasive scanb) report about the pancreas or ampullary duct, such as: • ‘Mass’ • ‘Thickening’ • ‘Suspicious of tumor’ |
| Triple-positive | Double-positive AND endoscopic US-guided biopsy confirming malignancy |
Table 2
Demographics, presenting complaints, and biochemical markers of single-positive and triple-negative patients with double-duct sign
| Single-positive patients | Triple-negative patients | |
|---|---|---|
| Basic demographics | ||
| No. of patients | 19 | 20 |
| Median age (yr) | 70 | 69 |
| Sex (F:M [% female]) | 13:6 (68) | 18:2 (90) |
| Presenting complaint | ||
| Jaundice | 3 (16) | 0 (0) |
| Abdominal pain | 7 (37) | 15 (75) |
| Asymptomatic | 3 (16) | 0 |
| Weight loss | 4 (21) | 5 (25) |
| Vomiting | 0 (0) | 0 (0) |
| Abdominal distension | 0 (0) | 0 (0) |
| Haematemesis | 1 (5) | 0 (0) |
| Confusion | 1 (5) | 0 (0) |
| Biochemical markers | ||
| Anaemiaa) | 7 (37) | 7 (35) |
| Hyperbilirubinaemia and/or deranged liver enzymesb) | 19 (100) | 0 |
| CA19-9 performed | 8 (42) | 6 (30) |
| Abnormal CA19-9c) | 3 (16) | 0 (0) |
| CA125 performed | 0 (0) | 2 (10) |
| Abnormal CA125d) | NA | 0 (0) |
| No tumor markers | 9 (47) | 12 (60) |
Table 3
Demographics, presenting complaints, and biochemical parkers of patients with double duct sign and malignant versus non-malignant pathologies
| Malignant | Non-malignant | p-value | |
|---|---|---|---|
| Basic demographics | |||
| No. of patients | 52 (50.5) | 51 (49.5) | |
| Median age (yr) | 71 | 69 | 0.26 |
| Sex (F:M [% female]) | 32:20 (62) | 47:14 (73) | 0.33 |
| Presenting complaint | |||
| Jaundice | 33 (63) | 4 (8) | < 0.01 |
| Abdominal pain | 10 (19) | 30 (59) | < 0.01 |
| Asymptomatic | 4 (8) | 5 (10) | > 0.99 |
| Weight loss | 2 (4) | 9 (18) | 0.05 |
| Vomiting | 1 (2) | 0 (0) | > 0.99 |
| Abdominal distension | 0 (0) | 1 (2) | > 0.99 |
| Haematemesis | 0 (0) | 1 (2) | > 0.99 |
| Biochemical markers | |||
| Anaemiaa) | 18 (35) | 17 (33) | 0.76 |
| Hyperbilirubinaemia and/or deranged liver enzymesb) | 35 (67) | 21 (41) | < 0.01 |
| CA19-9 performed | 27 (52) | 15 (29) | N/A |
| Abnormal CA19-9c) | 22 (42) | 3 (6) | < 0.01 |
| CA125 performed | 3 (6) | 4 (8) | N/A |
| Abnormal CA125d) | 2 (4) | 0 (0) | 0.14 |
| No tumor markers | 19 (37) | 29 (57) | N/A |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download