Abstract
Backgrounds/Aims
Parenchymal-sparing anatomical hepatectomy (Ps–AH) based on portal ramification of the right anterior section (RAS) is a new technique to avoid unnecessarily transecting too much liver parenchyma, especially in cases of major anatomical hepatectomy.
Methods
We prospectively assessed 26 patients with primary hepatic malignancies having undergone major Ps–AH based on portal ramification of the RAS from August 2018 to August 2022 (48 months). The perioperative indications, clinical data, intra-operative index, pathological postoperative specimens, postoperative complications, and follow-up results were retrospectively evaluated.
Results
Among the 26 patients analyzed, there was just one case that had intrahepatic cholangiocarcinoma The preoperative level of α–Fetoprotein was 25.2 ng/mL. All cases (100%) had Child–Pugh A liver function preoperatively. The ventral/dorsal RAS was preserved in 19 and 7 patients, respectively. The mean surgical margin was 6.2 mm. The mean surgical time was 228.5 minutes, while the mean blood loss was 255 mL. In pathology, 5 cases (19.2%) had microvascular invasion, and in the group of HCC patients, 92% of all cases had moderate or poor tumor differentiation. Six cases (23.1%) of postoperative complications were graded over III according to the Clavien–Dindo system, including in three patients resistant ascites or intra-abdominal abscess that required intervention.
Primary liver cancer has become a worldwide program, being the third cause of cancer death, and hepatocellular carcinoma (HCC) is one of two major subtypes that accounts for 80% of all cases globally [1]. Although multidisciplinary approach and treatments have been developed, anatomical hepatectomy (AH) still plays a particularly important role. AH is a type of liver resection that is based on portal vein territory that basically includes dissection of the parenchymal, following the isolating and transecting of Glisson’s pedicle [2]. Due to the tumor-bearing portal territory including both the tumor, and the micro-metastases known as satellites, and the mechanism of HCC progression and metastasis being via portal veins, AH showed superior results in overall survivals vs. other local-regional therapies in very early and early HCC [3,4]. Otherwise, recent evidence has confirmed that AH could bring more survival benefits than other types of treatment in selective intermediate and advanced HCC patients [5-9].
In addition, with increasingly expanded surgical indications, more volume of resected liver is required to accomplish the radical standard and negative margins of AH. In contrast, due to most primary liver tumors having developed in chronic liver disease, maximum parenchymal preservation and sparing in AH, especially major hepatectomy, is required to preserve postoperative hepatic function. Hence a preoperative comprehensive assessment of the hepatic anatomy and surgical strategy is crucial to calculating adequate parenchymal resection volume and achieving the final double standards of negative margin and postoperative hepatic function. One of the most popular liver classifications based on dual vascular (portal veins and hepatic arteries system) inflow and biliary drainage was first described by Couinaud [10], then more deeply investigated by Takasaki et al. [2] based on the famous Glissonean pedicle (GP) transection and hepatic resection method. However, another ventral–dorsal anatomical concept of right anterior section (RAS), which was introduced by Hjortsjo [11] in 1951, could maximize the remnant parenchymal volume while requiring oncologic standards. This division of RAS was based on the vertical plane named the ventral segment fissure (VSF), or the anterior fissure vein (AFV)–hepatic venous branch of the middle or the right hepatic vein (HV). After that, Kogure et al. [12] and Cho et al. [13] reproposed Hjortsjo’s segmental anatomy based on the portal ramification of right anterior section. Because of the lack of identified tools of the VSF, as well as preoperative portal ramifications, few studies have evaluated the efficacy, as well as the safety, of this concept of liver resection. So our research analyzed the contributions of Hjortsjo and Cho’s concept in major AH with a multicenter experience of short-term outcomes.
After Institution Review Board approval (BM.6735271), all patients with a histologically proven diagnosis of primary hepatic malignancies (HCC; ICC, intrahepatic cholangiocarcinoma; cHCC−ICC, combined hepatocellular cholangiocarcinoma; etc.) who underwent major parenchymal-sparing anatomical hepatectomy (Ps–AH) based on the RAS Portal Ramification at the Department of Gastrointestinal and Hepato-Pancreato-Biliary Surgery, Bach Mai Hospital; the Department of Hepatobiliary-Pancreatic Surgery, Vietnam National Cancer Hospital; and the Department of Hepatobiliary Surgery, National Hospital of Tropical Disease, from August 2018 to August 2022 (48 months) were enrolled. A multidisciplinary meeting was previously held in all cases including surgeons, radiologists, clinical pathologists, and oncologists for final strategy. Written informed consent for publication of their clinical details and clinical images was obtained from the patients’ family.
Patients’ data were prospectively collected, including; general and preoperative information: demographic characteristics (sex, age, height, weight, body mass index, medical history), tumor characteristics on the preoperative imaging workup (CT, computed tomography; and MRI, magnetic resonance imaging). Child–Pugh classification, and patient’s general physical status according to the American Society of Anesthesiologists (ASA) physical status classification system [14]. Intraoperative data and short-term outcomes: types of approach hepatectomy (anterior or convenient approach hepatectomy), types of Ps–AH, blood loss, operative time, tumor dimension, pathological postoperative specimens, resected margins status, length of hospital stay, postoperative morbidity and 90 days (d) mortality. The margin status was defined as follows: no cancer cells are identified microscopically at any of the resected margins (R0), presence of microscopic tumor (R1), and gross residual tumor, as determined by the surgeon intraoperatively (R2). However, there has been no evidence base or consensus yet regarding the definition of R1 resection for liver cancer resections; therefore, the distances between the tumor cells and resection margin have been included in this study [15]. Postoperative complications were revised and classified based on Clavien–Dindo [16].
The concept of dorsal–ventral classification of the RAS was first introduced by Hjortsjo [11] in 1951, and was based on the anatomical landmark called the “ventral segment fissure” (VSF) or “anterior fissure vein (AFV)’’ [17]. According to the author, the VSF was the vertical plane that has a branch of the middle or the right HV or an independent HV. After over a half of century, Kogure et al. [12] in 2002 and Cho et al. [13] in 2005 have given a different definition of RAS dorsal–ventral classification that is based on the ramifications of portal RAS bifurcation. These authors believed that the RAS portal vein bifurcates into the ventral dorsal branches, instead of the anterosuperior and anteroinferior ones as in Couinaud’s classification; but other research by Kurimoto et al. [17] used multi-detector computed tomography (MDCT) for scanning and stimulation the types of RAS poral bifurcation preoperatively, and revealed that the ventral–dorsal type occurred in 26% of patients, while the Couinaud’s type occurred in a half of all patients [12,13]. In surgical aspect, Kanemura et al. [18] in 2000 were the first to apply this anatomical concept in Ps–AH.
A J-shape laparotomy was conducted. The right hepatic and middle hepatic were dissected and taped. In all cases of our study, we have controlled vascular inflow by applying the Takasaki–Glissonean pedicle isolation and transection method with a comprehensive surgical hepatic anatomy based on Laennec’s capsule: Dissection of the cystic plate and umbilical plate (thickened parts of the GP comprising collagen-rich connective) and resection of the anchors (the thin, cord-like structures located at the orifices of the GP that connect with Laennec’s capsule), isolation of the right and left hepatic and RAS pedicles, then maximum movement of these pedicles [2,19]. After that, identifying the type of RAS pedicles bifurcation was conducted intraoperatively and intra-parenchymal, liver parenchymal around the liver hilum was transected, especially the RAS pedicle, then the portal perfusion of each ventral or dorsal section was confirmed by temporary occlusion before division. After that, liver parenchymal transection was conducted following the demarcation lines in the liver surface.
With cases of large tumor (> 5 cm) or tumor with retro-extrahepatic organ invasion, we used anterior approach hepatectomy (AAH) with the liver hanging maneuver (LHM) to avoid squeezing of the tumor cells, and to control bleeding [20-22]. In all cases, the Harmonic scalpel was used to crack the Laennec’s capsule and transect the hepatic parenchyma within 1.5 cm under Laennec’s capsule, then 5 mm LigaSure was used for deeper parenchymal transection. The vascular outflow was controlled by maintaining the central venous pressure below 5 mmHg during liver parenchymal transection [23]. In the case of associating complete caudate lobectomy, we used the combined right–left approach [24].
We proceeded with the following three types of major Ps–AH being applied for the new portal ramification - Ryu and Cho’s classification, or what may be known as the ventral–dorsal type [13,25]:
Type 1: Ventral segment sparing right hepatectomy (Vs–RH) with or without caudate lobectomy (Fig. 1, 2) was an extended right posterior sectionectomy that combined resection of the right posterior section (RPS) and dorsal RAS with or without caudate lobectomy (resection of hepatic segments 6, 7, d5, and d8 ± 1, according to Couinaud’s classification).
Type 2: Dorsal segment sparing meso-hepatectomy (Ds–MH) with or without caudate lobectomy (Fig. 3, 4) was an extended left medial sectionectomy that combined resection of the left medial section and ventral RAS with or without caudate lobectomy (resection of hepatic segments 4, v5, and v8 ± 1, according to Couinaud’s classification).
Type 3: Dorsal segment sparing left trisectionectomy (Ds–LT) with or without caudate lobectomy (Fig. 5-7) was an extended left hepatectomy that combined resection of the left liver and ventral RAS with or without caudate lobectomy (resection of hepatic segments 2, 3, 4, v5, and v8 ± 1, according to Couinaud’s classification).
Segment 8 sparing right hepatectomy (S8s–RH) or Segment 8 sparing left trisectionectomy (S8s–LT) with or without caudate lobectomy (Fig. 8) was an extended right posterior sectionectomy or left hepatectomy that combined resection of the RPS, segment 5 with/without caudate lobectomy (resection of hepatic segments 5, 6, and 7 ± 1) or the left liver, segment 5 with/without caudate lobectomy (resection of hepatic segments 5, 2, 3, and 4 ± 1), respectively. These types of major Ps–AH were applied for the classic portal ramification–Couinaud’s classification, or may be known as the cranio–caudal type [10].
Categorical variables contain a finite number of categories or distinct groups, and are presented as a percentage of each value. Continuous variables are numeric variables that have an infinite number of values between any two values, and are expressed as the mean ± standard deviation, or median (interquartile range) if without normal distribution. Continuous variables were compared by means of the t-test of the Wilcoxon rank sum test when appropriate. Various nominal variables were analyzed using the chi-square test or Fisher’s exact test, when appropriate. Each data was analyzed using SPSS for Windows, version 22.0 (IBM Corp.). All methods were carried out in accordance with the relevant guidelines and regulations.
From August 2018 to August 2022 (48 months), there were a total of 26 cases of primary liver malignancies that underwent major Ps–AH based on the portal ramification of the RAS in the three centers. General and preoperative information (Table 1), and intraoperative data and short-term outcomes (Table 2) were prospectively collected and analyzed.
Among the 26 patients analyzed, there was just one case that had ICC. The underlying cause of liver disease was hepatitis B virus infection or hepatitis C virus infection in 88.4% of all cases. The preoperative level of α–Fetoprotein was 25.2 ng/mL. All cases (100%) had Child–Pugh A liver function preoperatively: 21 cases (80.8%) had Child–Pugh classification of A5, while 5 cases (19.2%) had Child–Pugh classification of A6.
In 26 patients undergoing parenchyma-sparing hepatectomy of RAS, the ventral-and-dorsal RAS was preserved in 19 and 7 patients, respectively. There were 6 cases (23.1%) of AAH with the LHM [20]. All 26 patients received curative resection (R0 resection), with the mean of surgical margin being 6.2 mm. The median tumor diameter was 6.0 cm, with the largest one being 18 cm. Nine cases (34.6%) had satellite lesions, with the median number of lesions being one. The mean surgical time was 228.5 minutes, and the mean blood loss was 255 mL. In pathology, 5 cases (19.2%) had microvascular invasion, and in the group of HCC patients, 92% of all cases had moderate or poor tumor differentiation.
The median postoperative hospital stay was 15 d (range, 8−65 d). There were no major complications requiring additional surgery. There were 6 cases (23.1%) of postoperative complications that were graded over III according to the Clavien–Dindo system, including resistant ascites or intra-abdominal abscess that requiring intervention in three patients [16]. There were two patients of partial thrombosis of the right portal vein (RPV), and one of them had associated infected ascites leading to multi-organ dysfunction and mortality.
Among the 26 patients analyzed, there was just one case that had ICC. The preoperative level of α–Fetoprotein was 25.2 ng/mL. All cases (100%) had Child–Pugh A liver function preoperatively. The ventral and dorsal RASs were preserved in 19 and 7 patients, respectively. The mean of surgical margin was 6.2 mm. The mean surgical time was 228.5 minutes, and the mean blood loss was 255 mL. In pathology, 5 cases (19.2%) had microvascular invasion, and in the group of HCC patients, 92 % of all cases had moderate or poor tumor differentiation. There were 6 cases (23.1%) of postoperative complications that were graded over III according to the Clavien–Dindo system, including resistant ascites or intra-abdominal abscess that required intervention in three patients.
The anatomic concept of dorsal and ventral parts of the RAS was one of three types of RAS bifurcation, besides the cranio-caudal type and multiple type, and accounted for about 23% to 26% of all cases following previous studies [17,26]. One of the most important questions in this Ps–AH was how to identify the type of RAS bifurcation. There have been several ways to identify the type of RAS bifurcation. Hjortsjo CH first introduced the ventral–dorsal concept in 1951, and the landmark of these two parts of RAS was a vertical plane named the VSF–a plane that had a hepatic venous branch of the middle or right HV, or an independent HV. This venous branch was called the “anterior fissure vein” (AFV) first by Cho et al. [13], and according to Kobayashi et al. [26], AFV was termed V8—the hepatic venous branch that passes through segment VIII following Couinaud’s Classification. However, the AFV or V8 was not always identified, as well as not constant: It could enter either the right HV, the middle HV, or directly into the inferior vena cava. Moreover, in Kobayashi et al.’s study [26], V8 functioned as a landmark of the boundary between the ventral and dorsal segments in just 64 % of all cases.
Hence the most common way to evaluate the ramification patterns of the portal branches in the RAS was using MDCT scanning preoperatively. Other advantages of preoperative MDCT scanning were estimating the location of tumor to predict the type of AH, as well as the resected and remnant parenchyma volume. In all our 26 cases, MDCT scanning was conducted, and the ventral–dorsal RAS bifurcation was identified preoperatively. Intraoperatively, we used Takasaki’s method based on the GP approach, transected the liver parenchymal around the liver hilum, especially the RAS pedicle, then confirmed the portal perfusion of each ventral or dorsal section by temporary occlusion before division. After that, depending on the type of dorsal or ventral segment sparing AH, all either ventral or dorsal branches of RAS Glisson’s pedicle were sutured and divided, and then liver parenchymal transection was conducted following the demarcation lines in the liver surface. By using Takasaki’s method based on the GP approach, we have maximumly reduced the blood loss intraoperatively. Without using Cavitron Ultrasonic Surgical Aspirator, the mean blood loss intraoperatively was 255 mL, which was superior to other research [17,27].
Ventral or dorsal sparing hepatectomy can preserve about 20% of total liver volume following other research [17,28,29]. According to previous several studies, the volumes of the ventral segment and the dorsal segment were quite similar, at about 200 mL [28], but in Kobayashi et al. [26] and Kurimoto et al.’s studies [17], the volume of ventral segment was significantly greater than the dorsal segment. No matter how, these parts of liver are very important to avoid unnecessarily transecting too much liver parenchyma, especially in the cases of major AH. In our 26 patients undergoing parenchyma-sparing hepatectomy of the RAS, the ventral and dorsal RAS was preserved in 19 and 7 patients, respectively. All 26 patients received curative resection (R0 resection), with the mean surgical margin being 6.2 mm. Two of the most important factors influencing the oncological outcome are the negative liver parenchymal margin and the distance from the tumor to the liver parenchymal margin. We have considered this as one of the standards to be achieved after surgery. Therefore, in some cases where the tumor was located close to the liver parenchymal ischemic margin as determined by Takasaki’s method based on the GP approach, we decided to cut the liver parenchyma slightly larger than the ischemic area, thereby achieving standards for the liver parenchymal margin.
In our study, the situation is that our hospitals have not been able to systematically measure liver volume, so this method has been applied for the purpose of maximum patient safety. No patient has liver dysfunction postoperatively, the morbidity rate was acceptable, and the case of operative mortality was not related to liver dysfunction. All these results have shown favorable oncological short-term outcomes and safety. The in-hospital mortality of the surgery is about 4% (1/26 patients). Our research had a small sample size, so some results may not be representative. There have been two cases of acute RPV thrombosis after the surgery, due to high factors that are thought to contribute to thrombosis (Virchow triad) in these two cases. The case of mortality had partial thrombosis of the RPV associated with infected ascites leading to multi-organ dysfunction and mortality; therefore, it is more related to the issue of resuscitation and postoperative care, than to the technical cause of surgery. The sample size was relatively small with short study duration with limitations of experience. Further investigations and follow-up with a larger sample and control group or multi-center design must be conducted to evaluate the long-term outcomes of our technique.
In conclusions, Ps–AH based on RAS Portal Ramification is a feasible and safe method to achieve R0-resection with favorable short-term outcomes in major liver resection. Further investigations and follow-up must be conducted to evaluate the long-term outcomes of this technique.
ACKNOWLEDGEMENTS
The authors were thankful to the board and colleagues of the Department of Gastrointestinal and Hepato-pancreato-biliary surgery, Bach Mai Hospital; the Department of Hepatobiliary-pancreatic Surgery, Vietnam National Cancer Hospital and the Department of Hepatobiliary Surgery, National Hospital of Tropical Diseases, Hanoi, Vietnam for their assistance during the time of in-hospital treatment and observation of our patients.
REFERENCES
1. Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, et al. 2022; Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 161:108–118. DOI: 10.1016/j.ejca.2021.11.023. PMID: 34942552.
2. Takasaki K, Kobayashi S, Tanaka S, Saito A, Yamamoto M, Hanyu F. 1990; Highly anatomically systematized hepatic resection with Glissonean sheath code transection at the hepatic hilus. Int Surg. 75:73–77.
3. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. 2018; AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 67:358–380. DOI: 10.1002/hep.29086. PMID: 28130846.

4. Yang S, Lin H, Song J. 2021; Efficacy and safety of various primary treatment strategies for very early and early hepatocellular carcinoma: a network meta-analysis. Cancer Cell Int. 21:681. DOI: 10.1186/s12935-021-02365-1. PMID: 34923980. PMCID: PMC8684647.

5. Chen L, Sun T, Chen S, Ren Y, Yang F, Zheng C. 2020; The efficacy of surgery in advanced hepatocellular carcinoma: a cohort study. World J Surg Oncol. 18:119. DOI: 10.1186/s12957-020-01887-8. PMID: 32487104. PMCID: PMC7268283.

6. Yang B, Zheng B, Yang M, Zeng Z, Yang F, Pu J, et al. 2018; Liver resection versus transarterial chemoembolization for the initial treatment of Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Hepatol Int. 12:417–428. DOI: 10.1007/s12072-018-9888-4. PMID: 30073454.

7. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. 2016; Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 65:938–943. DOI: 10.1016/j.jhep.2016.05.044. PMID: 27266618.
8. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. 2017; Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 66:510–517. DOI: 10.1002/hep.29225. PMID: 28437844.
9. Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. 2014; Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 61:82–88. DOI: 10.1016/j.jhep.2014.03.012. PMID: 24650695.
10. Couinaud C. 1989; Surgical anatomy of the liver revisited. C. Couinaud 15 rue Spontini F 75116 Paris. 25–28.
11. Hjortsjo CH. 1951; The topography of the intrahepatic duct systems. Acta Anat (Basel). 11:599–615. DOI: 10.1159/000140534. PMID: 14829155.
12. Kogure K, Kuwano H, Fujimaki N, Ishikawa H, Takada K. 2002; Reproposal for Hjortsjo's segmental anatomy on the anterior segment in human liver. Arch Surg. 137:1118–1124. DOI: 10.1001/archsurg.137.10.1118. PMID: 12361415.

13. Cho A, Okazumi S, Miyazawa Y, Makino H, Miura F, Ohira G, et al. 2005; Proposal for a reclassification of liver based anatomy on portal ramifications. Am J Surg. 189:195–199. DOI: 10.1016/j.amjsurg.2004.04.014. PMID: 15720989.

14. Doyle DJ, Goyal A, Garmon EH. American Society of Anesthesiologists Classification [Internet]. 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441940/. cited 2023 Jul 11.
15. Wyatt J, Hübscher S, Goldin R, Tiniakos D. Dataset for histopathological reporting of liver resection specimens (including gallbladder) and liver biopsies for primary and metastatic carcinoma [Internet]. 2022. Available from: https://www.rcpath.org/resourceLibrary/dataset-for-histopathological-reporting-of-liver-resection-specimens-and-liver-biopsies-for-primary-and-metastatic-carcinoma.html. cited 2023 Jul 11.
16. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. 2009; The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 250:187–196. DOI: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912.
17. Kurimoto A, Yamanaka J, Hai S, Kondo Y, Sueoka H, Ohashi K, et al. Parenchyma-preserving hepatectomy based on portal ramification and perfusion of the right anterior section: preserving the ventral or dorsal area. J Hepatobiliary Pancreat Sci. 2016; 23:158–166. DOI: 10.1002/jhbp.317. PMID: 26744104.

18. Kanemura E, Togo S, Shizawa R, Tanaka K, Shimada H. 2000; Subdivision of liver anterior segment into two units according to hepatic venous drainage. Hepato-gastroenterology. 47:1056–1059.
19. Sugioka A, Kato Y, Tanahashi Y. 2017; Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci. 24:17–23. DOI: 10.1002/jhbp.410. PMID: 28156078. PMCID: PMC5299460.

20. Li L, Wang HQ, Wang Q, Yang J, Yang JY. 2014; Anterior vs conventional approach hepatectomy for large liver cancer: a meta-analysis. World J Gastroenterol. 20:17235–17243. DOI: 10.3748/wjg.v20.i45.17235. PMID: 25493040. PMCID: PMC4258596.

21. Liddo G, Buc E, Nagarajan G, Hidaka M, Dokmak S, Belghiti J. 2009; The liver hanging manoeuvre. HPB (Oxford). 11:296–305. DOI: 10.1111/j.1477-2574.2009.00068.x. PMID: 19718356. PMCID: PMC2727082.

22. Liu PH, Su CW, Hsu CY, Hsia CY, Lee YH, Huang YH, et al. 2016; Solitary large hepatocellular carcinoma: staging and treatment strategy. PLoS One. 11:e0155588. DOI: 10.1371/journal.pone.0155588. PMID: 27176037. PMCID: PMC4866714.

23. Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. 2004; The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 187:398–402. DOI: 10.1016/j.amjsurg.2003.12.001. PMID: 15006570.

24. Nguyen HH, Nguyen TK, Le VD, Luong TH, Dang KK, Nguyen VQ, et al. 2022; Isolated complete caudate lobectomy with Glissonean pedicle isolation using Takasaki's technique and right-left approach: preliminary experience from two case reports. World J Surg Oncol. 20:31. DOI: 10.1186/s12957-022-02496-3. PMID: 35115011. PMCID: PMC8815180.

25. Ryu M, Cho A. New liver anatomy: portal segmentation and the drainage vein. Springer;2009. DOI: 10.1007/978-4-431-95993-9.
26. Kobayashi T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. 2017; Study on the segmentation of the right anterior sector of the liver. Surgery. 161:1536–1542. DOI: 10.1016/j.surg.2016.12.020. PMID: 28126253.

27. Ogiso S, Ikai I, Narita M, Murakami T, Hata H, Yamaguchi T, et al. 2013; Parenchyma-sparing anatomical liver resection based on Hjortsjo's concept. Langenbecks Arch Surg. 398:751–758. DOI: 10.1007/s00423-013-1069-2. PMID: 23446710.

28. Tanaka K, Matsumoto C, Takakura H, Matsuo K, Nagano Y, Endo I, et al. 2010; Technique of right hemihepatectomy preserving ventral right anterior section guided by area of hepatic venous drainage. Surgery. 147:450–458. DOI: 10.1016/j.surg.2009.04.020. PMID: 19744462.

29. Shindoh J, Satou S, Aoki T, Beck Y, Hasegawa K, Sugawara Y, et al. 2012; Hidden symmetry in asymmetric morphology: significance of Hjortsjo's anatomical model in liver surgery. Hepatogastroenterology. 59:519–525. DOI: 10.5754/hge11529. PMID: 22024038.

Fig. 1
Schema of ventral segment sparing right hepatectomy (resection of hepatic segments 6, 7, d5, and d8). (A) Preoperative. (B) Postoperative.
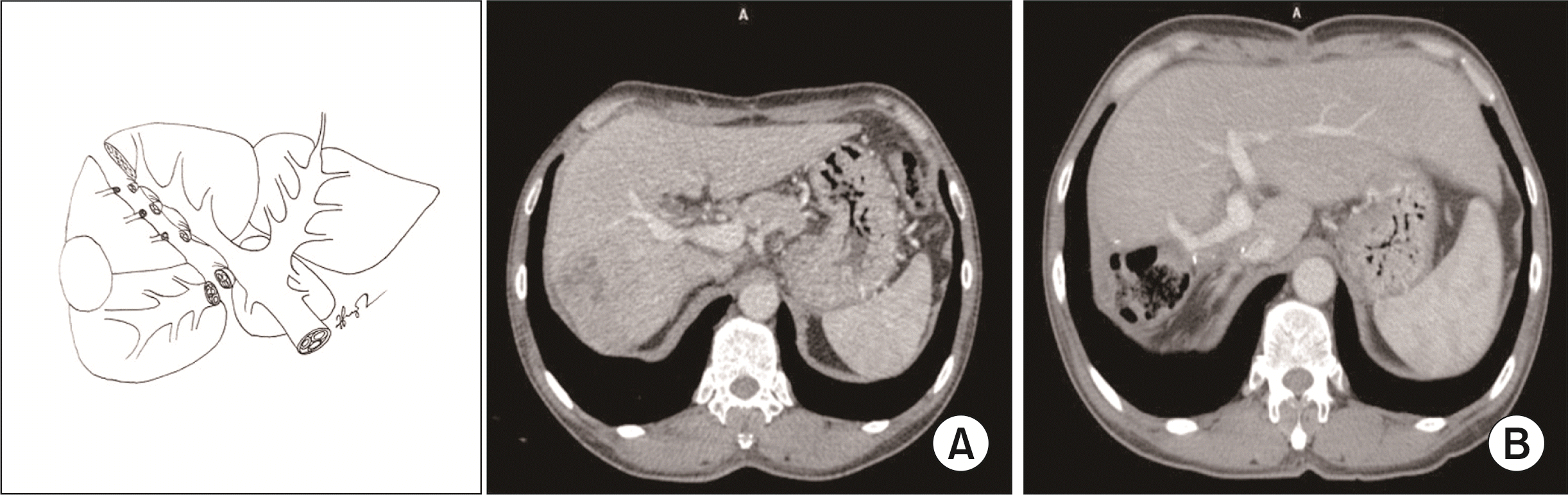
Fig. 3
Schema of dorsal segment sparing mesohepatectomy (resection of hepatic segments 4, v5, and v8 ± 1). (A) Preoperative. (B) Postoperative.
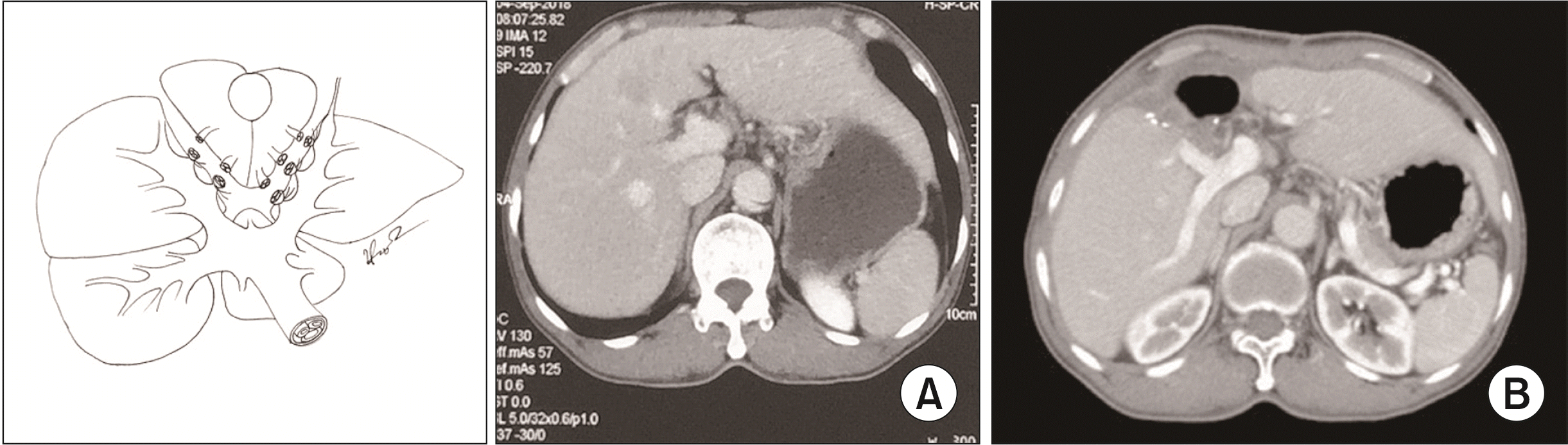
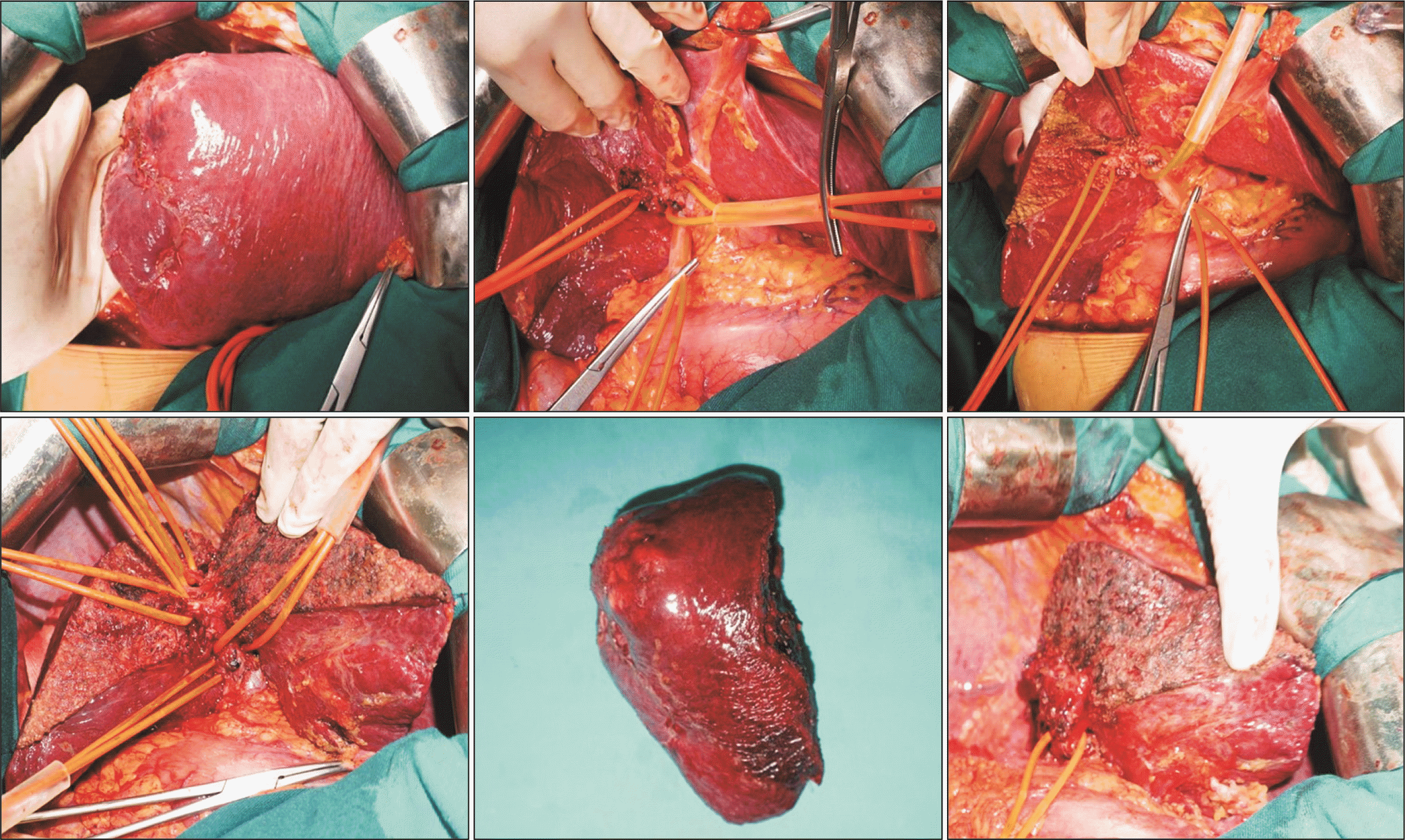
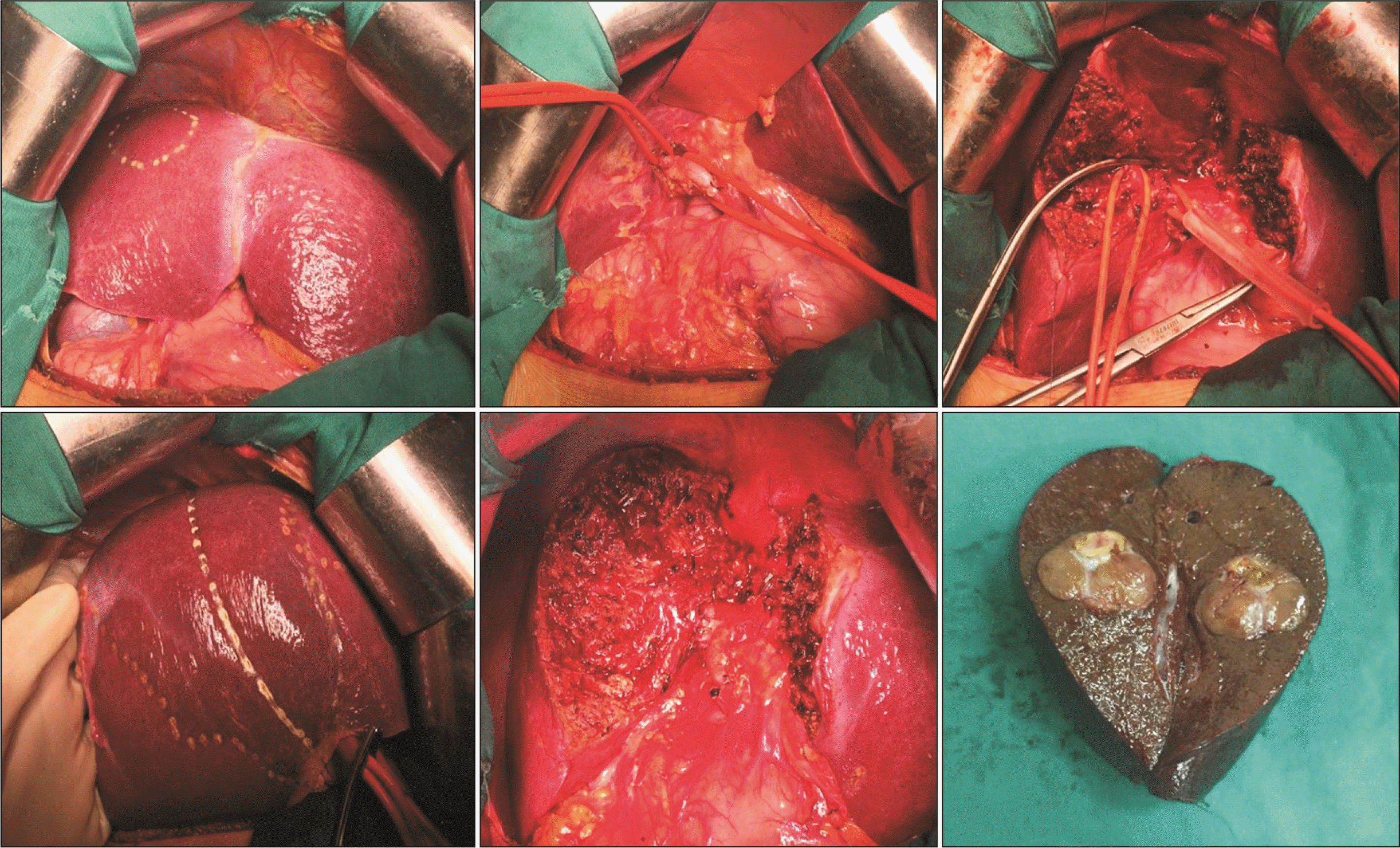
Fig. 5
Schema of dorsal segment preserving left trisectionectomy (resection of hepatic segments 2, 3, 4, v5, and v8). (A) Preoperative. (B) Postoperative.
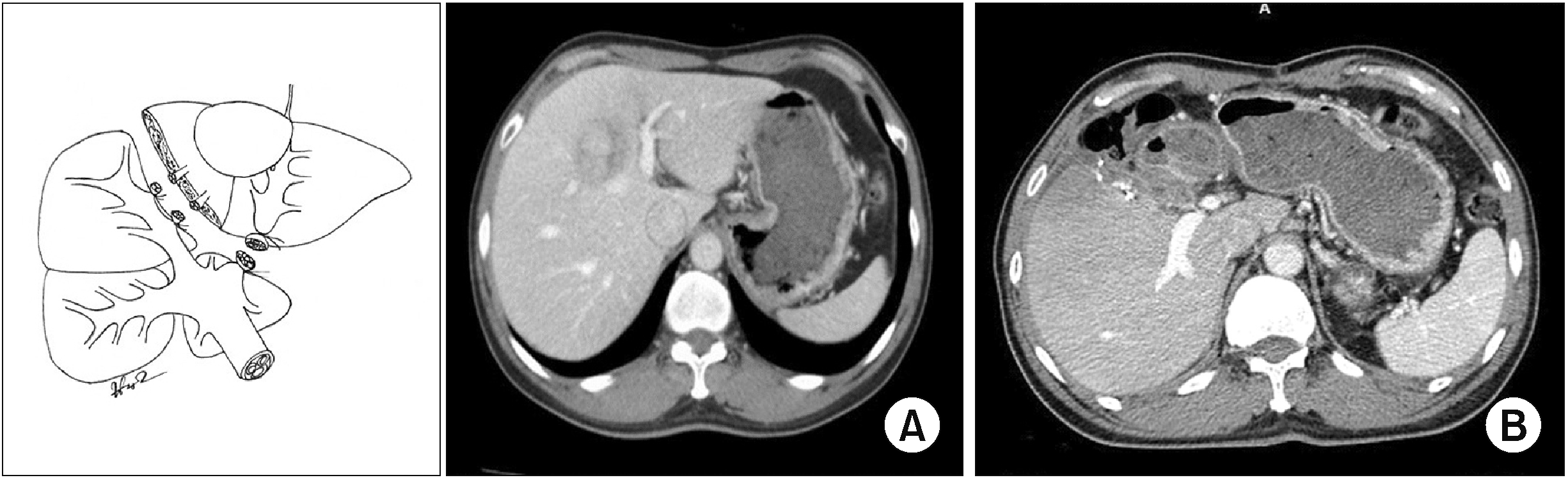
Fig. 6
Dorsal segment preserving left trisectionectomy (resection of hepatic segments 2, 3, 4, v5, and v8).
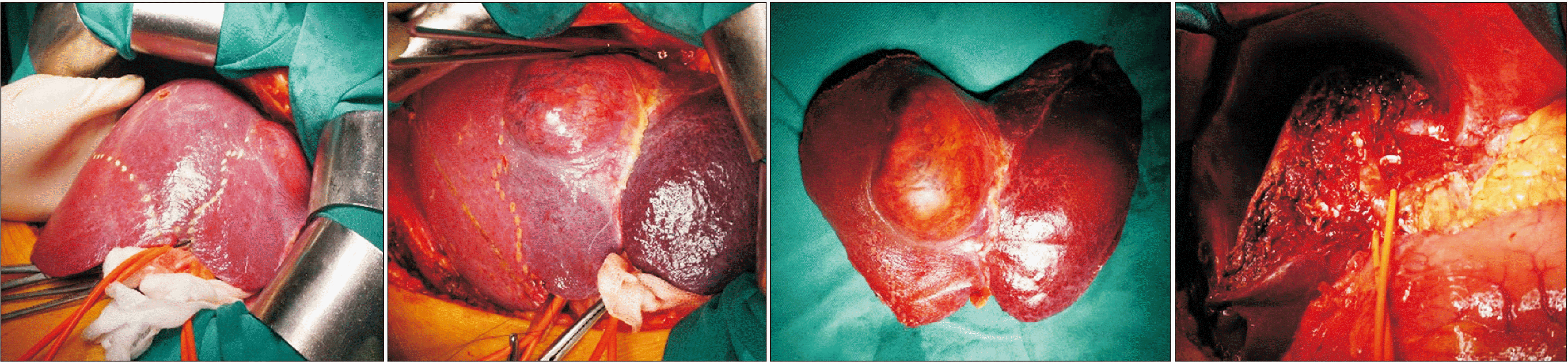
Fig. 7
Dorsal segment preserving left trisectionectomy and caudate lobectomy (resection of hepatic segments 1, 2, 3, 4, v5, and v8).
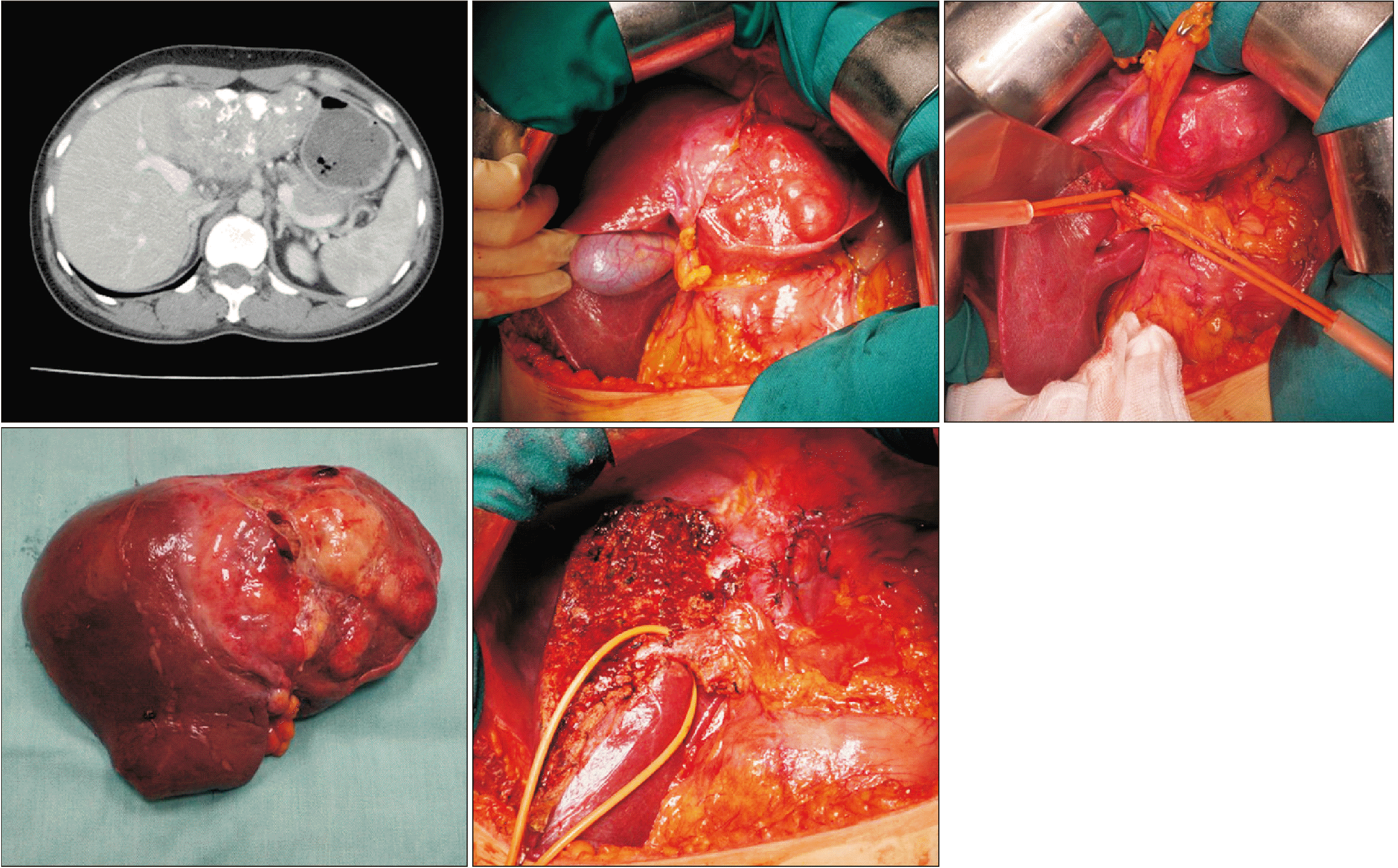
Fig. 8
Segment 8 sparing right hepatectomy with or without caudate lobectomy (resection of hepatic segments 5, 6, and 7 ± 1).
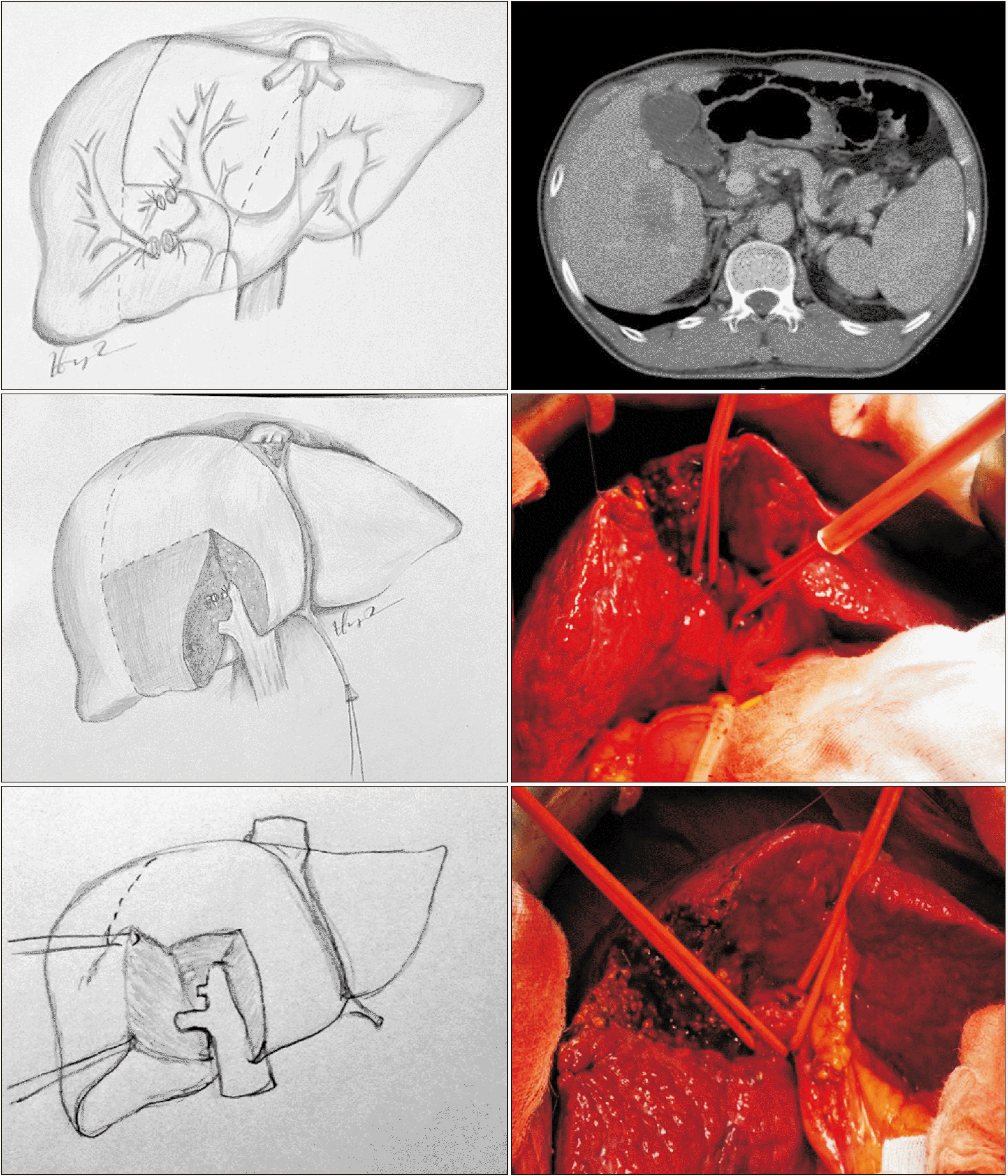
Table 1
General and preoperative information
Table 2
Intraoperative data and short-term outcomes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download