Abstract
Cholangiocarcinoma is a heterogeneous group of aggressive tumors that correspond to the second most common primary liver tumor. They can be classified according to their anatomical position concerning the biliary tree, and each subtype demonstrates different behavior and treatment. A 38-year-old male patient presenting solely right lumbar pain was diagnosed with a 7 cm hepatic tumor involving segments I, Iva, and VIII associated with involvement of the hepatic veins. He underwent a bloc resection of hepatic segments I, II, III, IV, partial V, partial VII, and VIII; right, middle, and left hepatic veins; and inferior vena cava segment, with perfusion of the remaining liver in situ with a preservation solution. As the patient had a large accessory inferior right hepatic vein draining the remaining liver, no reimplantation of hepatic veins was necessary. He remained clinically stable in outpatient follow-up, with excellent performance status—current survival of 2 years 6 months after surgical treatment.
Cholangiocarcinoma is a heterogeneous group of aggressive tumors that accounts for about 15% of all primary liver tumors, and after hepatocellular carcinoma, is the second most common primary liver tumor [1]. It arises from the epithelial cells of the intra and extrahepatic bile ducts and shows a peak incidence between 50 and 70 years of age, with predominance in male patients [1,2].
These tumors can be classified according to their anatomical position relative to the biliary tree: 1) Intrahepatic cholangiocarcinoma (iCCA): located on the periphery of the liver, proximal to the secondary bile ducts. 2) Perihilar cholangiocarcinoma: located at the level of the confluence of the hepatic ducts up to the insertion of the cystic duct into the common hepatic duct. 3) Extrahepatic cholangiocarcinoma: located below the cystic duct up to the level of Vater’s papilla.
Each subtype has different behavior and treatment. iCCA is an aggressive malignancy with 1- and 5-year survival rates of approximately 30% and 18%, respectively, corresponding to 10%−20% of cholangiocarcinoma. About a quarter of all cases are diagnosed through an incidental finding on an imaging exam. In 60%−70% of patients, iCCA presents as a single lesion [2].
In their early stages, iCCAs are usually asymptomatic. Jaundice is infrequent and is usually associated with advanced-stage disease. The gold standard treatment for this type of tumor is surgical resection associated with lymphadenectomy of the hepatic hilum [3]. Unfortunately, most patients have a late diagnosis with criteria of unresectability or inoperability, such as locally advanced disease or distant metastases. In this case, liver biopsy is mandatory for follow-up to systemic therapy [1,3,4].
Some factors are essential for assessing the resectability and operability of patients affected with iCCA. These include tumor size, invasion of important vascular structures, presence of metastases, patient’s functional capacity to support the surgical procedure, and adequate surgical planning [2,3].
In this case report, surgical treatment was possible even given the complex location, which compromised the three hepatic veins, due to the presence of an accessory inferior right hepatic vein (or vein of Makuuchi), which allowed for drainage of the remaining liver after extended left hepatectomy with resection of the inferior vena cava segment, and reconstruction with a graft from a deceased donor [5-7].
A 38-year-old male patient sought medical emergency for right lumbar pain with no other associated symptoms. He presented no comorbidities, previous surgeries, or continuous medication use. He denied drinking or smoking habits.
During the diagnostic investigation, computed tomography of the abdomen in an external service showed a hepatic nodule in segment VIII, with heterogeneous contrast enhancement, measuring 5.5 cm along its longest axis, while magnetic resonance imaging of the abdomen showed one hepatic nodule within defined limits. Moreover, the tomography of the nodule showed lobulated contours with a predominant hyper signal on T2-weighted sequences, heterogeneous contrast enhancement without significant washout in the late phases, without a frank intermingling adipose component, and measured approximately 5.8 cm along its longest transverse axis, centered on segment VIII (Fig. 1).
As the investigation continued, he underwent esophagogastroduodenoscopy and colonoscopy, both of which proved normal. Tumor markers were requested at the beginning of the investigation: alpha-fetoprotein = 1.7 ng/mL; carcinoembryonic antigen = 2.3 ng/mL; CA19-9 = 2 U/mL.
A percutaneous biopsy was performed with anatomopathological examination and confirmation by immunohistochemistry, which confirmed moderately differentiated adenocarcinoma (cholangiocarcinoma).
At the service of origin, surgical resection was not indicated, as justified by the location of the lesion, which was positioned in the topography of the right, middle, and left hepatic veins, with the tumor being considered unresectable. The patient was referred to palliative systemic therapy.
He sought our service nine months after the initial diagnosis, bringing a new abdominal magnetic resonance imaging that showed an enlargement of the lesion, and described as an expansive hepatic focal lesion, measuring 7.0 cm along its longest axis, involving segments I, IVa, and VIII. Disagreeing with the previous evaluation from another service, we contraindicated chemotherapy, as we considered the tumor susceptible to resection, and began surgical planning.
In this case, the great challenge was the involvement of the hepatic veins, making surgical resection a complex option, due to the need for reconstruction of the right hepatic vein after resection for venous drainage of the remaining liver. Fortunately, this patient had a large inferior right hepatic vein—or vein of Makuuchi—which was responsible for draining hepatic segments VI and VII directly into the vena cava; therefore, reimplantation of the right hepatic vein would not be necessary (Fig. 2).
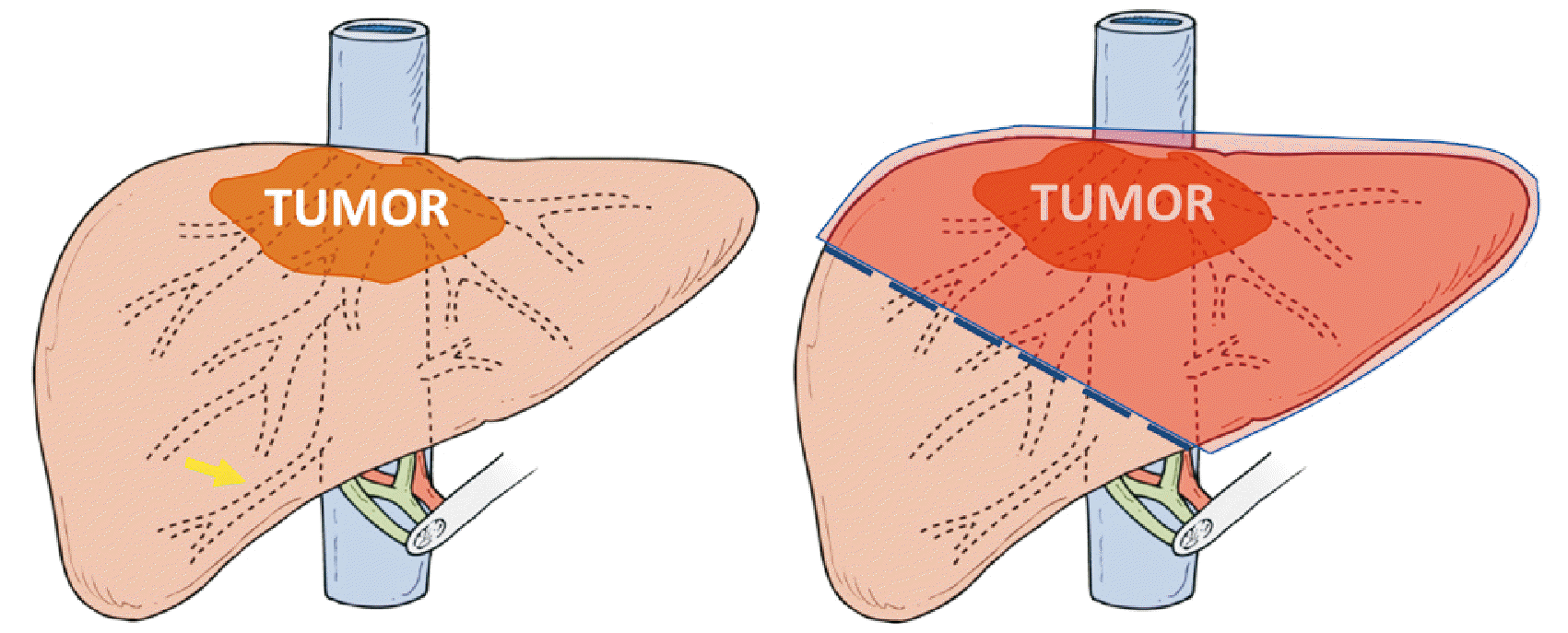
After careful evaluation of the image, and extensive discussion by our team of surgeons, the possibility of resectioning the lesion through an extended left hepatectomy (Fig. 3) with resection of the inferior vena cava segment above the inferior right hepatic vein, followed by reconstruction, was defined.
The proposal was widely discussed with the patient, who agreed to undergo surgery, aware of the risks inherent in the procedure and its high complexity.
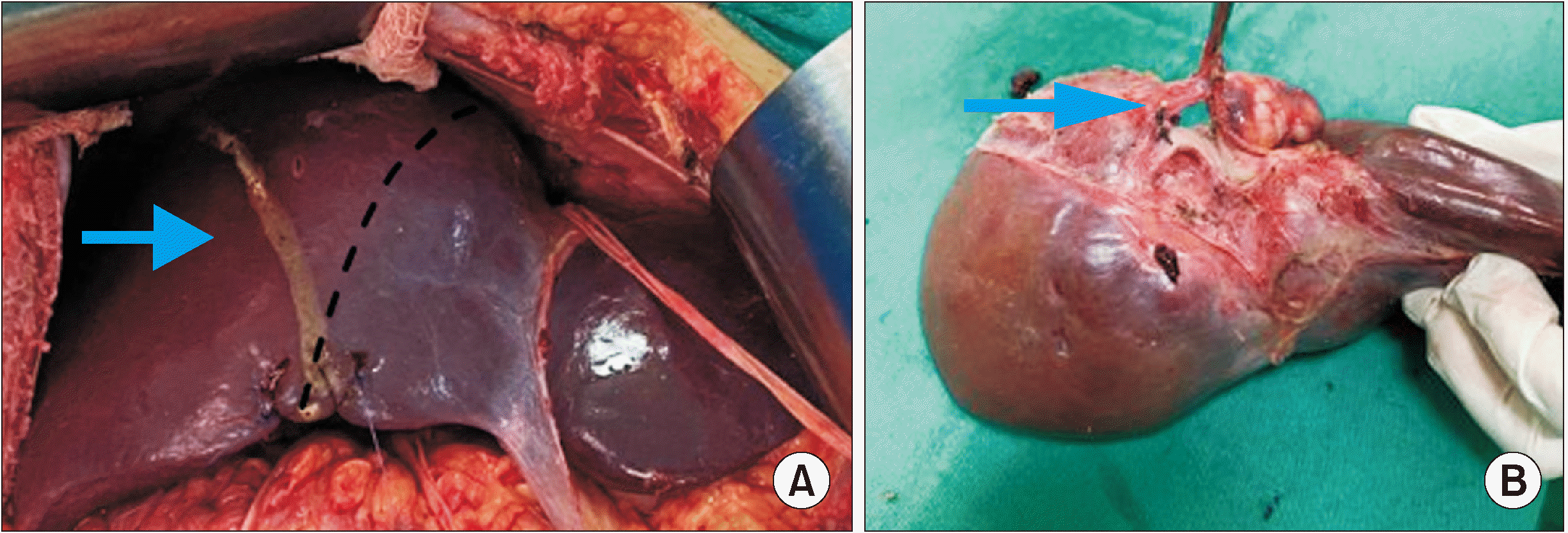
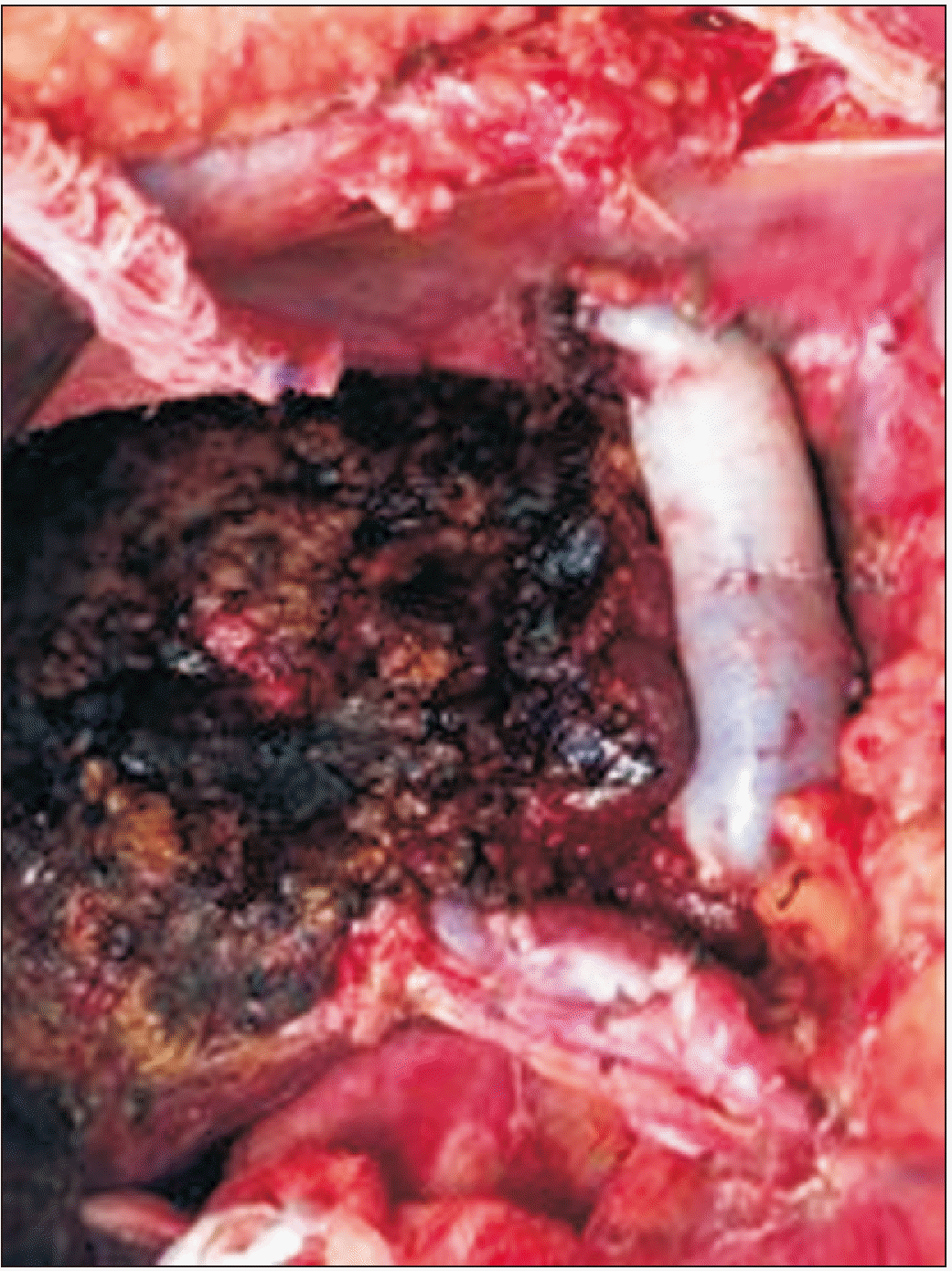
Experienced liver surgeons performed the surgical procedure. An extended left hepatectomy was performed, and during dissection of the hepatic veins, these veins involvement by the tumor was verified, with no possibility of separation of the structures. Thus, the surgery proceeded with the en bloc resection of hepatic segments I, II, III, IVa, IVb, partial V, partial VII, and VIII; right, middle and left hepatic veins; and inferior vena cava segment (Fig. 4).
Intraoperatively, a total clamp of the vena cava was performed in the segment located between the inferior right hepatic vein and the diaphragm. The estimated reconstruction time was 1 hour, so it was decided to perfuse the remaining liver in situ through the portal vein with a preservation solution (Custodiol®), to reduce the damage caused by hepatic ischemia. For this, after sectioning the left portal branch, a venotomy of the proper portal vein was performed, and the catheter was directed to the right portal branch; 1 liter of preservation solution was administered, and a venotomy of the vena cava was performed to drain the solution, in addition to the provision of ice slush in the abdominal cavity. The inferior vena cava segment was reconstructed using a homologous graft with ABO compatibility (Fig. 5).
There was no reconstruction of the right hepatic vein. During the surgical procedure, the inferior right hepatic vein was preserved, and was responsible for draining the remaining liver. Standard lymphadenectomy was performed.
Surgical time was approximately 400 minutes, with a blood transfusion of 1 packed red blood cells bag being administered intraoperatively, and 1 more in the immediate postoperative period. After the end of the procedure, the patient was transferred to the intensive care unit (ICU) on mechanical ventilation, using intravenous noradrenaline at a dose of 0.3 mcg/kg/min.
On the first postoperative day, he was extubated with a significant reduction in vasoactive drugs; and on the second postoperative day, was discharged from the ICU. He had grade IVa surgical complications on the Clavien-Dindo scale [7], such as transitory hepatic dysfunction of the remaining liver (jaundice and ascites), pneumonia, and dialytic renal failure, which evolved with improvement after clinical treatment, and with multiple antimicrobials.
On the 25th postoperative day, the patient was discharged from the hospital with significant clinical improvement. He recovered his renal function, and three months after the surgical procedure, was released from renal replacement therapy.
Anatomopathological examination of the surgical specimen confirmed moderately differentiated intrahepatic small duct cholangiocarcinoma, without vascular invasion, measuring 9.0 cm × 7.0 cm × 6.0 cm, with margins free of neoplasia, and no regional lymph node metastasis. Pathological staging was pT1b pN0.
He underwent adjuvant chemotherapy with capecitabine. However, given that he had severe hand-foot syndrome, his regimen was changed to a combination of leucovorin, 5-fluorouracil, and oxaliplatin, totaling six months of systemic therapy.
He remained clinically stable in outpatient follow-up, with excellent performance status. He was disease-free up to 1 year 2 months after the surgical procedure, when he presented two smaller than 2 cm hepatic nodules suggestive of secondary disease, and systemic therapy was restarted—current survival of 2 years 6 months after surgical treatment.
The patient underwent radioablation in both lesions, presenting complete remission. It has now been 6 months without signs of active disease.
Among primary malignant liver tumors, hepatocellular carcinoma and iCCA are the most common, accounting for 70% and 15% of cases, respectively. In recent decades, the incidence of iCCA has increased [1,2].
Complete surgical resection of the lesion is currently the only well-established treatment option for iCCA. However, compared to other hepatobiliary neoplasms, iCCA generally presents a lower degree of resectability and cure rates, and a suboptimal postoperative prognosis in patients who are candidates for resection [1,3].
In a large multicenter study with 584 patients who underwent complete resection of an iCCA, the probability of cure was approximately 9.7%, higher in patients with small tumors (< 5 cm), well-differentiated, and without lymph node metastases. Even in young patients with early lesions, liver resection with free margins is achieved in less than 30% of cases [8].
In many cases, large-volume liver resections are required, and are associated with a higher rate of postoperative complications.
An extended left hepatectomy associated with resection of the hepatic veins and the inferior vena cava segment is one of the most complex and challenging procedures for the liver surgeon. The need for reimplantation or reconstruction of the right hepatic vein makes the surgery even more challenging; for a very aggressive disease with high recurrence rate, it raises questions about the indication of a procedure of this magnitude.
The inferior right hepatic vein is almost always present, even in small dimensions. The presence of a large right inferior hepatic vein can bring benefits. In cases of inferior vena cava obstruction and the existence of a large inferior right hepatic vein, it allows hemodynamic adaptation that can protect the liver for some time.
In patients who will undergo major liver resections, drainage of the right liver can be performed through the inferior right hepatic vein, without needing reconstruction of the right hepatic vein [9].
The case in question details the presence of the inner right hepatic vein, which can be preserved, making the procedure more feasible, and less questionable. Even so, despite performing major surgery with free margins, the patient evolved with disease recurrence one year 2 months later, showing the aggressive nature of the disease. Notably, patients with unresectable iCCA have an overall survival of less than one year, bringing the benefit of surgery to the patient’s survival and quality of life.
Extensive liver resections can be highly complex, being mainly associated with vascular resections of large vessels that require reconstruction. The presence of the inferior right hepatic vein allows the extended left hepatectomy with resection of hepatic veins, without the need for reconstruction of the drainage route, leading to a shorter surgical time. iCCA is an aggressive disease that, even with the possibility of a gold standard treatment, has high recurrence rates and poor prognosis.
REFERENCES
1. Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, et al. 2021; Cholangiocarcinoma. Pathologica. 113:158–169. DOI: 10.32074/1591-951X-252. PMID: 34294934. PMCID: PMC8299326.

2. El-Diwany R, Pawlik TM, Ejaz A. 2019; Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 28:587–599. DOI: 10.1016/j.soc.2019.06.002. PMID: 31472907.

3. Zhang H, Yang T, Wu M, Shen F. 2016; Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 379:198–205. DOI: 10.1016/j.canlet.2015.09.008. PMID: 26409434.

4. Massarweh NN, El-Serag HB. 2017; Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 24:1073274817729245. DOI: 10.1177/1073274817729245. PMID: 28975830. PMCID: PMC5937247.

5. Suzuki T, Ebata T, Yokoyama Y, Mizuno T, Igami T, Yamaguchi J, et al. 2019; Left trisectionectomy combined with resection of the right hepatic vein and inferior vena cava after right hepatic vein embolization for advanced intrahepatic cholangiocarcinoma. Surg Case Rep. 5:98. DOI: 10.1186/s40792-019-0655-0. PMID: 31214903. PMCID: PMC6582073.

6. Cawich SO, Naraynsingh V, Pearce NW, Deshpande RR, Rampersad R, Gardner MT, et al. 2021; Surgical relevance of anatomic variations of the right hepatic vein. World J Transplant. 11:231–243. DOI: 10.5500/wjt.v11.i6.231. PMID: 34164298. PMCID: PMC8218342.

7. Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, et al. 2015; Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 121:3998–4006. DOI: 10.1002/cncr.29619. PMID: 26264223.

8. Dindo D, Demartines N, Clavien PA. 2004; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213. DOI: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542. PMCID: PMC1360123.
9. Champetier J, Haouari H, Le Bas JF, Létoublon C, Alnaasan I, Farah I. 1993; Large inferior right hepatic vein. Surg Radiol Anat. 15:21–29. DOI: 10.1007/BF01629857. PMID: 8488431.

Fig. 1
Preoperative abdominal computed tomography showing a 7.0 cm sized liver tumor in segment VIII.
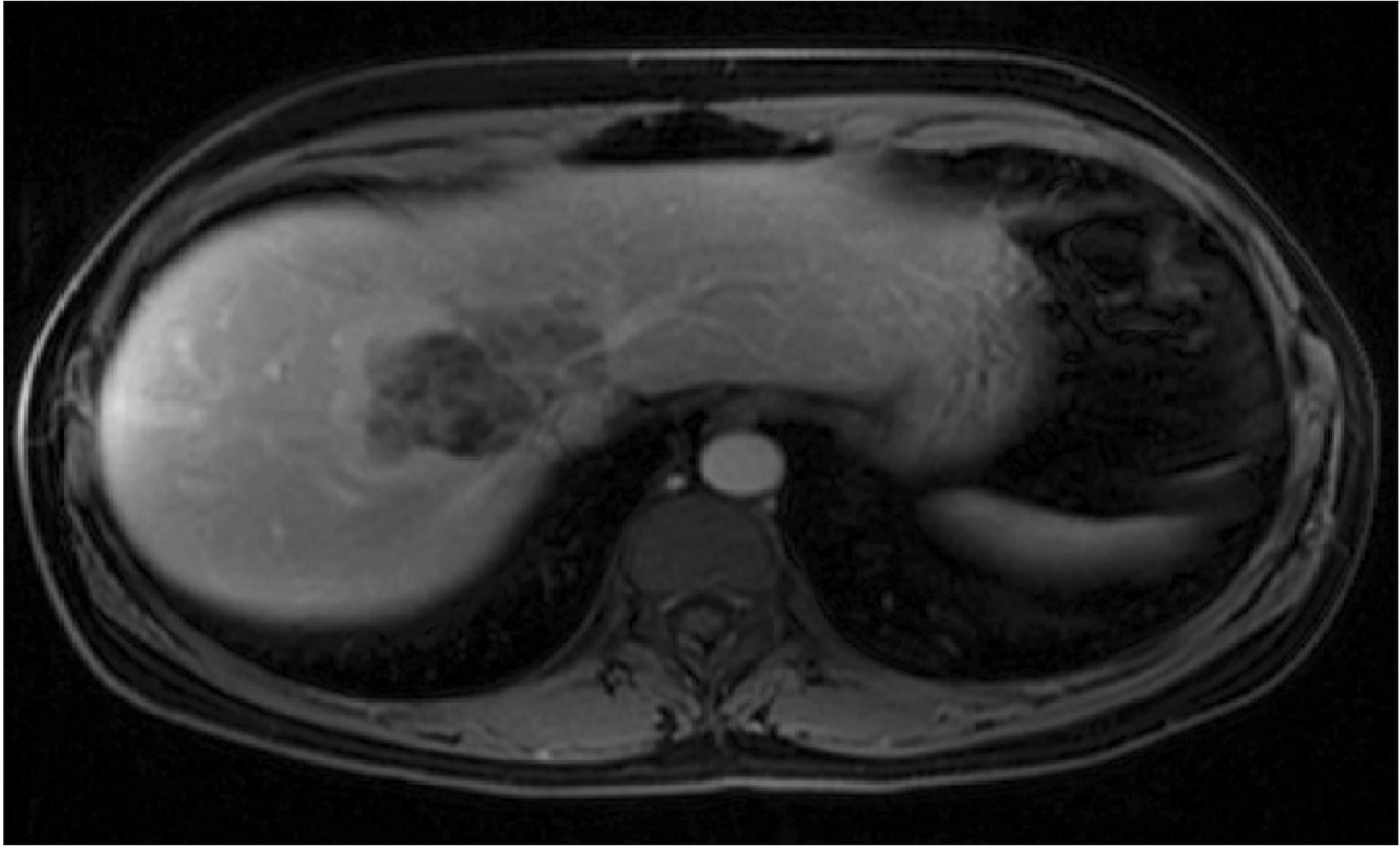
Fig. 2
Abdominal magnetic resonance imaging showing inferior right hepatic vein–Makuuchi vein (arrow) draining segments VI and VII into the inferior vena cava.
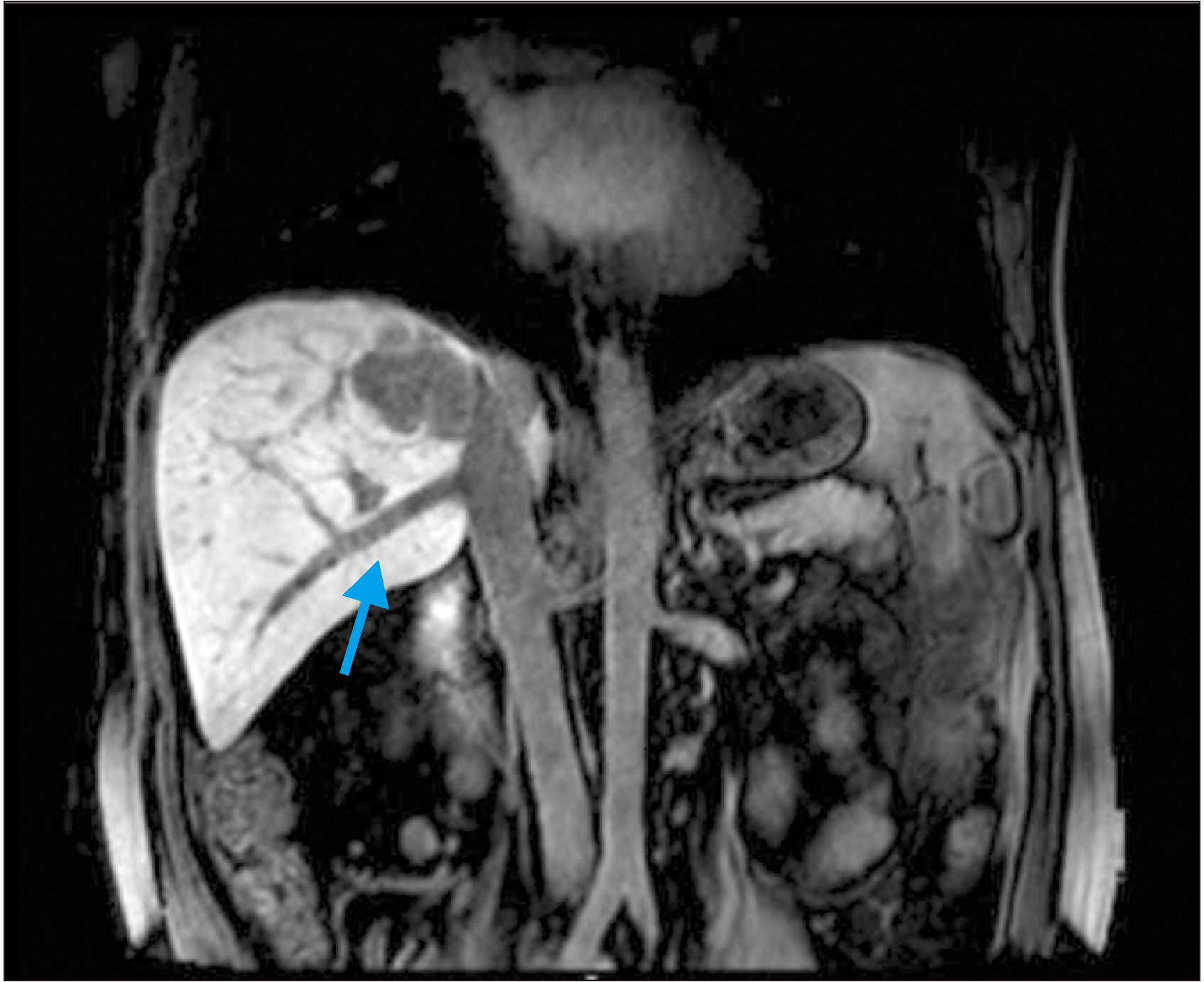
Fig. 3
Representation of surgical planning with the markings of liver resection (dashed), inferior hepatic vein (arrow), and final hepatectomy area–extended left hepatectomy (shaded).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download