Abstract
References
Fig. 1
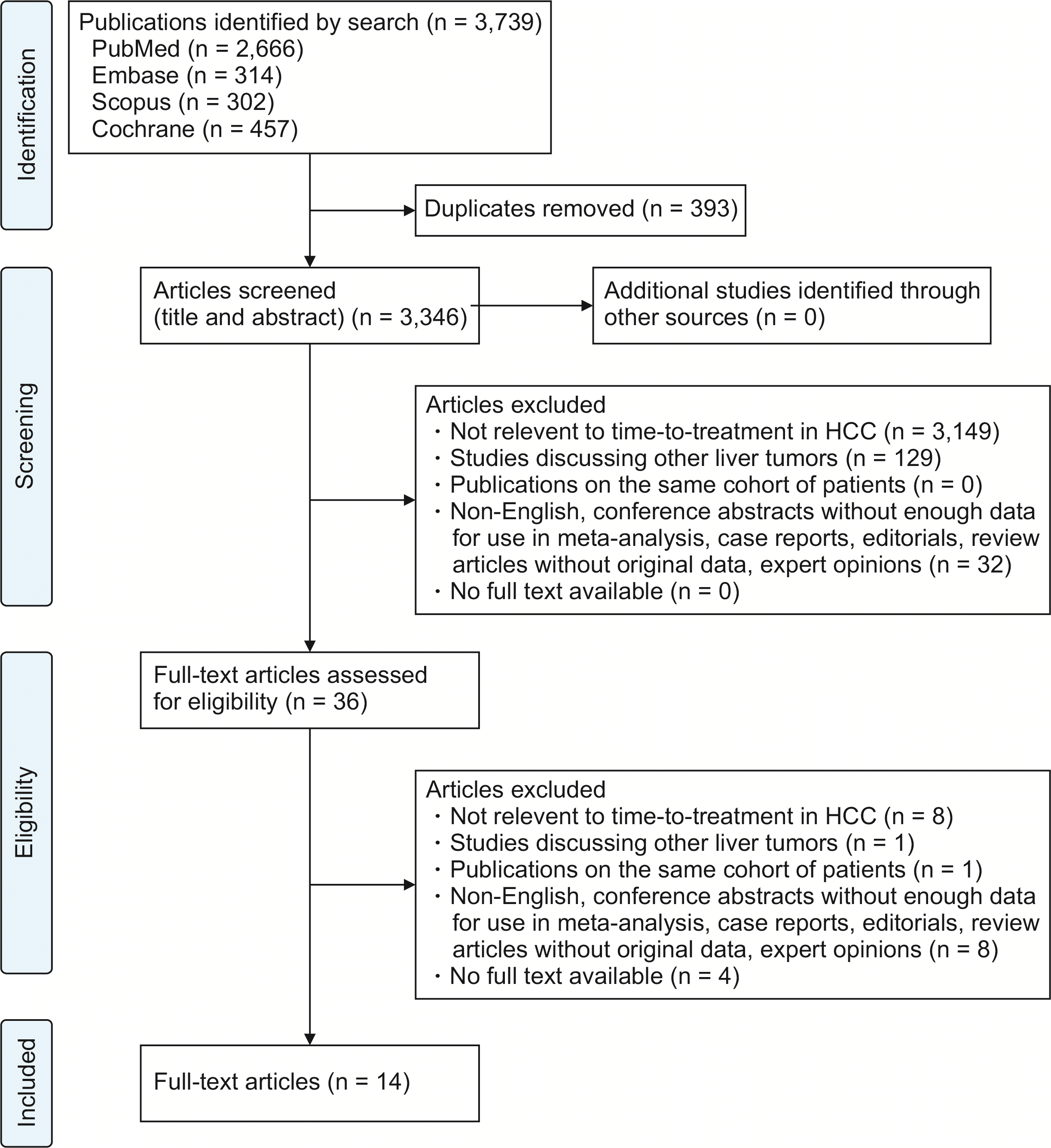
Fig. 2
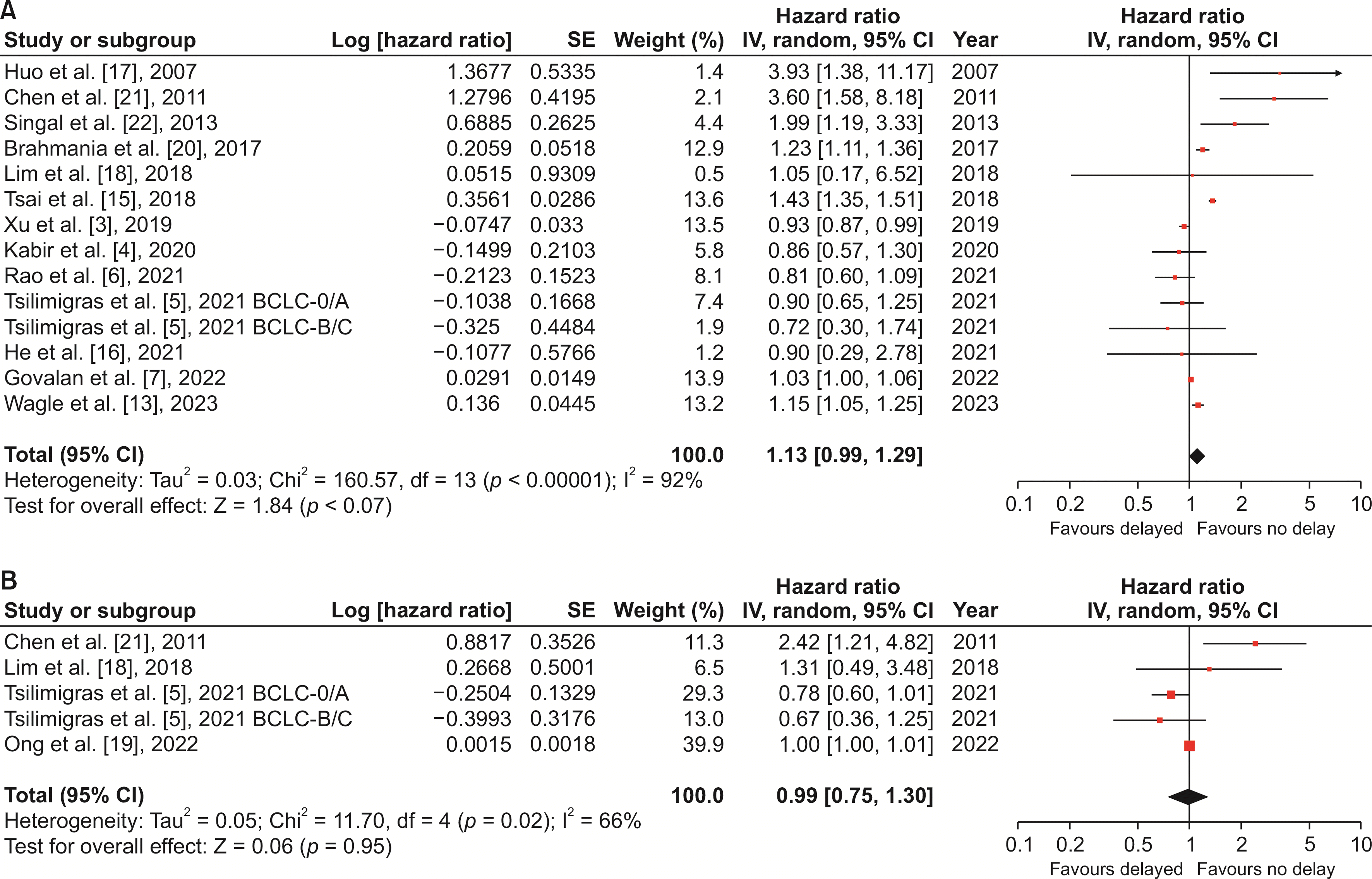
Fig. 3
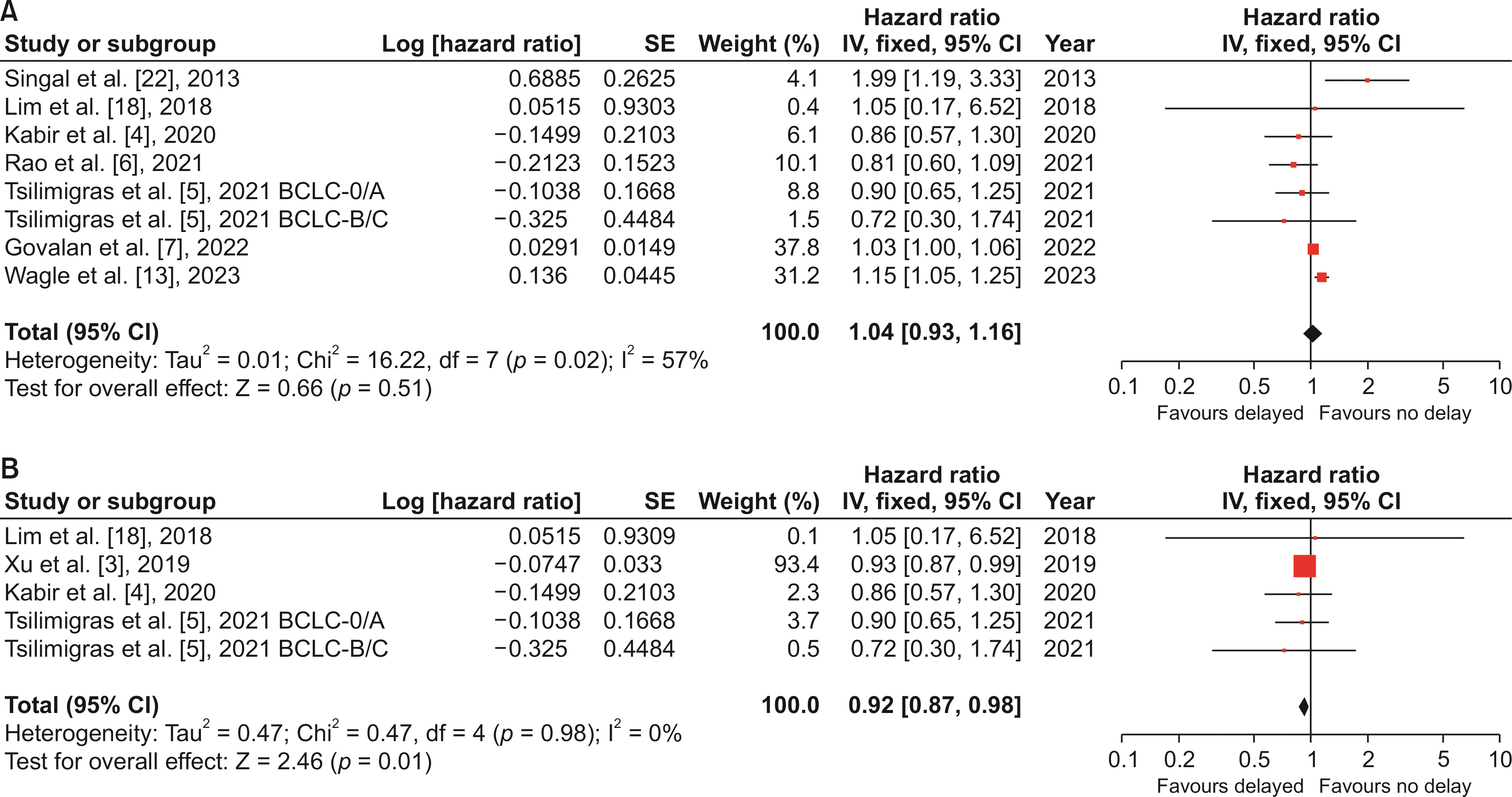
Table 1
| No | Author, year | Study design | Study period | Country | Sample size | Age (yr) | Male | Child-Pugh (A/B) | AFP (ng/mL) | Tumor size (cm) | MELD Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Brahmania et al. [20], 2017a) | Retrospective | Jul 2010–Dec 2013 | Canada |
No delay: 110 Delayed: 109 |
NR | NR | NR | NR | NR | NR |
| 2 | Chen et al. [21], 2011 | Prospective | Jan 2004–Jul 2007 | Taiwan |
No delay: 100 Delayed: 21 |
No delay: 64.8 ± 10.3 Delayed: 66.2 ± 9.7 |
No delay: 59 Delayed: 21 |
No delay: 88/12 Delayed: 15/6 |
No delay: 162 ± 402 Delayed: 70.5 ± 129 |
NR | NR |
| 3 | Govalan et al. [7], 2022 | Retrospective | 2010–2017 | United States |
No delay: 71,845 Delayed: 16,307 |
No delay: 60 ± 10 Delayed: 63 ± 9 |
No delay: 54,802 Delayed: 12,445 |
NR |
Positive: No delay: 41,060 Delayed: 9,110 |
No delay: 4.0 (2.5–7.0) Delayed: 3.2 (2.2–5.0) No delay: 4.5 ± 3.3g) Delayed: 3.5 ± 2.1g) |
No delay: 11 (8–17) Delayed: 11 (8–16) No delay: 12.0 ± 6.7g) Delayed: 11.7 ± 5.9g) |
| 4 | He et al. [16], 2021b) | Retrospective | 2012–2018 | China |
No delay: 215 Delayed: 17 |
NR | NR | NR | NR | NR | NR |
| 5 | Huo et al. [17], 2007 | Retrospective | Feb 1998–Apr 2003 | Taiwan |
No delay: 96 Delayed: 48 |
No delay: 68.0 ± 9.4 Delayed: 67.6 ± 10.4 |
No delay: 71 Delayed: 35 |
No delay: 65/31 Delayed: 34/14 |
NR |
> 5 cm: No delay: 16 Delayed: 7 |
No delay: 11.1 ± 2.5 Delayed: 12.3 ± 1.8 |
| 6 | Kabir et al. [4], 2020c,d) | Retrospective | 2000–2015 | Singapore |
No delay: 781 Delayed: 82 |
No delay: 61.7 ± 12.0 Delayed: 60.3 ± 12.0 |
No delay: 610 Delayed: 66 |
No delay: 737/44 Delayed: 77/5 |
NR |
No delay: 5.6 ± 4.7 Delayed: 5.1 ± 4.1 |
NR |
| 7 | Lim et al. [18], 2018c) | Retrospective | Jan 2006–Jun 2016 | France |
No delay: 50 Delayed: 50 |
No delay: 65 (59–72) Delayed: 65 (58–73) No delay: 65.3 ± 9.9g) Delay: 65.3 ± 11.5 g) |
No delay: 41 Delayed: 36 |
NR |
No delay: 12 (4–112) Delayed: 9 (5–34) |
No delay: 3.7 (2.5–6.4) Delayed: 3.4 (2.6–6.3) No delay: 4.2 ± 3.0g) Delayed: 4.1 ± 2.8g) |
No delay: 8 (6–9) Delayed: 7 (6–9) No delay: 7.67 ± 2.29g) Delayed: 7.33 ± 2.29g) |
| 8 | Ong et al. [19], 2022 | Retrospective | Jan 2011–Jul 2017 | Singapore |
No delay: 106 Delayed: 109 |
No delay: 68.49 ± 10.68 Delayed: 68.62 ± 9.43 |
No delay: 83 Delayed: 77 |
NR | NR | NR | NR |
| 9 | Rao et al. [6], 2021 | Retrospective | Jan 2008–Jul 2017 | Unites States |
No delay: 500 Delayed: 104 |
NR | NR | NR | NR | NR | NR |
| 10 | Singal et al. [22], 2013 | Retrospective | Jan 2005–Jun 2012 | United States |
No delay: 50 Delayed: 115 |
NR |
No delay: 38 Delayed: 93 |
No delay: 50/50 Delayed: 72/42 |
NR | NR | NR |
| 11 | Tsai et al. [15], 2018e,f) | Retrospective | 2004–2010 | Taiwan |
No delay: 21,123 Delayed: 2,124 |
No delay: 63.03 ± 12.17 Delayed: 64.59 ± 12.04 |
NR | NR | NR | NR | NR |
| 12 | Tsilimigras et al. [5], 2021 | Retrospective | 2000–2017 | United States |
No delay: 537 Delayed: 238 |
No delay: 68 (59–74) Delay: 67 (59–74) No delay: 67.0 ± 11.2g) Delayed: 66.7 ± 11.1g) |
No delay: 391 Delayed: 186 |
NR |
400: No delay: 87 Delayed: 35 |
No delay: 5.0 (3.0–8.5) Delayed: 4.5 (3.0–7.5) No delay: 5.5 ± 4.1g) Delayed: 5.0 ± 3.4g) |
NR |
| 13 | Wagle et al. [13], 2022 | Retrospective | 2001–2015 | United States |
No delay: 7,245 Delayed: 1,205 |
NR |
No delay: 4,863 Delayed: 812 |
NR | NR | NR | NR |
| 14 | Xu et al. [3], 2019b) | Retrospective | 2004–2012 | United States and Puerto Rico |
No delay: 7,115 Delayed: 4,987 |
No delay: 62.8 ± 11.8 Delayed: 62.0 ± 10.3 |
No delay: 5,055 Delayed: 3,716 |
NR |
Elevated: No delay: 3,446 Delayed: 2,628 |
> 5 cm: No delay: 2,584 Delayed: 1,269 |
No delay: 13.0 ± 8.6 Delayed: 13.1 ± 8.5 |
All continuous variables are expressed in mean ± standard deviation or median (interquartile range) unless otherwise stated. All categorical variables are expressed as n (%) unless otherwise stated.
AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; MELD, Model for End-stage Liver Disease; NR, not reported.
a)Values included in this study are from the hazard ratio for wait time expressed per 30 days. b)Values included in this study are from the cohort defining treatment delay as > 60 days (data comparing other cut-offs were excluded). c)Values included in this study is obtained after propensity score matching. d)Values included in this study are from the cohort defining treatment delay as > 90 days (data comparing other cut-offs were excluded). e)Values included in this study are from the data sets of cohorts ≤ 30 days and 61–180 days (other data sets were excluded). f)Histology of liver cancer in this study does not explicitly mention HCC, assumed that study includes only HCC and excludes other liver cancers based on reading of article. g)Mean and standard deviation were calculated from median and range/interquartile range using methods described by Wan et al. [10].
Table 2
| No | Study variables and/or outcomes | No. of data sets | Total number of patients (delayed/no delay) | No. of patients (%) | Effect size, OR (95% CI)/MD (95% CI)/HR (95% CI)a) | p-value | I2 (%) | Model used | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Delayed | No delay | ||||||||
| Demographics and histopathological findings | |||||||||
| 1 | Age (yr) | 9 | 125,719 (23,966/101,753) | NA | –0.17 (–1.09, 0.76) | 0.72 | 90 | RE | |
| 2 | Male | 10 | 111,087 (23,162/87,925) | 17,487 (75.5) | 66,013 (75.1) | 1.07 (0.95, 1.20) | 0.26 | 65 | RE |
| 3 | Child’s B cirrhosis | 4 | 1,293 (266/1,027) | 82 (30.8) | 113 (11.0) | 1.49 (0.62, 3.61) | 0.37 | 77 | RE |
| 4 | Tumor size (cm) | 4 | 89,890 (16,677/73,213) | NA | –0.70 (–1.14, –0.26) | 0.002* | 59 | RE | |
| 5 | BCLC-0/A staging | 3 | 1,040 (403/637) | 322 (79.9) | 547 (85.9) | 0.75 (0.29, 1.92) | 0.55 | 79 | RE |
| 6 | MELD score | 4 | 100,498 (21,392/79,106) | NA | 0.12 (–0.39, 0.62) | 0.65 | 86 | RE | |
| 7 | Surgical resection | 6 | 102,157 (21,779/80,378) | 6,667 (30.6) | 18,946 (23.6) | 1.10 (0.20, 5.99) | 0.92 | 89 | RE |
| 8 | RFA | 3 | 88,438 (16,443/71,995) | 2,842 (17.3) | 10,611 (14.7) | 1.22 (1.16, 1.27) | < 0.0001* | 0 | FE |
| Outcomes | |||||||||
| 9 | Overall survival | 13 | 134,955 (25,298/109,657) | NA | 1.13 (0.99, 1.29) | 0.07 | 92 | RE | |
| 10 | Disease-free survival | 4 | 1,211 (418/793) | NA | 0.99 (0.75, 1.30) | 0.95 | 66 | RE | |
| 11 | Overall survival with TTT defined as 90 days | 7 | 99,109 (18,101/81,008) | NA | 1.04 (0.93, 1.16) | 0.51 | 57 | RE | |
| 13 | Overall survival in surgical delay | 4 | 13,840 (5,357/8,483) | NA | 0.92 (0.87, 0.98) | 0.01* | 0 | FE | |
BCLC, Barcelona Liver Cancer Clinic; CI, confidence interval; FE, fixed-effects; HR, hazard ratio; I2, heterogeneity; MD, mean difference; MELD, Model for End-stage Liver Disease; NA, not applicable; OR, odds ratio; RE, random-effects; RFA, radiofrequency ablation; TTT, time-to-treatment.
Table 3
| No | Author, year | Treatment delay cut-off | Treatment modalities | Time-to-treatment | Treatment complications | 1-year OS (%) | 1-year DFS (%) | 5-year OS (%) | 5-year DFS (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Brahmania et al. [20], 2017 | 96 daysc) |
RFA: No delay: 110 Delayed: 109 |
96 days (75–139) | NR | NR | NR | NR | NR |
| 2 | Chen et al. [21], 2011 | 5 wk |
RFA: No delay: 100 Delayed: 21 |
NR | NR | NR | NR | NR | NR |
| 3 | Govalan et al. [7], 2022 | 90 days |
LT: No delay: 6,299 Delayed: 1,696 LR: No delay: 10,459 Delayed: 1,287 Any type of ablation: No delay: 10,507 Delayed: 2,809 |
NR | NR | NR | NR | NR | NR |
| 4 | He et al. [16], 2021 | 60 daysd) | NR | NR | NR | NR | NR | NR | NR |
| 5 | Huo et al. [17], 2007 | 60 days |
TACE: No delay: 48 Delayed: 56 PAI: No delay: 35 Delayed: 29 PEI: No delay: 18 Delayed: 15 |
NR | NR |
No delay: 97 Delayed: 86 |
NR | NR | NR |
| 6 | Kabir et al. [4], 2020a) | 90 dayse) |
LR: No delay: 781 Delayed: 82 |
NR | NR | NR | NR | NR | NR |
| 7 | Lim et al. [18], 2018a) | 90 days |
LR: No delay: 50 Delayed: 50 |
3 mon (1.8–4.6) |
No delay: 8 Delayed: 18 |
No delay: 100 Delayed: 96 |
No delay: 94 Delayed: 88 |
No delay: 80 Delayed: 81 |
No delay: 48 Delayed: 37 |
| 8 | Ong et al. [19], 2022 | 42 days | NR | 42 days (0–445) | NR | NR | NR | NR | NR |
| 9 | Rao et al. [6], 2021 | 90 days | NR | 46 days (29–74) | NR | NR | NR | NR | NR |
| 10 | Singal et al. [22], 2013 | 90 days |
LT: No delay: 5 Delayed: 1 LR: No delay: 4 Delayed: 23 TACE: No delay: 34 Delayed: 53 RFA: No delay: 4 Delayed: 12 Systemic therapy: No delay: 3 Delayed: 26 |
1.7 mon (0.1–42.5) | NR |
No delay: 89.80 Delayed: 63.70 |
NR | NR | NR |
| 11 | Tsai et al. [15], 2018b) | < 30 days vs. 61–180 daysf) | NR | NR | NR | NR | NR | NR | NR |
| 12 | Tsilimigras et al. [5], 2021 | 90 days |
LR: No delay: 537 Delayed: 238 |
60 days (34–100) |
No delay: 197 Delayed: 79 |
NR | NR |
No delay: 63.70 Delayed: 64.90 |
No delay: 33.50 Delayed: 42.40 |
| 13 | Wagle et al. [13], 2023 | 90 days | NR | 1 mon (1–3) | NR | NR | NR | NR | NR |
| 14 | Xu et al. [3], 2019 | 60 daysd) |
LR: No delay: 7,115 Delayed: 4,987 |
50 days (29–86) | NR | NR | NR | NR | NR |
All continuous variables are expressed in mean ± standard deviation, or median (interquartile range) unless otherwise stated. All categorical variables are expressed as n (%) unless otherwise stated.
RFA, radiofrequency ablation; DFS, disease-free survival; NR, not reported; LR, liver resection; LT, liver transplantation; OS, overall survival; PAI, percutaneous acetic acid injection; PEI, percutaneous ethanol injection; TACE, transarterial chemoembolization.
a)Values included is this study is obtained after propensity score matching. b)Histology of liver cancer in this study does not explicitly mention HCC, assumed that study includes only HCC and excludes other liver cancers based on reading of article. c)Values included in this study are from the hazard ratio for wait time expressed per 30 days. d)Values included in this study are from the cohort defining treatment delay as > 60 days (data comparing other cut-offs were excluded). e)Values included in this study are from the cohort defining treatment delay as > 90 days (data comparing other cut-offs were excluded). f)Values included in this study are from the data sets of cohorts ≤ 30 days and 61–180 days (other data sets were excluded).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download