Abstract
Despite debates regarding the safety of well-selected left-sided pancreatic cancer, minimally invasive distal pancreatosplenectomy is considered safer and more effective than open distal pancreatosplenectomy in well-selected patients. Previous studies have shown that minimally invasive surgery yields comparable oncologic outcomes to open surgery. While patients who undergo minimally invasive distal pancreatosplenectomy also experience recurrences and metastases after surgery, port-site metastasis is particularly rare. In this report, we report an extremely rare case of port-site metastasis following minimally invasive distal pancreatosplenectomy for left-sided pancreatic cancer.
Despite the controversies, with advancements in surgical techniques and equipment, the safety and oncological effectiveness of minimally invasive pancreatosplenectomy have been demonstrated. As a result, laparoscopic surgery or robot-assisted surgery is being considered as one of the treatment options for well-selected left-sided pancreatic cancer.
Pancreatic cancer is known for its high early recurrence rates after surgery, with the liver being the most common site of recurrence, followed by the lungs, bones, and brain [1]. While cutaneous recurrence and metastasis can occur, they are extremely rare and are often associated with disseminated pancreatic cancer [2].
This report presents a rare case of metastasis at the port site used during minimally invasive distal pancreatosplenectomy for left-sided pancreatic cancer. This will provide a new perspective on the prognosis observation of pancreatic cancer patients who underwent minimally invasive surgery.
A 51-year-old female patient visited our hospital with a palpable mass in the left abdominal area. She had a history of laparoscopic distal pancreatosplenectomy for left-sided pancreatic cancer on August 18, 2015 (Fig. 1A, 1B). At the time of the initial preoperative evaluation, an endoscopic ultrasound-guided fine needle biopsy for the pancreatic mass was performed, but a pathological diagnosis could not be confirmed. The preoperative CA 19-9 levels was 223.5 U/mL. A laparoscopic distal pancreatosplenectomy was performed following a previously reported method [3]. The postoperative pathological results revealed pancreatic ductal adenocarcinoma. No postoperative complications were observed, and she was discharged on the seventh postoperative day. Starting from the twenty-eighth postoperative day, she underwent adjuvant chemotherapy (Gemcitabine) and completed the prescribed cycles. During follow-up, the serum CA 19-9 level decreased to 11.6 U/mL.
Eight months after the surgery, she reported a palpable mass around the site of the previous drain insertion. Her serum CA 19-9 level increased to 40.0 U/mL, and positron emission tomography-computed tomography (PET-CT) showed an uptake signal in the area around the port site of the previous drain after laparoscopic distal pancreatosplenectomy (Fig. 1C). There was no other evidence suggesting peritoneal recurrence. The surgery involved excision of the lesion with a 1.5 cm margin (Fig. 1D, 1E). The postoperative pathological results confirmed that the mass, measuring approximately 2 cm × 1 cm × 1 cm, was a metastatic adenocarcinoma originating from the pancreas (Fig. 1F).
We have retrospectively reviewed the data from a single center. From June 2007 to October 2021. A total of 316 patients underwent distal pancreatectomy for left-sided pancreatic cancer. The mean age of the patients was 64.55 ± 9.77 years. Males comprised 178 patients (56.3%), while females accounted for 138 patients (43.7%) were females. Among them, open distal pancreatectomy was performed in 137 patients (43.4%), and 179 patients (56.6%) underwent minimally invasive distal pancreatectomy. Upfront surgery was performed in 238 patients (75.3%) and 78 patients (24.7%) underwent distal pancreatectomy following neoadjuvant treatment. Overall survival of minimally invasive distal pancreatectomy was median of 32.36 months (95% confidence interval [CI], 27.21–37.52).
Among the 179 patients who underwent minimally invasive distal pancreatectomy, 8 cases (4.5%) had port-site metastasis (Table 1). When comparing within the group of patients with metastasis, the median survival duration of port-site metastasis was similar to that of other site recurrences (21.125 months [CI, 12.67–29.58] vs. 26.110 months [CI, 23.23–28.99], p = 0.34) (Fig. 2).
Since 2007, we have been applying our selection criteria, the Yonsei criteria, to perform minimally invasive distal pancreatosplenectomy for the treatment of left-sided pancreatic cancer [3-5]. According to our institutional findings, bloodless and margin-negative resection were identified as independent prognostic factors for left-sided pancreatic cancer [6]. When performing minimally invasive distal pancreatosplenectomy, the following factors, observed on preoperative CT scan, enable bloodless and margin-negative resection: 1) a tumor confined within the pancreas, 2) intact fascia between the left pancreas and left adrenal gland and left kidney, and 3) being at least 1–2 cm away from the major vessels [7]. Recently, it was reported that minimally invasive radical distal pancreatectomy for pancreatic cancer within Yonsei criteria resulted in very excellent oncologic outcomes [3]. With the accumulation of experience in minimally invasive surgery and advancements in surgical techniques and equipment, it is now possible to perform minimally invasive pancreatectomy for advanced pancreatic cancer following neoadjuvant chemotherapy [8-10].
With the increasing adoption of minimally invasive surgery in the field of clinical oncology, the number of reported cases of port-site metastasis is also rising. Specifically, port-site metastasis has been previously reported in gallbladder cancer and colon cancer after minimally invasive surgery [11,12]. Among them, port-site metastasis of gallbladder cancer in hepatobiliary and pancreatic surgery has been reported as a case report and raised concerns [13-15]. Therefore, the use of a bag during minimally invasive surgery has been recommended [16]. However, cases of port-site metastasis in pancreatic cancer after minimally invasive pancreatectomy are rare. The present case is a port-site metastasis from pancreatic cancer after minimally invasive distal pancreatosplenectomy, which is rarely reported in the literature.
Upon reviewing the literature, skin metastasis after radical pancreatectomy for left-sided pancreatic cancer has been reported in several cases, mostly associated with disseminated pancreatic cancer [17], In our case, there was no evidence of systemic metastasis, suggesting a less likely association with disseminated pancreatic cancer. Nevertheless, considering the possibility of systemic metastasis, the patient required additional adjuvant chemotherapy. However, the patient declined to undergo adjuvant chemotherapy. Subsequent follow-up revealed multiple peritoneal metastases four months after the removal of the lesion at the port site.
Among our eight cases, surgical resection was performed in only two cases for the port-site metastatic lesions, and in these cases, pathological findings confirmed metastatic adenocarcinoma, clinically originating from the pancreas, directly confirming metastatic findings at the port site. The pathological characteristics of the primary pancreatic cancer in the two cases were mostly moderately differentiated ductal adenocarcinoma, and at the time of surgery, the resection margins were free. There was no evidence of perineural invasion, lymphovascular invasion, or lymph node involvement. In six cases, systemic metastasis was already confirmed at the time of discovery, and surgical treatment was not performed. In these cases, the locations found on PET-CT and CT scans were around the port site on the abdominal wall used in the previous surgery or along the path of port insertion. Although tissue confirmation through resection or biopsy was not performed, the location of the metastatic lesion and the progression of the disease were observed before observing port-site metastasis. Subsequently, these patients underwent adjuvant chemotherapy.
After minimally invasive pancreatosplenectomy, the following factors are considered as possible causes of port-site metastasis in pancreatic cancer: 1) peritoneal seeding due to the preoperative endoscopic ultrasound-guided fine needle biopsy (EUS-FNAB). Although it is known that preoperative EUS-FNAB does not significantly impact prognosis, reports have shown needle tract and peritoneal seeding after EUS-guided fine needle aspiration biopsy in pancreatic cancer [18]. 2) Pathological findings indicating invasion of pancreatic cancer into the surrounding soft tissue may lead to shedding during the mobilization or delivery of resected pancreatic cancer. 3) The tumor biology of pancreatic cancer may also play a role. In a previous study comparing SUVmax determined based on preoperative PET-CT and SMAD4 expression, we observed a correlation between high SUVmax values and loss of SMAD4, which we considered to reflect the characteristics of systemic metastasis in pancreatic cancer [19].
According to our study, similar to previously reported studies, there is no significant difference in long-term survival and other oncologic outcomes between minimally invasive distal pancreatectomy and open distal pancreatectomy for well-selected left-sided pancreatic cancer [2]. Therefore, minimally invasive distal pancreatectomy is oncologically safe and effective. In our study, port-site metastasis was found in 4.5% of cases; however, there was no significant difference in oncologic adverse effects compared to other recurrence patterns. Therefore, there is no sufficient evidence to hold negative opinions about minimally invasive distal pancreatectomy regarding the potential oncologic risk of port-site metastasis. Nonetheless, considering the limited number of reports available, further multicenter studies are needed to address this issue in the future.
In particular, considering the survival of patients with port-site metastasis (21.125 months), tumor biology appears to be similar to that of conventional resectable pancreatic cancer, suggesting that the maintenance of potent chemotherapy has played a significant role. Therefore, it is necessary to consider aggressive treatment options. Especially, FOLFIRINOX and nab-paclitaxel have been found to be effective in pancreatic cancer with systemic metastasis [20]. Based on this, additional chemotherapy should be considered for favorable outcomes in patients with port-site metastasis, and various institutions need to collaborate for further research in this regard.
Port-site metastasis can occur in minimally invasive pancreatectomy. However, the oncologic risk is not different from other metastasis patterns. Therefore, there is no evidence to suggest a negative opinion against minimally invasive surgery. Reports of port-site metastasis in pancreatic cancer are rare, but further research is needed.
REFERENCES
1. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. 2015; Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 26 Suppl 5:v56–68. DOI: 10.1093/annonc/mdv295. PMID: 26314780.

2. Aida T, Iwase R, Usuba T, Kumagai Y, Furukawa K, Onda S, et al. 2023; Successful resection of port site recurrence of pancreatic ductal adenocarcinoma after laparoscopic distal pancreatectomy. Surg Case Rep. 9:35. DOI: 10.1186/s40792-023-01607-w. PMID: 36867254. PMCID: PMC9984651.

3. Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. 2014; Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 28:2848–2855. DOI: 10.1007/s00464-014-3537-3. PMID: 24853839.

4. Choi SH, Kang CM, Hwang HK, Lee WJ, Chi HS. 2012; Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg. 16:868–869. DOI: 10.1007/s11605-012-1825-6. PMID: 22258879.

5. Choi SH, Kang CM, Lee WJ, Chi HS. 2011; Laparoscopic modified anterior RAMPS in well-selected left-sided pancreatic cancer: technical feasibility and interim results. Surg Endosc. 25:2360–2361. DOI: 10.1007/s00464-010-1556-2. PMID: 21298529.

6. Kang CM, Kim DH, Lee WJ. 2010; Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 24:1533–1541. DOI: 10.1007/s00464-009-0806-7. PMID: 20054579.

7. Kang CM, Lee SH, Lee WJ. 2014; Minimally invasive radical pancreatectomy for left-sided pancreatic cancer: current status and future perspectives. World J Gastroenterol. 20:2343–2351. DOI: 10.3748/wjg.v20.i9.2343. PMID: 24605031. PMCID: PMC3942837.

8. Hong SS, Hwang HK, Lee WJ, Kang CM. 2020; Feasibility and safety of laparoscopic radical distal pancreatosplenectomy with adrenalectomy in advanced pancreatic cancer. Ann Surg Oncol. 27:5235–5236. DOI: 10.1245/s10434-020-08670-9. PMID: 32474822.

9. Kim SJ, Park JY, Hwang HS, Kang CM. 2021; Complete response of locally advanced left-sided pancreatic cancer after modified FOLFIRINOX chemotherapy followed by conversion surgery: a case report. Ann Hepatobiliary Pancreat Surg. 25:390–394. DOI: 10.14701/ahbps.2021.25.3.390. PMID: 34402441. PMCID: PMC8382870.

10. Kim YS, Kim JS, Kim SH, Hwang HK, Lee WJ, Kang CM. 2022; Laparoscopic radical distal pancreatosplenectomy with celiac axis excision following neoadjuvant chemotherapy for locally advanced pancreatic cancer. Ann Hepatobiliary Pancreat Surg. 26:118–123. DOI: 10.14701/ahbps.21-097. PMID: 34907094. PMCID: PMC8901982.

11. Bărbulescu M, Alecu L, Boeţi P, Popescu I. 2012; Port-site metastasis after laparoscopic surgery for colorectal cancer--still a real concern? Case report and review of the literature. Chirurgia (Bucur). 107:103–107.
12. Lupinacci RM, Santana A, Dias AR. 2014; Metastatic gallbladder adenosquamous carcinoma to the skin. J Surg Case Rep. 2014:rju130. DOI: 10.1093/jscr/rju130. PMID: 25480835. PMCID: PMC4256706.

13. Berger-Richardson D, Chesney TR, Englesakis M, Govindarajan A, Cleary SP, Swallow CJ. 2017; Trends in port-site metastasis after laparoscopic resection of incidental gallbladder cancer: a systematic review. Surgery. 161:618–627. DOI: 10.1016/j.surg.2016.08.007. PMID: 27743715.

14. Maker AV, Butte JM, Oxenberg J, Kuk D, Gonen M, Fong Y, et al. 2012; Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol. 19:409–417. DOI: 10.1245/s10434-011-1850-9. PMID: 21698501.

15. Gao KJ, Yan ZL, Yu Y, Guo LQ, Hang C, Yang JB, et al. 2020; Port-site metastasis of unsuspected gallbladder carcinoma with ossification after laparoscopic cholecystectomy: a case report. World J Clin Cases. 8:5729–5736. DOI: 10.12998/wjcc.v8.i22.5729. PMID: 33344567. PMCID: PMC7716338.

16. Petryshyn N, Dražić T, Hogendorf P, Strzelczyk J, Strzałka A, Szwedziak K, et al. 2021; Port site metastases a year after initial laparoscopic cholecystectomy. Should the use of retrieval bags during laparoscopic cholecystectomy be the new gold standard? Pol Przegl Chir. 93:61–65.
17. Zhou HY, Wang XB, Gao F, Bu B, Zhang S, Wang Z. 2014; Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature. Oncol Lett. 8:2654–2660. DOI: 10.3892/ol.2014.2610. PMID: 25364444. PMCID: PMC4214468.

18. Chong A, Venugopal K, Segarajasingam D, Lisewski D. 2011; Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 74:933–935. DOI: 10.1016/j.gie.2010.10.020. PMID: 21951481.

19. Kang CM, Hwang HK, Park J, Kim C, Cho SK, Yun M, et al. 2016; Maximum standard uptake value as a clinical biomarker for detecting loss of SMAD4 expression and early systemic tumor recurrence in resected left-sided pancreatic cancer. Medicine (Baltimore). 95:e3452. DOI: 10.1097/MD.0000000000003452. PMID: 27124039. PMCID: PMC4998702.

20. Rajeshkumar NV, Yabuuchi S, Pai SG, Tong Z, Hou S, Bateman S, et al. 2016; Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br J Cancer. 115:442–453. DOI: 10.1038/bjc.2016.215. PMID: 27441498. PMCID: PMC4985357.

Fig. 1
Port-site metastasis after laparoscopic radical distal pancreatosplenectomy. (A) Preoperative Abdominal-Pelvic CT scan. (B) Preoperative PET-CT scan. (C) Eight months after, postoperative PET-CT, previous port site metastatic lesion. (D) Port site metastatic lesion excision. (E) Excisional gross mass, metastatic adenocarcinoma. (F) Pathological analysis, metastatic adenocarcinoma. CT, computed tomography; PET-CT, positron emission tomography-CT.
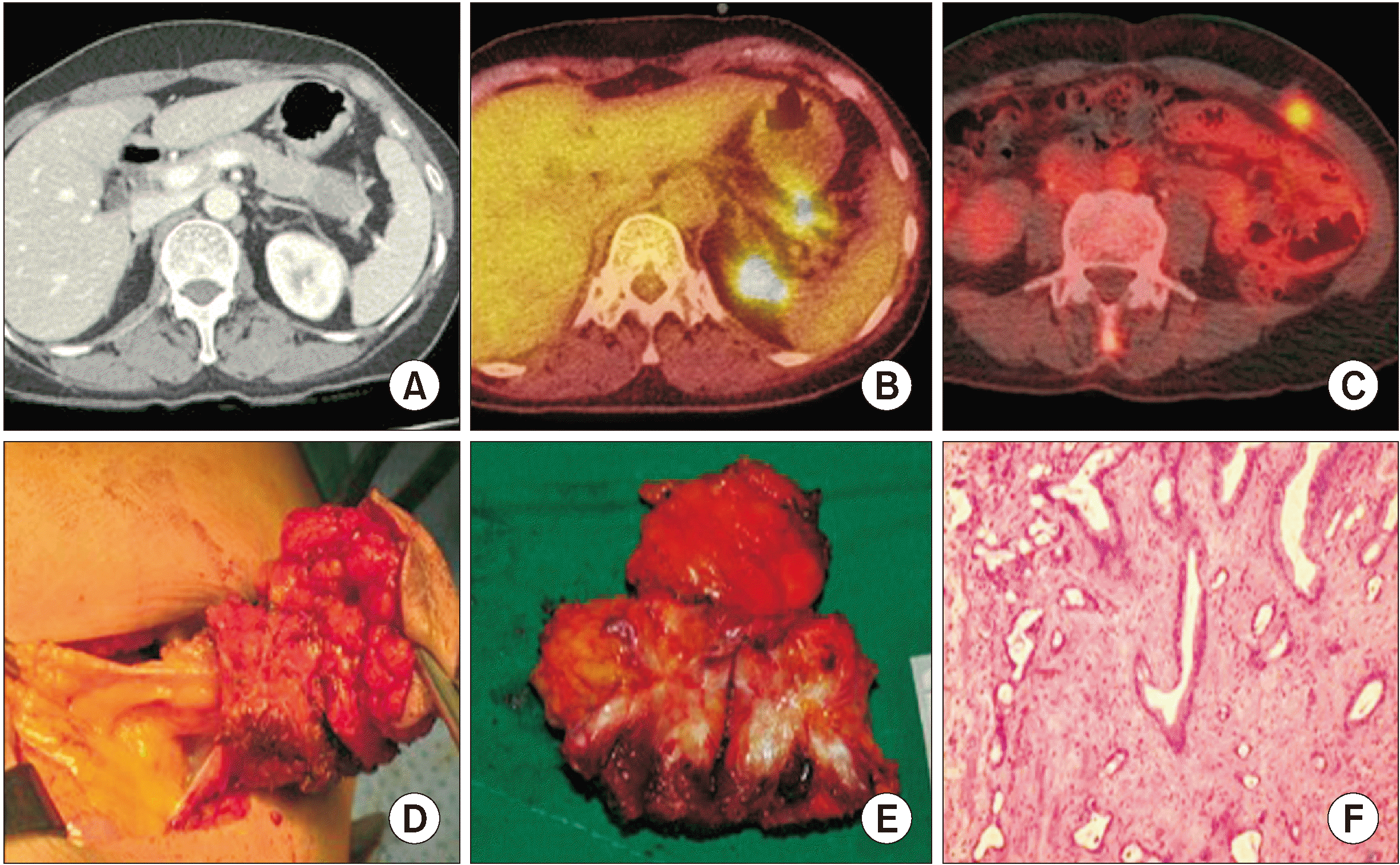
Fig. 2
A comparison of survival rates between patients with port-metastasis and patients with metastasis in other sites.
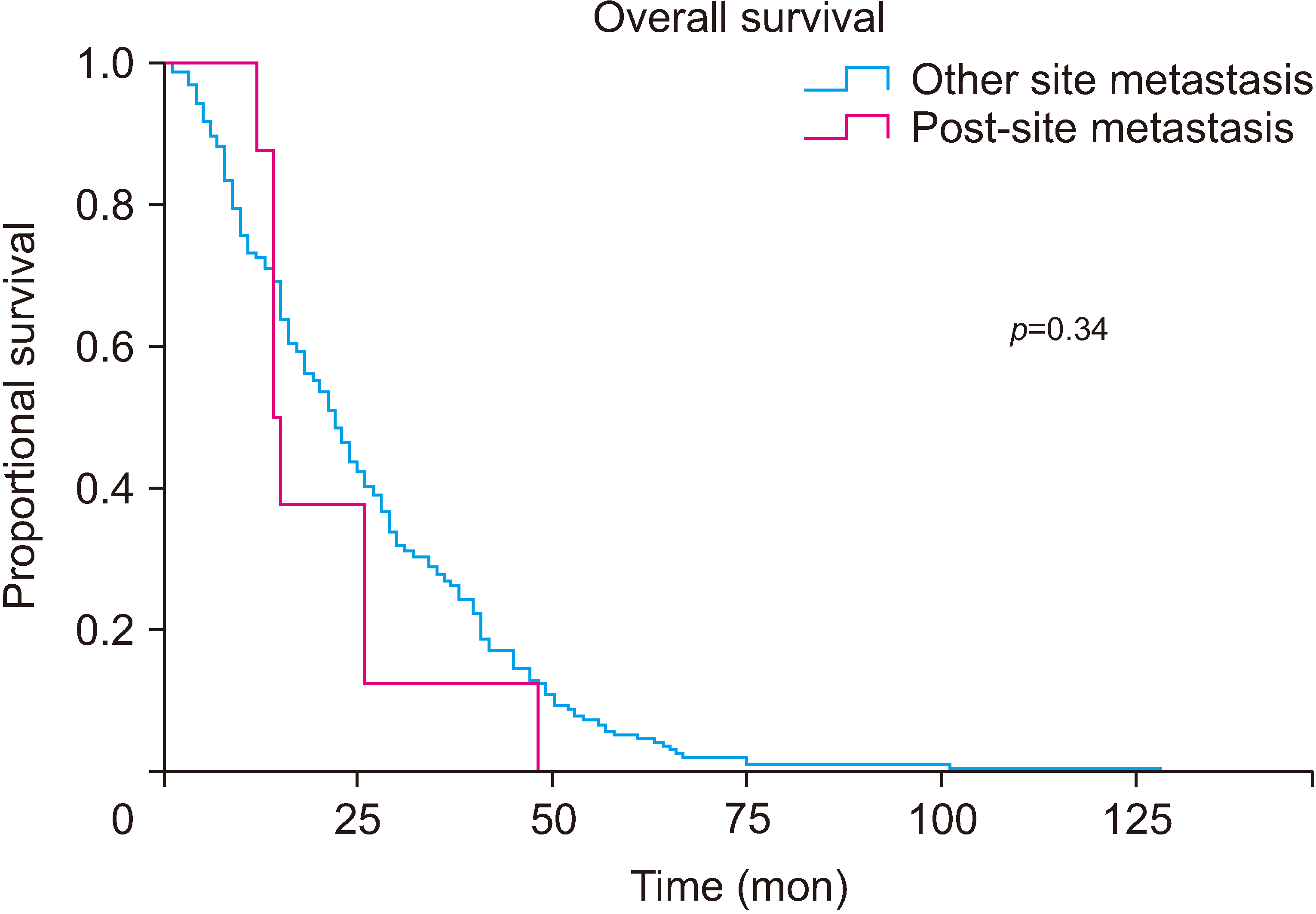
Table 1
Summary of patients with port-site metastasis following laparoscopic radical distal pancreatectomy




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download