Abstract
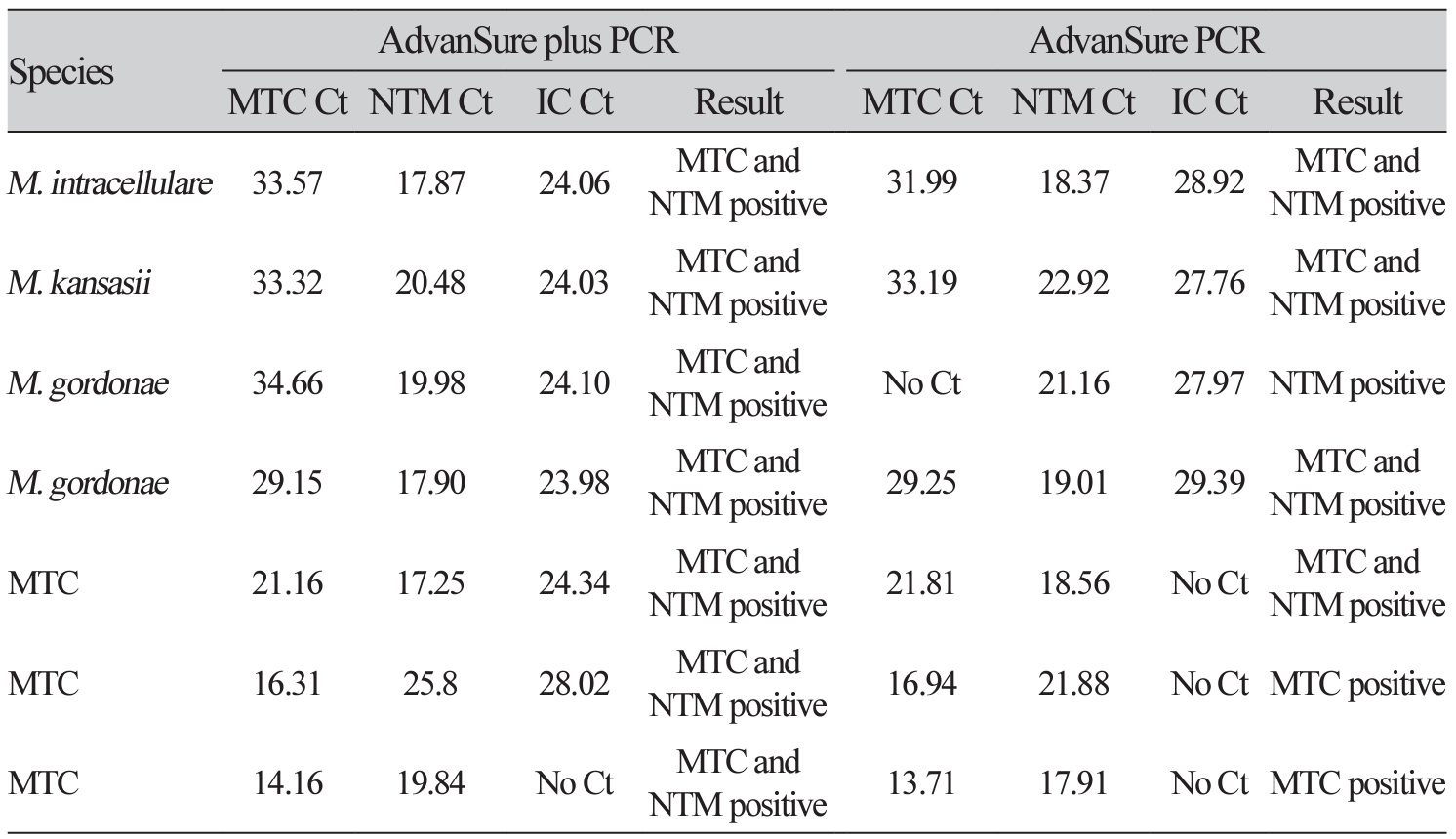
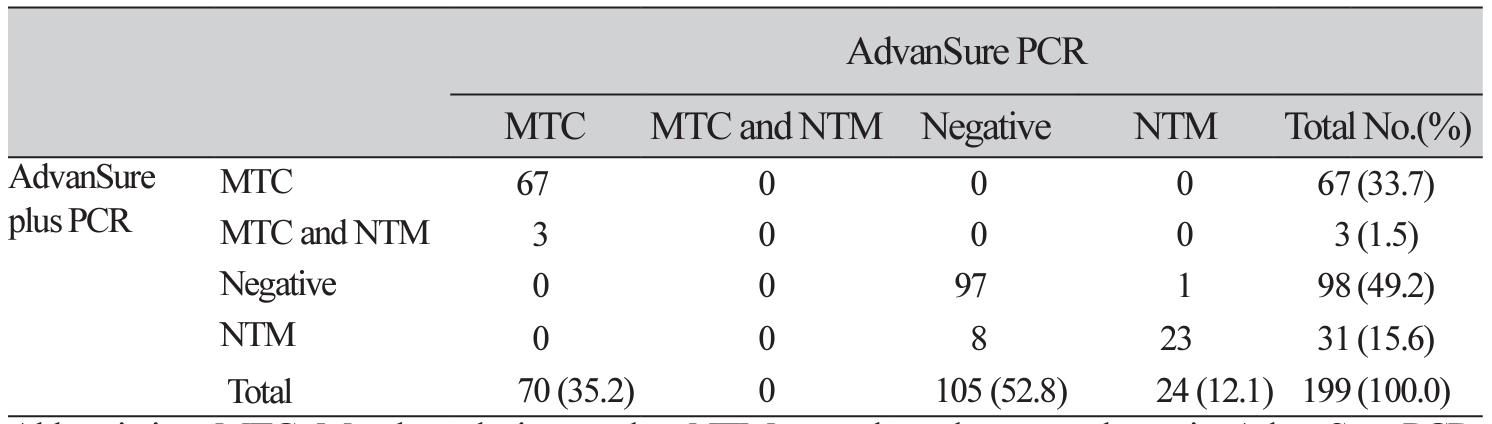
Background: The AdvanSure TB/NTM plus real-time PCR (AdvanSure plus PCR; LG Chem., Seoul, Korea) assay has been developed to increase the diagnostic sensitivity of nontuberculous mycobacteria (NTM) compared with the currently used The AdvanSure TB/NTM real-time PCR (AdvanSure PCR; LG Chem., Seoul, Korea) assay. In this study, we aimed to evaluate the performance of the AdvanSure plus PCR comparing the results with mycobacterial culture and the AdvanSure. Methods: Patients (n=199) with suspected NTM or Mycobacterium tuberculosis complex (MTC) were tested using AdvanSure plus PCR, AdvanSure, acid-fast bacilli staining, and mycobacteria culture. Additionally, 200 DNA samples (n=100, MTC and n=100, NTM) were obtained from positive MTC or NTM cultures for evaluation using the AdvanSure plus PCR assay. Results: The two real-time PCR systems showed a 94.0% (n=187/199) concordance rate (Kappa=0.94). Based on culture results, the sensitivity and specificity for the detection of v were 100% (45/45) and 83.8% (129/154) using AdvanSure plus PCR, and 100.0% (45/45) and 99.3% (153/154) using AdvanSure, respectively. The sensitivity and specificity for NTM detection were 68.0% (n=17/25) and 90.2% (n=157/174), respectively, with AdvanSure plus PCR, and 64.0% (n=16/25) and 95.4% (n=166/174), respectively, using AdvanSure. With culture-positive samples, AdvanSure plus PCR tested positive for 100% of both the MTC and NTM specimens. Seven (out of 200) culture-positive samples tested positive for both MTC and NTM using the AdvanSure plus PCR. Conclusion: AdvanSure plus PCR had increased sensitivity but decreased specificity compared with AdvanSure for the detection of NTM. The AdvanSure plus PCR assay can be used for the simultaneous detection of MTC and NTM in direct specimen and culture.
Go to : 
REFERENCES
1. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American thoracic society and the centers for disease control and prevention was adopted by the ATS board of directors, July 1999. This statement was endorsed by the council of the In fectious disease society of America, september 1999. Am J Respir Crit Care Med 2000;161:1376-95.
2. Lee H, Myung W, Koh WJ, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007-2016. Emerg Infect Dis 2019;25:569-72.
3. Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evo lution of tuberculosis control and prospects for reducing tu berculosis incidence, prevalence, and deaths globally. JAMA 2005;293:2767-75.
4. World Health Organization. Global tuberculosis report 2019. Geneva: World Health Organization. http://www.who.int/tb/publica tions/global_report/en/ [online] (last visited on 17 Oct 2019).
5. Levy H, Feldman C, Sacho H, van der Meulen H, Kallenbach J, Koornhof H. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest 1989;95:1193-7.
6. Ritchie SR, Harrison AC, Vaughan RH, Calder L, Morris AJ. New recommendations for duration of respiratory isolation based on time to detect Mycobacterium tuberculosis in liquid culture. Eur Respir J 2007;30:501-7.
7. Greco S, Rulli M, Girardi E, Piersimoni C, Saltini C. Diagnos tic accuracy of in-house PCR for pulmonary tuberculosis in smear-positive patients: meta-analysis and metaregression. J Clin Microbiol 2009;47:569-76.
8. Bartlett JM and Stirling D. A short history of the polymerase chain reaction. Methods Mol Biol 2003;226:3-6.
9. Kim SW, Kim SI, Lee SJ, Lee JH, Ryu YJ, Shim SS, et al. The effectiveness of real-time PCR assay, compared with microbiologic results for the diagnosis of pulmonary tuberculosis. Tuberc Respir Dis 2015;78:1-7.
10. Drosten C, Panning M, Kramme S. Detection of Mycobacte rium tuberculosis by real-time PCR using pan-mycobacterial primers and a pair of fluorescence resonance energy transfer probes specific for the M. tuberculosis complex. Clin Chem 2003;49:1659-61.
11. Jung CL, Kim MK, Seo DC, Lee MA. Clinical usefulness of real-time PCR and Amplicor MTC PCR assays for diagnosis of tuberculosis. Korean J Clin Microbiol 2008;11:29-33.
12. Ho TBL, Robertson BD, Taylor GM, Shaw RJ, Young DB. Comparison of Mycobacterium tuberculosis genomes reveals frequent deletions in a 20 kb variable region in clinical isolates. Int J Genomics 2000;1:272-82.
13. Talarico S, Durmaz R, Yang Z. Insertion- and deletion-associated genetic diversity of Mycobacterium tuberculosis phospholipase C-encoding genes among 106 clinical isolates from Turkey. J Clin Microbiol 2005;43:533-8.
14. Gordon SV, Brosch R, Billault A, Garnier T, Eiglmeier K, Cole ST. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol 1999;32:643-55.
15. Wang HY, Jin HW, Bang HE, Choi YI, Park EM, Koh WJ, et al. Evaluation of MolecuTech real MTC-ID for MTC/NTM detection using direct specimens. Korean J Clin Microbiol 2011;14:103-9.
16. Mäkinen J, Marjamäki M, Marttila H, Soini H. Evaluation of a novel strip test, GenoType Mycobacterium CM/AS, for species identification of mycobacterial cultures. Clin Microbiol Infect 2006;12:481-3.
17. Hwang S, Oh KJ, Jang IH, Uh Y, Yoon KJ, Kim HY, et al. Evalu ation of the diagnostic performance of the AdvanSure TB/NTM real-time PCR kit for detection of mycobacteria. Korean J Clin Microbiol 2011;14:55-9.
18. Mdivani N, Li H, Akhalaia M, Gegia M, Goginashvili L, Ker nodle DS, et al. Monitoring therapeutic efficacy by real-time detection of Mycobacterium tuberculosis mRNA in sputum. Clin Chem 2009;55:1694-700.
19. Rosso F, Michelon CT, Sperhacke RD, Verza M, Olival L, Conde MB, et al. Evaluation of realtime PCR of patient pleu ral effusion for diagnosis of tuberculosis. BMC Res Notes 2011;4:279.
20. Thomsen VO, Kok-Jensen A, Buser M, Philippi-Schulz S, Burkardt HJ. Monitoring treatment of patients with pulmonary tuberculosis: can PCR be applied? J Clin Microbiol 1999;37:36017.
21. Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson M, Salfinger M, et al. Practice guidelines for clinical microbiology baboratories: mycobacteria. Clin Microbiol Rev 2018;31:e00038-17.
22. Min JW, Yoon HI, Park KU, Song JH, Lee CT, Lee JH. Real-time polymerase chain reaction in bronchial aspirate for rapid de tection of sputum smear-negative tuberculosis. Int J Tuberc Lung Dis 2010;14:852-8.
Go to : 
Table 4
Results of AdvanSure plus PCR, AdvanSure PCR assay and AFB staining according to the results of culture
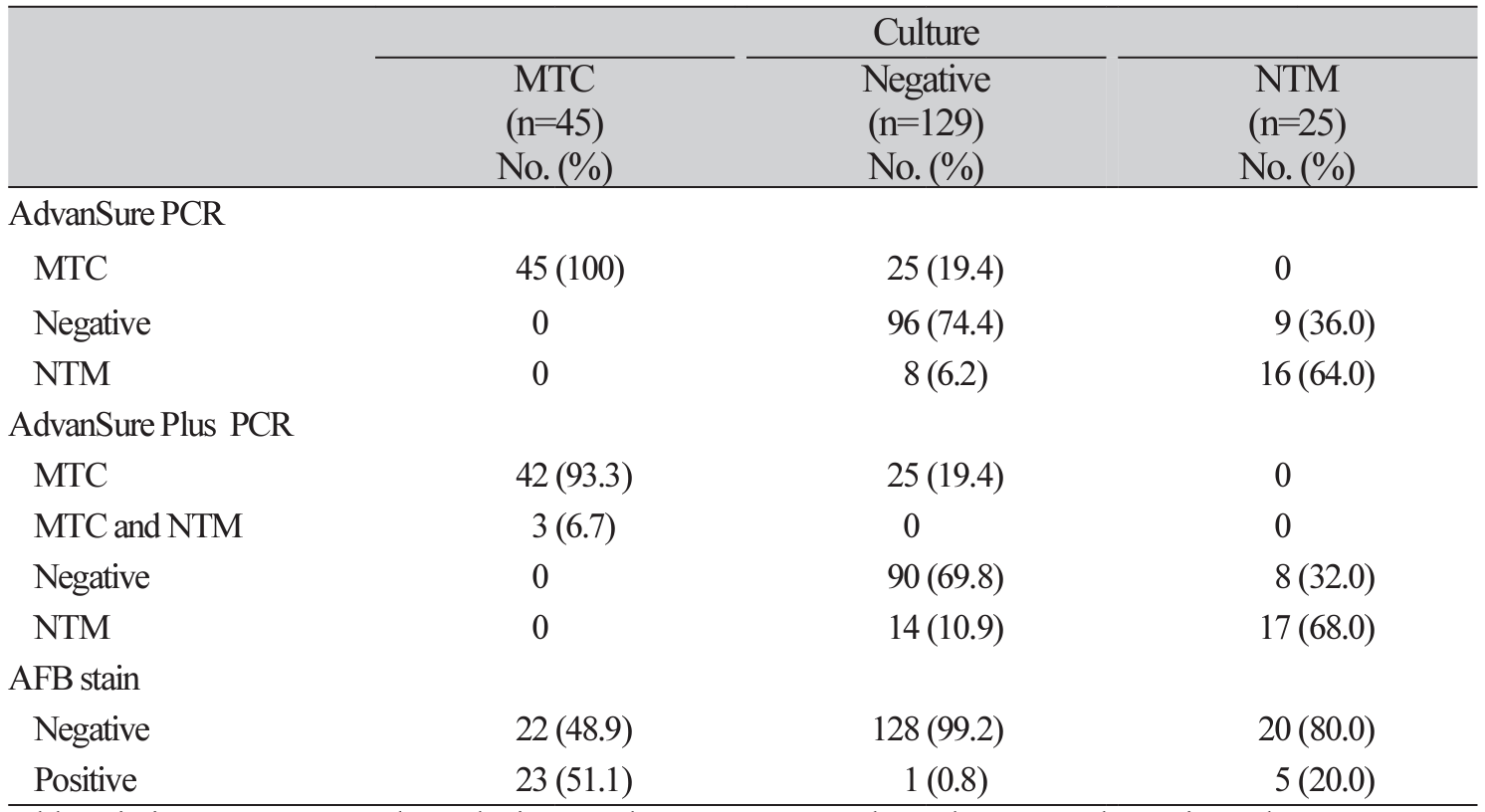
Table 5
The performance of AdvanSure plus PCR, AdvanSure PCR assay, and AFB stain for detection of MTC/NTM compared with mycobacterial culture
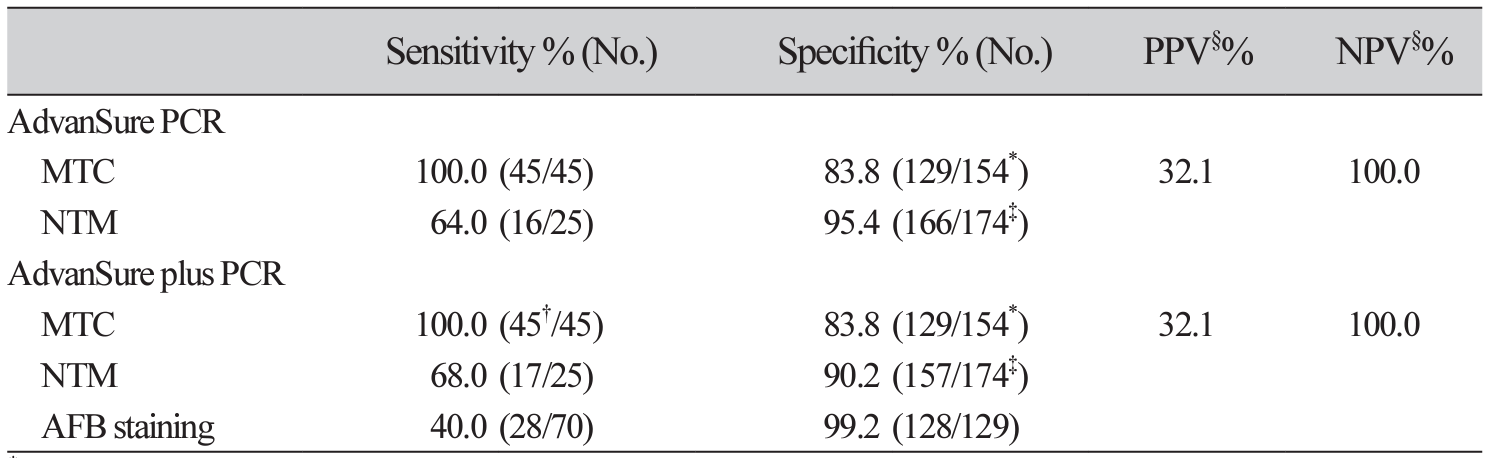
*Included negative culture samples (n=129) and NTM positive culture samples (n=25).†Included MTC & NTM co-positive samples (n=3) by AdvanSure plus PCR.‡Included negative culture samples (n=129) and MTC positive culture samples (n=45).§Calculated using Korean tuberculosis prevalence of 0.0768%.Abbreviation: MTC, M. tuberculosis complex; NTM, nontuberculous mycobacteria; AdvanSure PCR, AdvanSure TB/NTM real-time PCR; AdvanSure plus PCR, AdvanSure plus TB/NTM real-time PCR.




 PDF
PDF Citation
Citation Print
Print


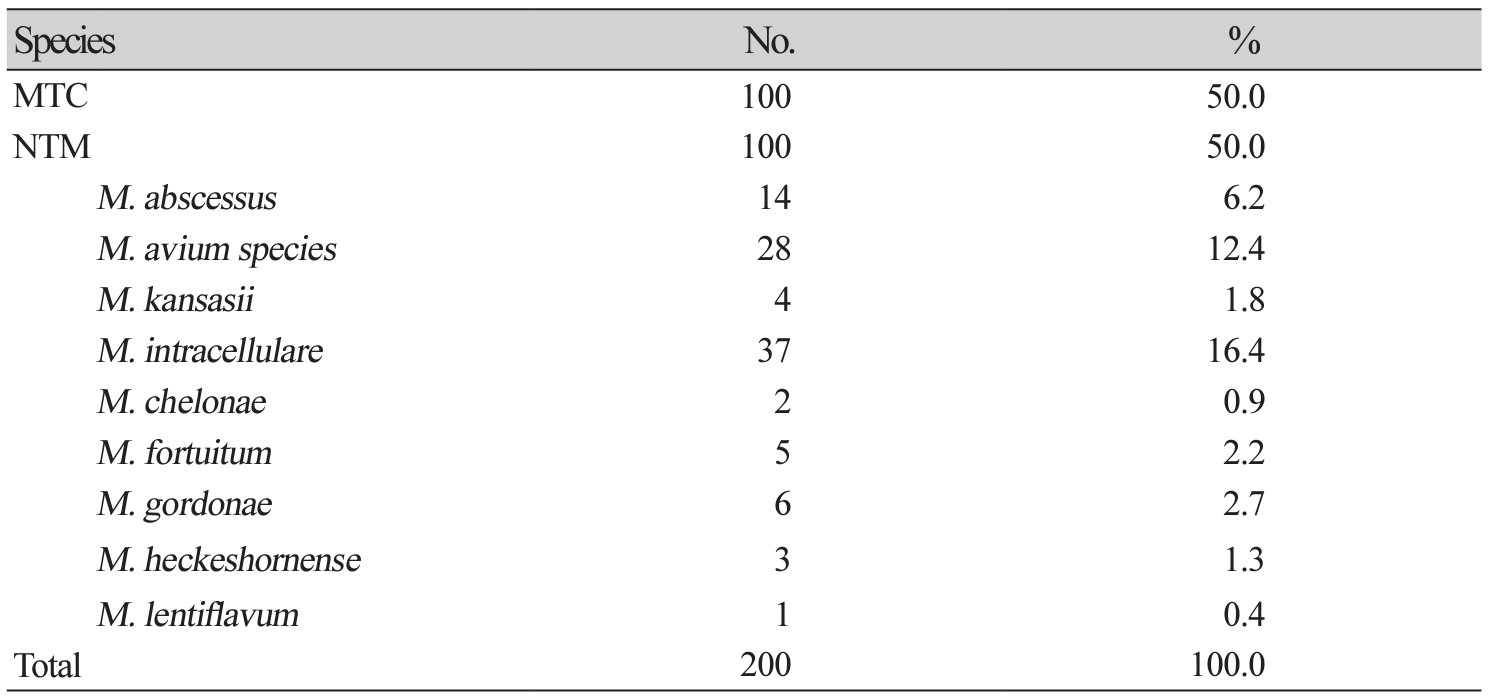
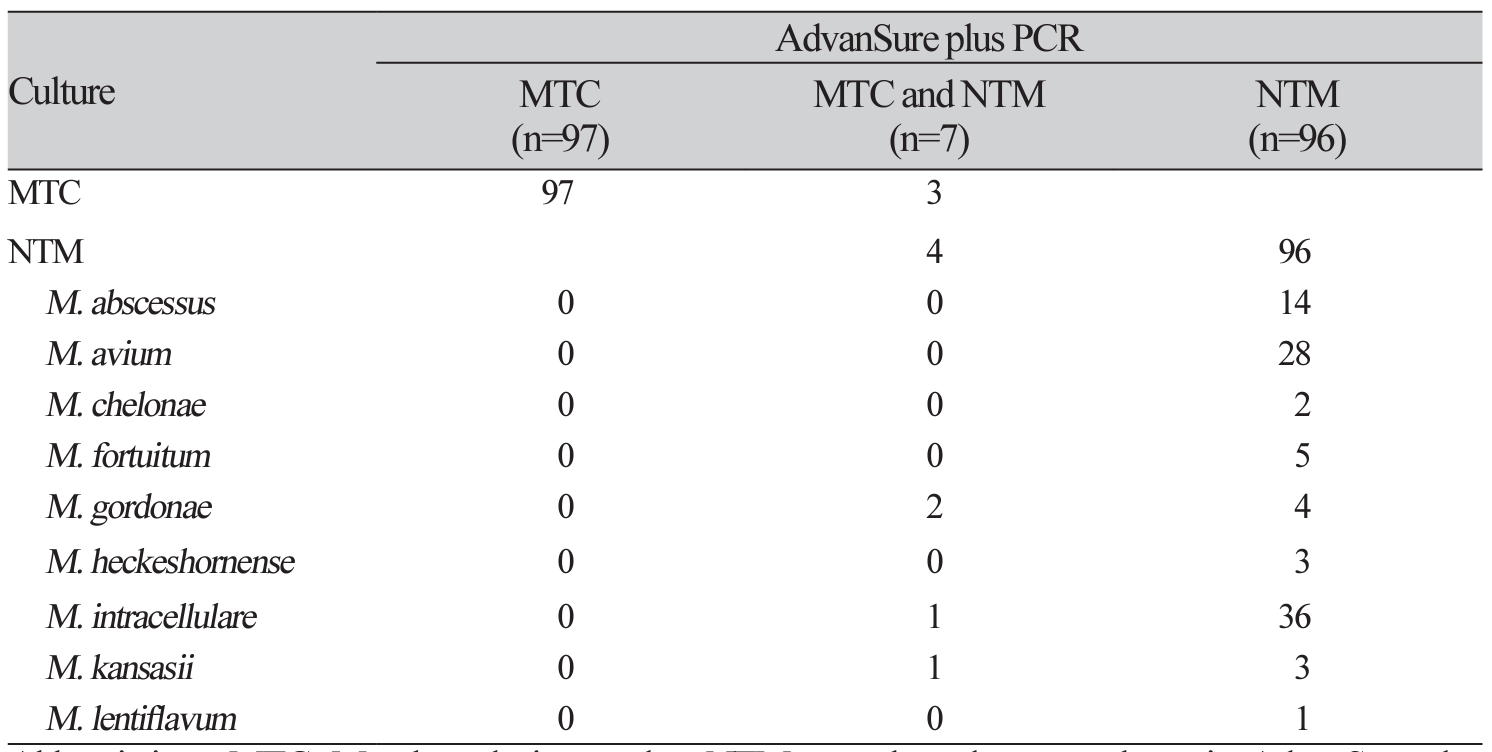
 XML Download
XML Download