Abstract
Background: Carbapenem-resistant Acinetobacter baumannii (CRAB) has emerged as an important nosocomial pathogen.The purpose of this study was to determine the effective methods for performing surveillance cultures of CRAB. Methods: Nasal and rectal swabs were obtained concurrently from hospitalized intensive care unit patients colonized with CRAB. All the samples were inoculated in CHROMagar Acinetobacter medium with CR102 (CHROMagar), MacConkey agar medium supplemented with 5 μg/mL imipenem (MCA-IPM),, and triptic soy broth medium supplemented with 5μg/mL imipenem (TSB-IPM). CRAB detection rates for each sample were compared. Results: The CRAB detection rate in either one of the nasal or rectal swabs from the 37 patients tested were 89.2% (33/37) with the use of CHROMagar, 78.4% (29/37) with the use of MCA-IMP, and 86.5% (32/37) with the use of TSB-IMP. Conclusion: We determined that concurrent use of both nasal and rectal swabs and CHROMagar could be an effective method for CRAB surveillance cultures.
Carbapenem-resistant Acinetobacter baumannii (CRAB) has emerged as an important nosocomial pathogen worldwide [1]. CRAB infections usually occur in intensive care units (ICUs), but it is very difficult to eliminate CRAB from the environment [2,3]. Surveillance cultures are used to detect CRAB in colonized patients [4]. During an outbreak, some hospitals carry out immediate surveillance cultures for the detection of CRAB, which can help in effective infection management [5].
As CRAB surveillance cultures, media containing antibiotics that inhibits the growth of susceptible bacteria and only allows resistant bacteria to grow, or selective media for A. baumannii that inhibits the growth of other bacteria are used [4,6,7]. For CRAB surveillance culture samples, skin, nasal, pharyngeal, or rectal swabs, among others, are used [8,9]. In the present study, using nasal and rectal swab samples, CRAB detection rates from cultures imipenem-contained plates and liquid media as well as in CRAB-selective chromogenic medium were compared to determine the most effective surveillance culture method.
In the medical ICU at a teaching hospital, paired samples (37 nasal and 37 rectal swabs) were collected from 37 patients, from whom CRAB was isolated in clinical samples within seven days. All of the collected samples were cultured in air at 37°C after being inoculated in each of the following media: CHROMagar Acinetobacter containing the MDR selective supplement, CR102 (CHROMagar, Paris, France; CHROMagar); MacConkey agar supplemented with 5 µg/mL imipenem (MCA-IPM); and triptic soy broth supplemented with 5 µg/mL imipenem (TSB-IPM). CHROMagar and MCA-IPM were examined after 24 hours and 48 hours. TSB-IPM were subcultred to MacConkey agar after 24 hours of incubation, and then the plates were examined after 24 hours. If red olonies formed on CHROMagar or if colorless colonies formed on MCA-IPM or subculture of TSB-IPM, species identification and antibiotic susceptibility tests were performed using MicroScan Walkaway-96 system and MicroScanNeg BP Combo 42 Panel (Siemens, West Sacramento, CA, USA). In the event that the bacteria proliferated in the liquid medium, TSB-IMP, they were subcultured onto MacConkey agar (MCA), and if colorless colonies formed on MCA, the identification and antibiotic susceptibility tests were performed. A statistical comparison of the detection rates was performed using the chi-square test and STATA 12 (STAT Corp., College Station, TX, USA), with significance level of 0.05.
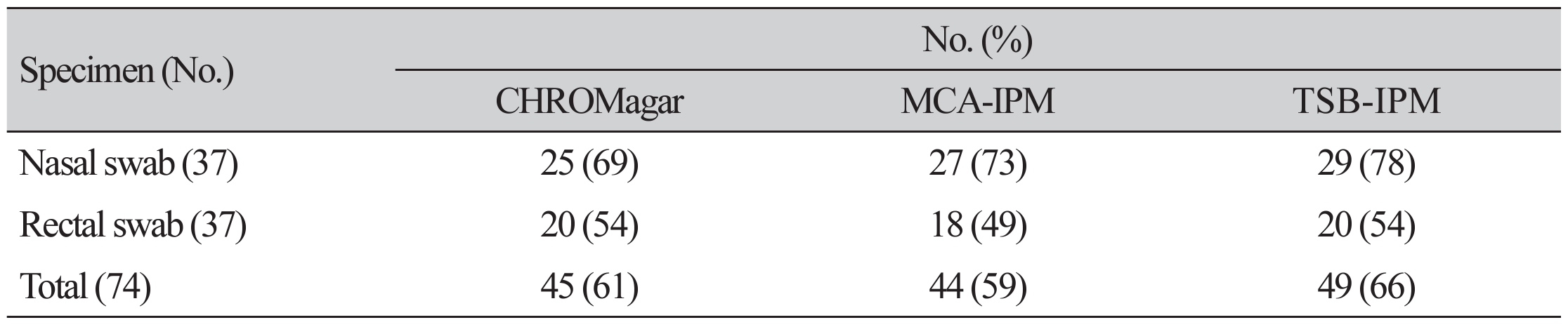
The CRAB detection rates from nasal swabs were 67.6% (25/37) with the use of CHROMagar, 73.0% (27/37) with the use of MCA-IMP, and 78.4% (29/37) with the use of TSB-IMP; whereas the CRAB detection rates from rectal swabs were 54.1% (20/37) with the use of CHROMagar, 48.6% (18/37) with the use of MCA-IMP, and 54.1% (20/37) with the use of TSB-IMP. For all 74 samples, the overall detection rates were 60.8% (45/74) with the use of CHROMagar, 60.8% (45/74) with the use of MCA-IMP, and 66.2% (49/74) with the use of TSB-IMP; the difference, however, was not statistically significant (Table 1). From the 37 patients, the CRAB detection rate from either one of the nasal or rectal swabs were 89.2% (33/37) with the use of CHROMagar, 78.4% (29/37) with the use of MCA-IMP, and 86.5% (32/37) with the use of TSB-IMP, with no statistically significant differences in the detection rates (Table 2).
In CHROMagar, 40 of the 45 CRAB isolates showed characteristic colonies in 24-h incubation, while five of the isolates required 48 h of incubation to form colonies. In MCA-IMP, 41 out of the 44 CRAB isolates formed colonies in 24 h incubation, and the remaining three isolates formed colonies after 48h. In TSB-IMP, all CRAB isolates formed colonies in 48 h subcutures. A single isolate of Burkholderia cepacia and a single strain of Stenotrophomonas maltophilia formed colonies that were suspected to be CRAB colonies in all three media, and the MicroScan Panel was used to determine the species. In cases where Enterobacteriaceae or other bacteria were mixed with CRAB and cultured, there were ten such isolates in MCA-IMP, four isolates in TSB-IMP, and one isolate in CHROMagar.
In a study that compared the use of different samples for the detection of CRAB colonization, axillary, pharyngeal, and rectal swabs were reported to yield similar detection rates [9]. Another study found no significant differences between rectal, nasal, or groin colonization of A. baumannii and suggested that a combination of rectal and nasal swabs improved detection rate [10]. A recent study comparing the suitability of nasal and rectal swabs for use in surveillance cultures using CHROMagar found that the use of nasal swabs allowed more sensitive detection than the use of rectal swabs, and that concurrently using nasal and rectal swabs can improve the detection rate for CRAB colonization [11]. In the present study, nasal swabs showed higher detection rate than rectal swabs when the culturing was performed with MCA-IMP and TSB-IMP. CRAB was detected only in the rectal swabs with the use of MCA-IMP in two cases, and there were three such cases with the use of TSB-IMP. Of the 33 patients that tested positive for CRAB in cultures with CHROMagar, 13 showed the presence of the organism only in nasal swabs and 8 showed the presence of the organism only in rectal swabs; thus, using nasal and rectal swabs concurrently resulted in a noticeable increase in the detection rate.
After 24h of incubation, the formation of colonies that were suspected to be CRAB colonies was observed in 40 out of 45 isolates with the use of CHROMagar and in 41 out of 44 isolates with the use of MCA-IMP. However, with the use of MCA-IMP, when the specimens were mixed with species of Enterobacteriaceae or other bacteria that formed colonies similar to CRAB colonies, additional subculture was necessary to perform antibiotic susceptibility tests and for final identification. When a single isolate of an Enterobacteriaceae species was cultured in CHROMagar, the colony could be easily distinguished from colonies of CRAB. In TSB-IMP, at least 48h of incubation was needed to obtain colonies that were suspected to be CRAB colonies, and in cases of mixed growth with other bacteria, additional subculture was needed.
In a study that compared the use of different media for the isolation of CRAB, the organism grew well in CHROMagar and MCA supplemented with imipenem, but the inhibition of carbapenem-susceptible A. baumannii (CSAB) could not be achieved in MCA supplemented with imipenem [12]. The authors in the previous study [12] used cultured strains and MCA supplemented with imipenem at a concentration of 1µg/mL; however, in the present study, clinical samples and a medium supplemented with 5 µg/mL of imipenem were used. For A. baumannii, the susceptible criteria of imipenem is ≤2 µg/mL and the resistant criteria is ≥8 µg/mL [13]. CSAB with minimal inhibitory concentration 2 or 4 µg/mL could grow in MCA supplemented with 1 µg/mL of imipenem. A. baumannii that grew in MCA-IMP and TSB-IMP, both supplemented with 5 µg/mL of imipenem, in this study were actually all CRAB.
True infection and colonization were not compared in this study. Another limitation of this study was that only A. baumannii strains were evaluated. It would be valuable to explore infected and colonized patients, and to evaluate other Acinetobacter species.
The use of nasal and rectal swabs, along with the use of CHROMagar, resulted in CRAB detection in 33 (89.2%) out of 37 patients with CRAB. Using CHROMagar allowed the presumptive identification of CRAB in 40 out of 45 isolates (89.9%) within 24 h. Therefore, we determined that the concurrent use of nasal and rectal swabs, along with the use of CHROMagar followed by incubation for 24 h, and up to 48 hours if necessary, represents the most effective method for surveillance cultures for the detection of CRAB colonization.
배경: 카베페넴 내성 Acinetobacter baumannii (CRAB)가 중요한 병원 감염의 원인균으로 대두되고 있다. 이 연구는 CRAB 감시배양의 효과적인 방법을 결정하기 위해 시행하였다.
방법: CRAB가 정착된 중환자실 입원환자에서 비강과 직장 도말을 동시에 실시하였다. 모든 검체는 CR102가 첨가된 CHROMagar Acinetobacter 배지(CHROMagar), 5 µg/mL 이미페넴이 첨가된 MacConkey 배지(MCA-IPM), 5 µg/mL 이미페넴이 첨가된 triptic soy broth 배지(TSB-IPM)에 접종하였고, CRAB 검출률을 비교하였다.
결과: 37명의 환자로부터 시행한 비강 혹은 직장 도말 중 한 곳에서라도 CRAB가 검출된 비율은 CHROMagar에서 89.2% (33/37), MCA-IPM에서 78.4% (29/37), TSB-IPM에서 86.5% (23/27)였다.
결론: CRAB 감시배양에서 CHROMagar를 이용하여 비강과 직장 도말을 동시에 시행하는 것이 효과적인 방법임을 알 수 있었다.
REFERENCES
1. Gilad J and Carmeli Y. Treatment options for multidrug-resistant Acinetobacter species. Drugs 2008;68:165-89.
2. Agusti C, Pujol M, Argerich MJ, Ayats J, Badia M, Dominguez A, et al. Short-term effect of the application of selective decontamination of the digestive tract on different body site reservoir ICU patients colonized by multi-resistant Acinetobacter baumannii. J Antimicrob Chemother 2001;49:205-8.
3. Heritier C, Dobouix A, Poirel L, Marty N, Nordmann P. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA58. J Antimicrob Chemother 2005;55:115-8.
4. Marchaim D, Navon-Venezia S, Schwartz D, Tarabeia J, Fefer I, Schwaber MJ, et al. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol 2007;45:1551-5.
5. Maragakis LL and Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment option. Clin Infect Dis 2008;15:1254-63.
6. Doi Y, Onuoha EO, Adams-Haduch JM, Pakstis DL, McGaha TL, Werner CA, et al. Screening for Acinetobacter baumannii colonization by use of sponges. J Clin Microbiol 2011;49:154-8.
7. Gordon NC and Wareham DW. Evaluation of CHROMagar Acinetobacter for detection of enteric carriage of multidrug-resistant Acinetobacter baumannii in samples from critically ill patients. J Clin Microbiol 2009;47:2249-51.
8. Fournier PE and Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006;42:692-9.
9. Ayats J, Corbella X, Ardanuy C, Dominguez MA, Ricart A, Ariza J, et al. Epidemiological significance of cutaneous, pharyngeal, and digestive tract colonization by multiresistant Acinetobacter baumannii in ICU patients. J Hosp Infect 1997;37:287-95.
10. Lortholary O, Fago JY, Hoi AB, Mahieu G, Gutmann L. Colonization by Acinetobacter baumannii in intensive-care-unit patients. Infect Cont Hosp Epidemiol 1998;19:188-90.
11. Song W, Lee J, Kim TK, Park MJ, Kim HS, Kim JS. Modified CHROMagar Acinetobacter medium for direct detection of multidrug-resistant Acinetobacter strains in nasal and rectal swab samples. Ann Lab Med 2013;33:193-5.
12. Moran-Gilad J, Adler A, Schwartz D, Navon-Venezia S, Carmeli Y. Laboratory evaluation of different agar media for isolation of carbapenem-resistant Acinetobacter spp. Eur J Clin Microbiol Infect Dis 2014;33:1909-13.
13. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 28th informational supplement. M100-S28. Wayne; PA: 2018.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download