Abstract
Background: The disease burden caused by Mycobacterium tuberculosis (MTB) complex continues to decrease in most countries. However, the diseases caused by the nontuberculous mycobacteria (NTM) become a public health problem. This study aimed to compare the diagnostic accuracy of three real-time PCR assays: Advansure TB/NTM real-time PCR kit (LG Chem., Korea), Genedia MTB/NTM detection kit (Green Cross MS, Korea), and PowerChek MTB/NTM Real-time PCR kit (Kogenebiotech, Korea) for the detection of MTB complex and NTM.
Methods: Total 102 acid-fast bacilli (AFB) smear-positive and 177 smear-negative specimens from Korea University Medical Center, Guro Hospital, were enrolled. The AFB smear-positive and negative specimens were collected from November 2016 to October 2017 and November to December 2018, respectively. DNA extraction was performed using Genedia Mycobacteria DNA prep Kit (Green Cross MS). The statistical analysis was performed using MedCalc 18.11.6 (MedCalc, Ostend, Belgium).
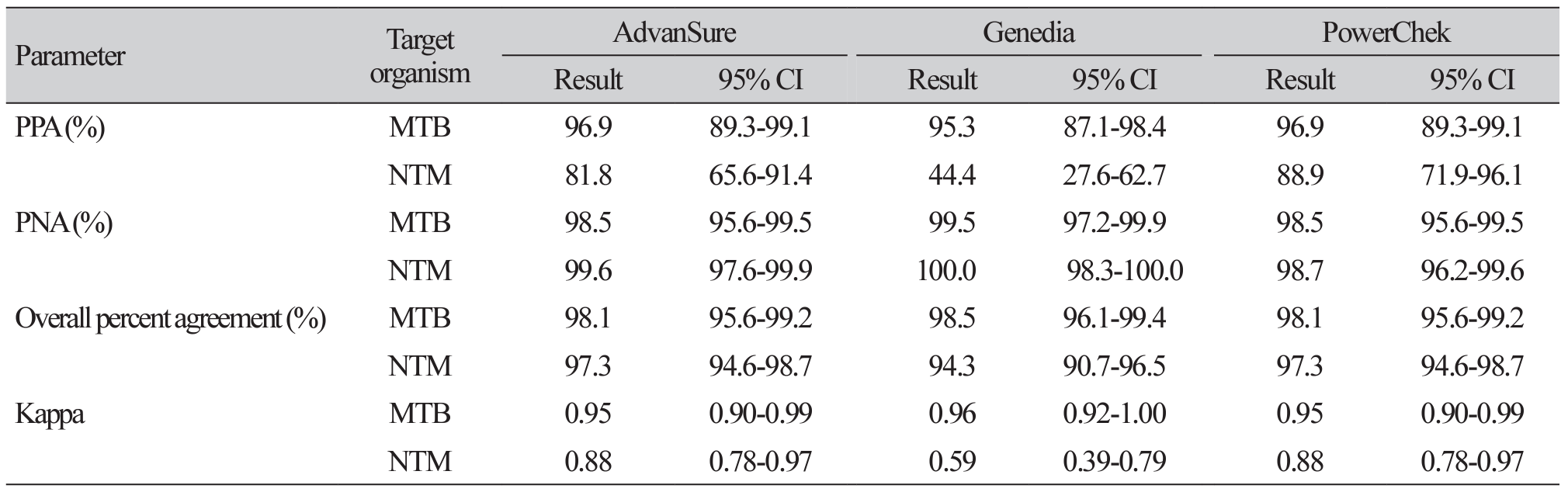
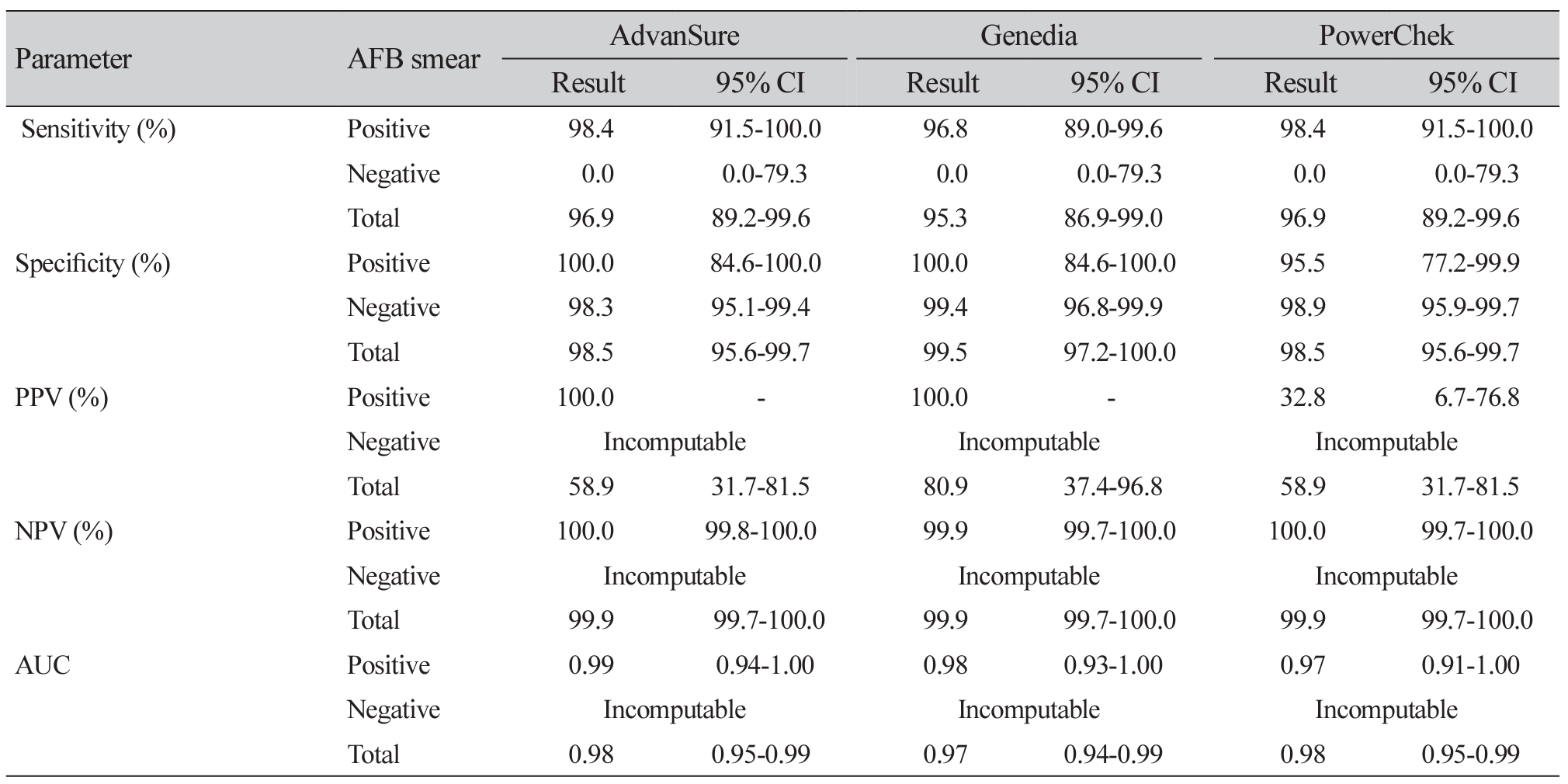
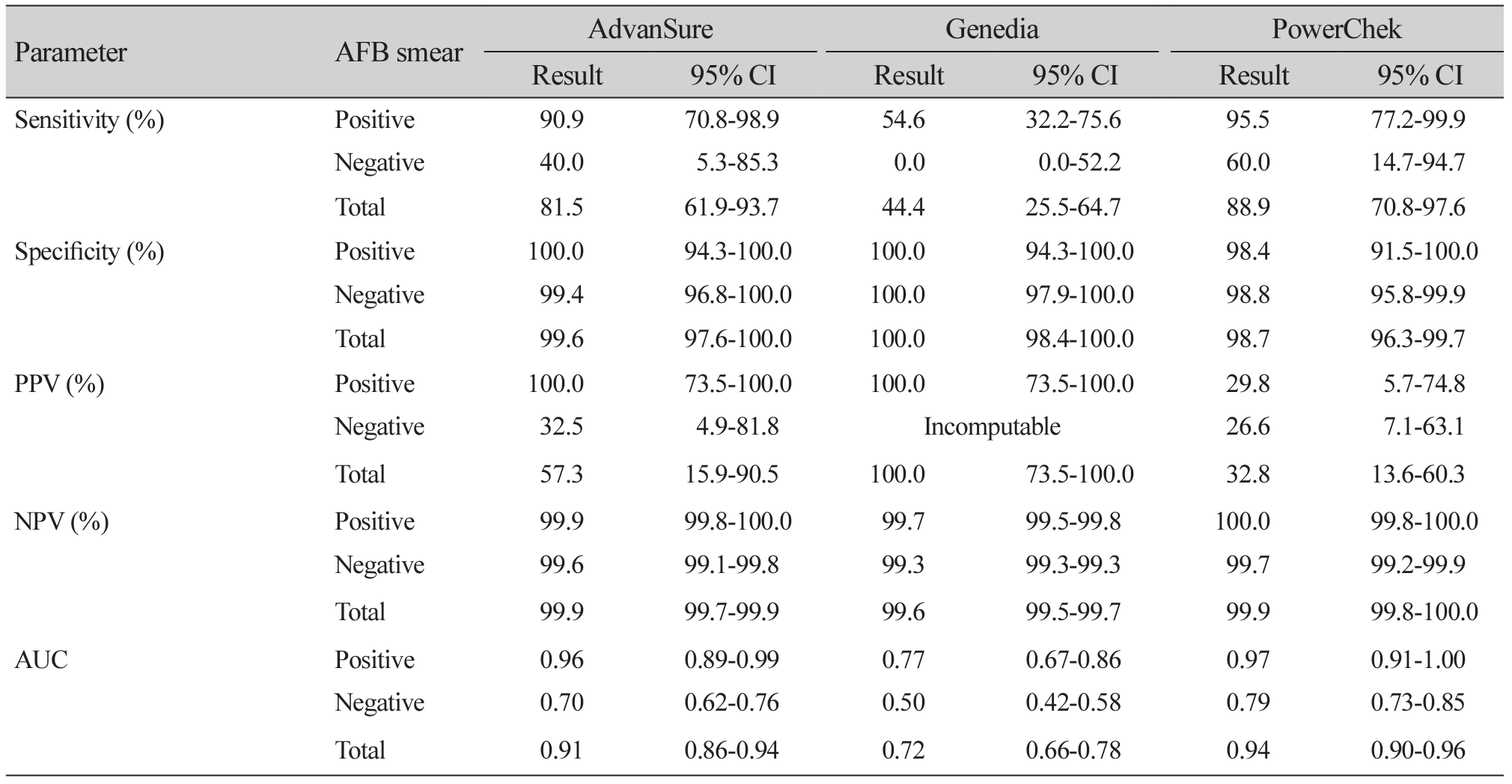
Results: Among 261 specimens, 64 showed MTB complex growth and 28 exhibited NTM growth. The sensitivity, specificity, positive predictive value, and negative predictive value of Advansure/Genedia/PowerChek kits for MTB were 96.9%/95.3%/96.9%, 98.5%/99.5%/98.5%, 58.9%/80.9%/58.9%, and 99.9%/99.9%/99.9%. Whereas those for NTM detection were 81.5%/44.4%/88.9%, 99.6%/100.0%/98.7%, 57.3%/100.0%/32.8% and 99.9%/99.6%/99.9%, respectively. The area under the receiver operating characteristic curve of Advansure and PowerChek for NTM detection was statistically different from that of Genedia (P<0.0001).
Conclusion: Three real-time PCR assays were reliable for MTB complex in AFB-positive and -negative specimens. There was a difference between these three reagents for the accuracy of NTM detection.
REFERENCES
1. WHO. Global tuberculosis report 2019. http://www.who.int/tb/publications/global_report/en/ [Online] (last visited on 18 November 2019).
2. Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis 2012;72:409-15.
3. Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis 2013;75:199-204.
4. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of nontuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 2010;14:1069-71.
5. Kwon YS and Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci 2016;31:649-59.
6. Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis 2016;79:74-84.
7. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American thoracic society/infectious diseases society of America/centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:111-5.
8. Centers for Disease Control and Prevention. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. Morb Mortal Wkly Rep 2009;58:7-10.
9. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci 2005;20:913-25.
10. Choi YJ, Kim HJ, Shin HB, Nam HS, Lee SH, Park JS, et al. Evaluation of peptide nucleic acid probe-based real-time PCR for detection of Mycobacterium tuberculosis complex and nontuberculous mycobacteria in respiratory specimens. Ann Lab Med 2012;32:257-63.
11. Lim JH, Kim CK, Bae MH. Evaluation of the performance of two real-time PCR assays for detecting Mycobacterium species. J Clin Lab Anal 2019;33:e22645.
12. Choe W, Kim E, Park SY, Chae JD. Performance evaluation of anyplex plus MTB/NTM and AdvanSure TB/NTM for the detection of Mycobacterium tuberculosis and nontuberculous mycobacteria. Ann Clin Microbiol 2015;18:44-51.
13. American Thoracic Society and Centers for Disease Control. Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis 1990;142:725-735.
14. Gaillard T, Fabre M, Martinaud C, Vong R, Brisou P, Soler C. Assessment of the SD bioline Ag MPT64 rapid and the MGIT TBc identification tests for the diagnosis of tuberculosis. Diagn Microbiol Infect Dis 2011;70:154-6.
15. Clinical and Laboratory Standards Institute (CLSI). User protocol for evaluation of qualitative test performance EP12-A2. Wayne; PA, 2008.
16. Kong KA. Statistical methods: reliability assessment and method comparison. Ewha Med J 2017;40:9.
17. Huh HJ, Kwon HJ, Ki CS, Lee NY. Comparison of the Genedia MTB detection kit and the Cobas TaqMan MTB assay for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol 2015;53:1012-4.
18. Cho WH, Won EJ, Choi HJ, Kee SJ, Shin JH, Ryang DW, et al. Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med 2015;35:356-61.
19. Kee SJ and Suh SP. Increasing burden of nontuberculous mycobacteria in Korea. J Korean Med Sci 2017;32:1215-6.
20. WHO. Xpert MTB/RIF implementation manual: technical and operational 'how-to': practical considerations. Geneva; Switzerland: World Health Organization, 2014.
21. Jeong JY, Lee SH, Jang S. A systematic review on the effectiveness of detection of M. tuberculosis and rifampin resistance using Xpert MTB/RIF. Ann Clin Microbiol 2014;17:4249.
22. Li S, Liu B, Peng M, Chen M, Yin W, Tang H, et al. Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: a systematic review and meta-analysis. PLoS One 2017;12:e0180725.
23. Jung CL, Kim M, Seo D, Lee M. Clinical usefulness of real-time PCR and Amplicor MTB PCR assays for diagnosis of tuberculosis. Korean J Clin Microbiol 2008;11:29-33.
Table 1
The AFB culture results for the specimens of AFB smear negative and MTB/NTM real-time PCR negative
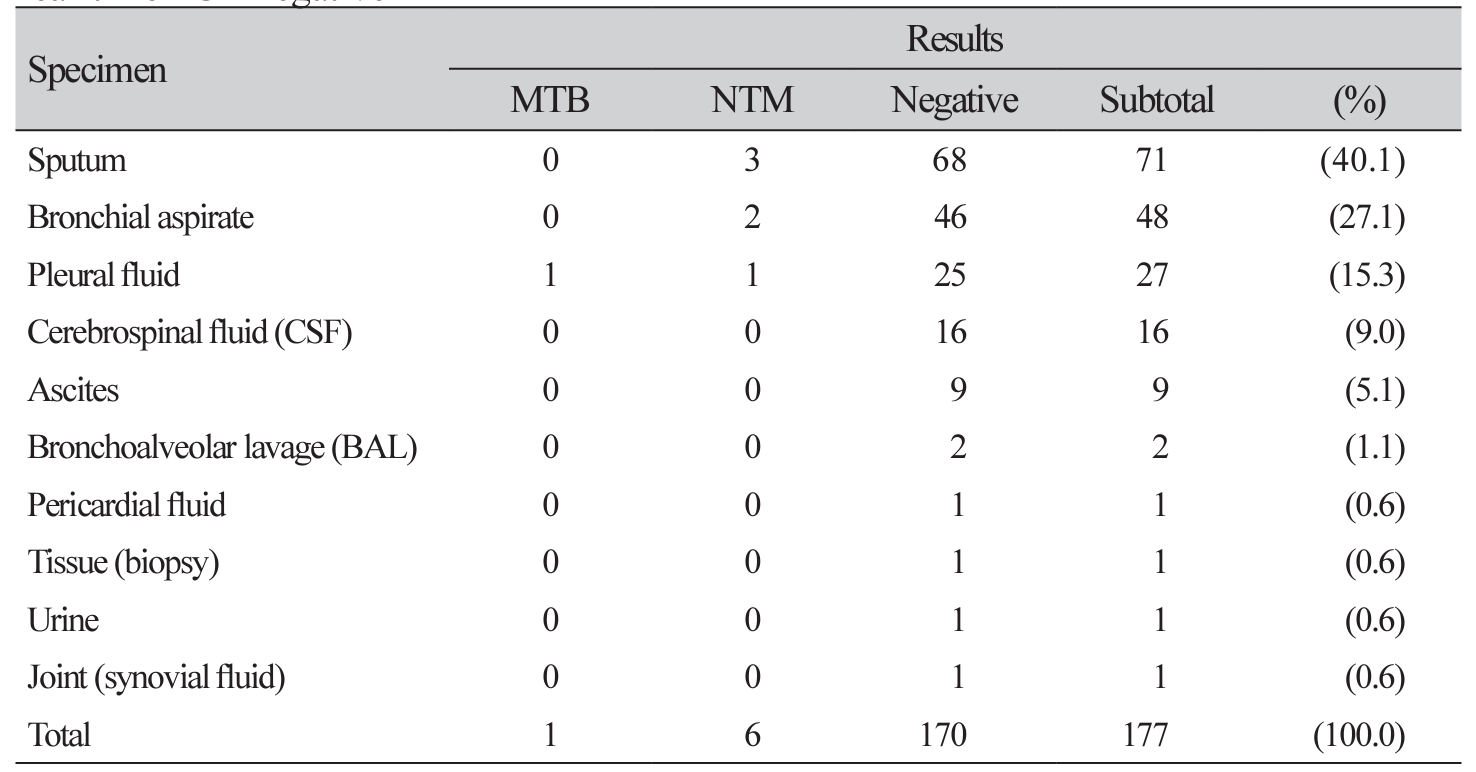
Table 2
Discrepant results among three real-time PCR assays and AFB culture in AFB smear positive specimens
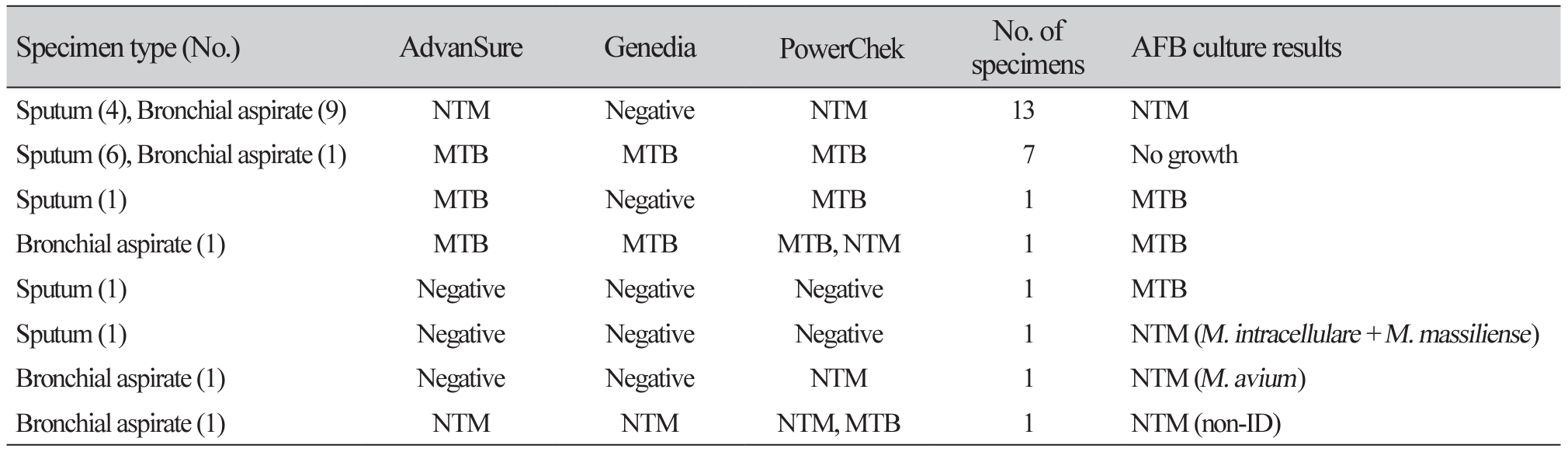
Table 3
Discrepant results between three real-time PCR assays and AFB culture in AFB smear negative specimens
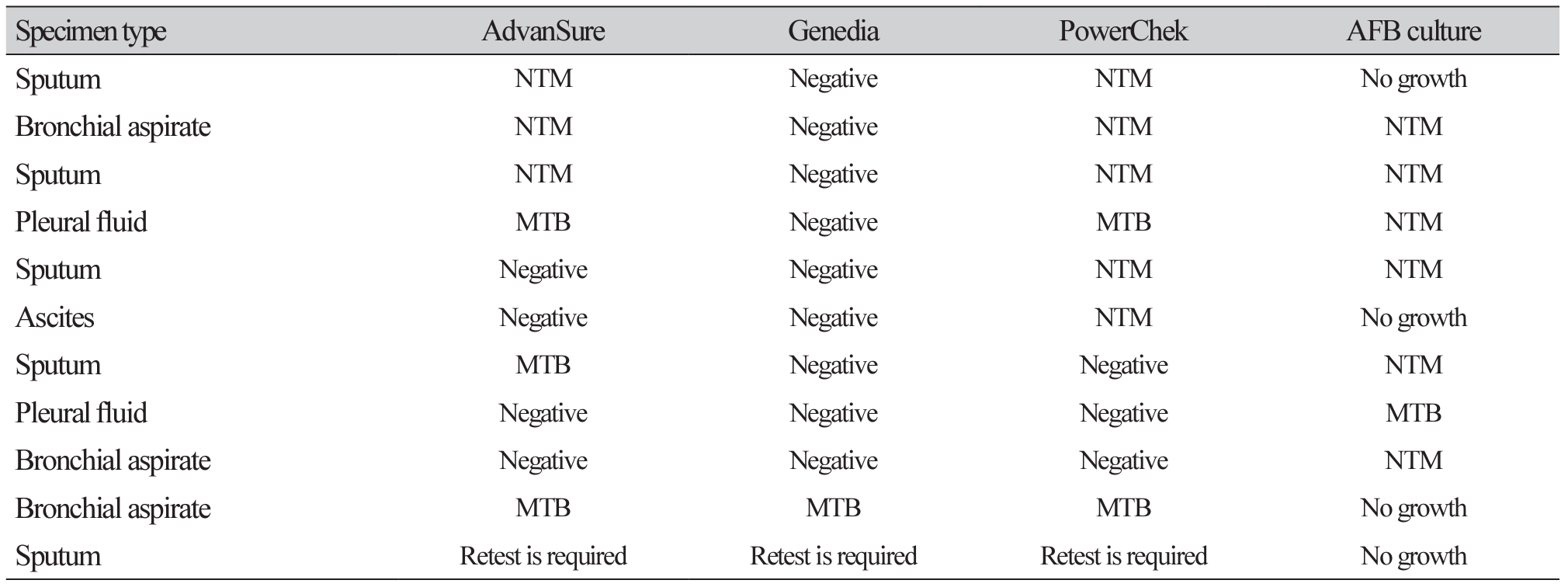
Table 4
Agreement between AFB culture tests and three real-time PCR assays for MTB and NTM detection (n=261)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download