Abstract
Background: The prevalence of carbapenemase-producing Enterobacteriaceae (CPE), especially the KPC-2-producing Klebisella pneumoniae, is rapidly increasing and becoming a menace to global public health. This study aims to present the molecular epidemiology of the KPC-2-producing K. pneumoniae isolates emerged in a tertiary hospital in South Korea and describe its clinical significance. Methods: This study included carbapenem-resistant K. pneumoniae isolates collected from a tertiary hospital from April to December in 2018. Antimicrobial susceptibility of K. pneumoniae isolates was tested using disk diffusion method. PCR and DNA sequence analyses were performed to identify the resistance genotype. In addition, the molecular epidemiology was investigated using pulsed-field gel electrophoresis (PFGE) and multilocus sequencing typing (MLST). Results: Total 100 KPC-2-producing K. pneumoniae isolates were collected, which were mainly classified into two pulsotypes according to the XbaI restriction digestion pattern by PGFE analysis (pulsotype A, n = 31; pulsotype B, n = 63). The isolates exhibiting pulsotype A belonged to ST395 and the remaining isolates exhibiting pulsotype B were attributed to ST307 by MLST analysis. Conclusion: This study investigated clinical information and molecular bacterial profiles for KPC-2-producing K. pneumoniae isolates. These findings indicate that the proper infection control activities are needed to prevent the spread of multidrug-resistant organisms such as CPE, which could cause high mortality in clinical field.
REFERENCES
1. Potter RF, D’Souza AW, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat 2016;29:30-46 .
2. Nordmann P, Naas T, Poirel L. Global spread of carbapenemaseproducing Enterobacteriaceae. Emerg Infect Dis 2011;17:1791-8. .
3. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011;55:4943-60. .
4. KCDC. Monitoring of antimicrobial resistance on general hospitals in Korea 2010. Public Health Weekly Report. 2010;34:565-9. .
5. Yoon EJ, Yang JW, Kim JO, Lee H, Lee KJ, Jeong SH. Carbapenemase-producing Enterobacteriaceae in South Korea: a report from the national laboratory surveillance system. Future Microbiol 2018;13:771–83. .
6. Iovleva A and Doi Y. Carbapenem-resistant Enterobacteriaceae. Clin Lab Med 2017;37:303-15.
7. Park YJ, Yu JK, Park KG, Park YG, Lee S, Kim SY, et al. Prevalence and contributing factors of nonsusceptibility to imipenem or meropenem in extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Diagn Microbiol Infect Dis 2011;71:87-9. .
8. Lee EJ, Lee SJ, Bahk HJ, Kim SN, Lee HM. Analysis of carbapenemase-producing Enterobacteriaceae (CPE) surveillance results for 2017 in Korea: comparison with the surveillance results of the previous 5 years (2012-2016). Division of healthcare associated infection control, center for infectious disease surveillance & response, KCDC. https://www.cdc.go.kr/board/board. es?mid=a20602010000&bid=0034&list_no=141909&act=view#. [Online] (last visited on 15 May 2020). .
9. Hong SK, Yong D, Kim K, Hong SS, Hong SG, Khosbayar T, et al. First outbreak of KPC-2producing Klebsiella pneumoniae sequence type 258 in a hospital in South Korea. J Clin Microbiol 2013;51:3877-9. .
10. Yoo JS, Kim HM, Yoo JI, Yang JW, Kim HS, Chung GT, et al. Detection of clonal KPC-2-producing Klebsiella pneumoniae ST258 in Korea during nationwide surveillance in 2011. J Med Microbiol 2013;62:1338-42. .
11. Jeong S, Kim JO, Yoon EJ, Bae IK, Lee W, Lee H, et al. Extensively drug-resistant Escherichia coli sequence type 1642 carrying an IncX3 plasmid containing the blaKPC-2 gene associated with transposon Tn4401a. Ann Lab Med 2018:38;17-22. .
12. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 28th informational supplement; M100-S28. Wayne; PA: 2018. .
13. Maida CM, Bonura C, Geraci DM, Graziano G, Carattoli A, Rizzo A. Outbreak of ST395 KPCproducing Klebsiella pneumoniae in a neonatal intensive care unit in Palermo, Italy. Infect Control Hosp Epidemiol 2018;39:496-8. .
14. Zheng B, Dai Y, Liu Y, Shi W, Dai E, Han Y, et al. Molecular epidemiology and risk factors of carbapenem-resistant Klebsiella pneumoniae infections in Eastern China. Front Microbiol 2017;8:1061. .
15. Chang YY, Chuang YC, Siu LK, Wu TL, Lin JC, Lu PL. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect 2015;48:219-25. .
16. American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med 1996;153:1711-25. .
17. Craven DE and Chroneou A. Nosocomial pneumonia. In: Mandell GL, Douglas RG, Bennett JE, eds. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7th ed. Philadelphia; Churchill Livingstone, 2010:3717-25 .
18. Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015;70:2133-43. .
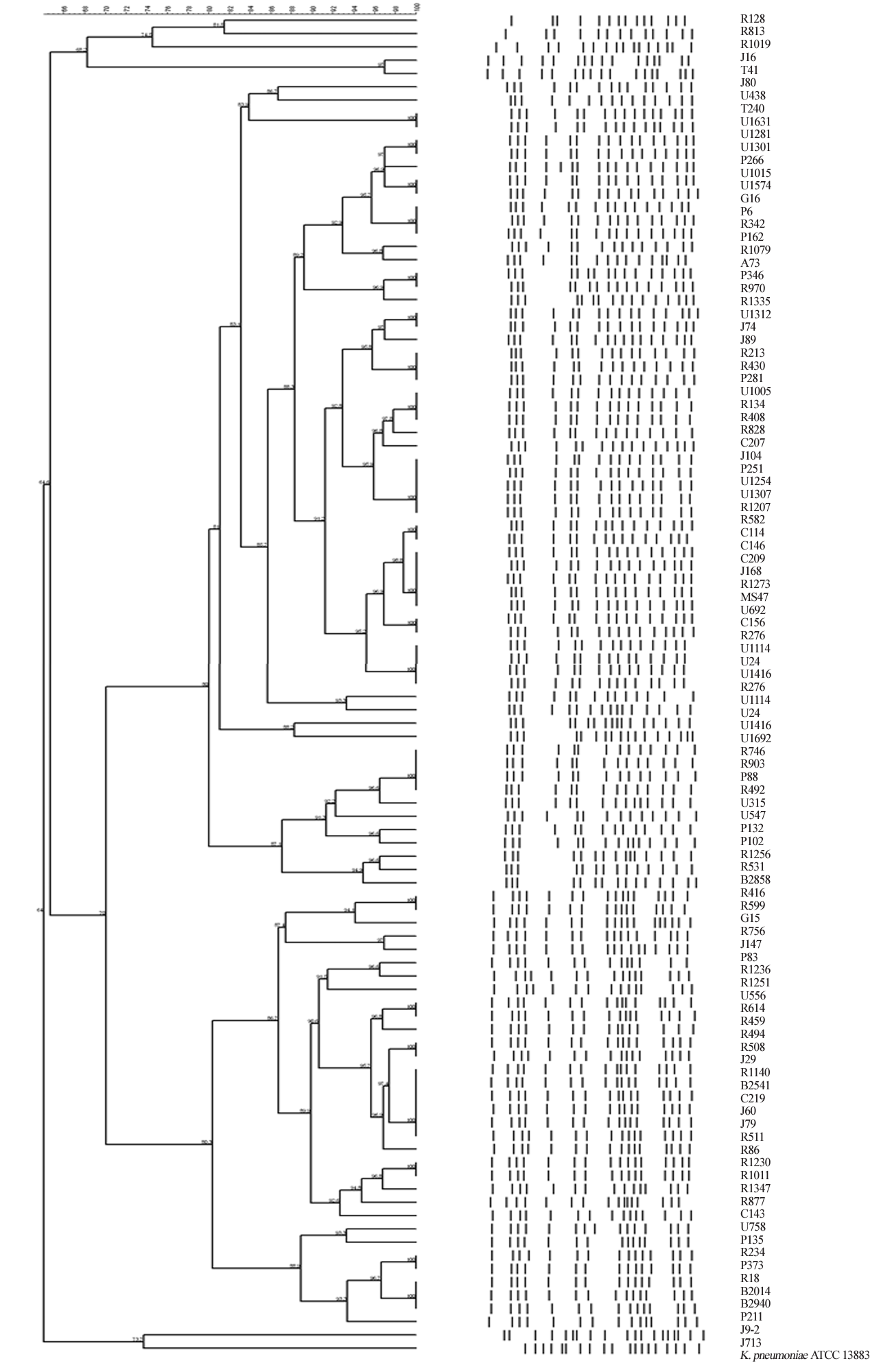
19. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995:33:2233-9.
Fig. 1.
Monthly isolation of KPC-2 producing K. pneumoniae isolates stratified by PFGE banding patterns. Bar graphs indicate the numbers of KPC-2 producing K. pneumoniae isolates collected in each nine month in 2018 and arrows indicate the activity performed for eradicating a KPC-type carbapenemase producing K. pneumoniae isolates outbreak. Yellow bar, pulsotype A; Blue bar, pulsotype B; Gray bar, other pulsotypes.
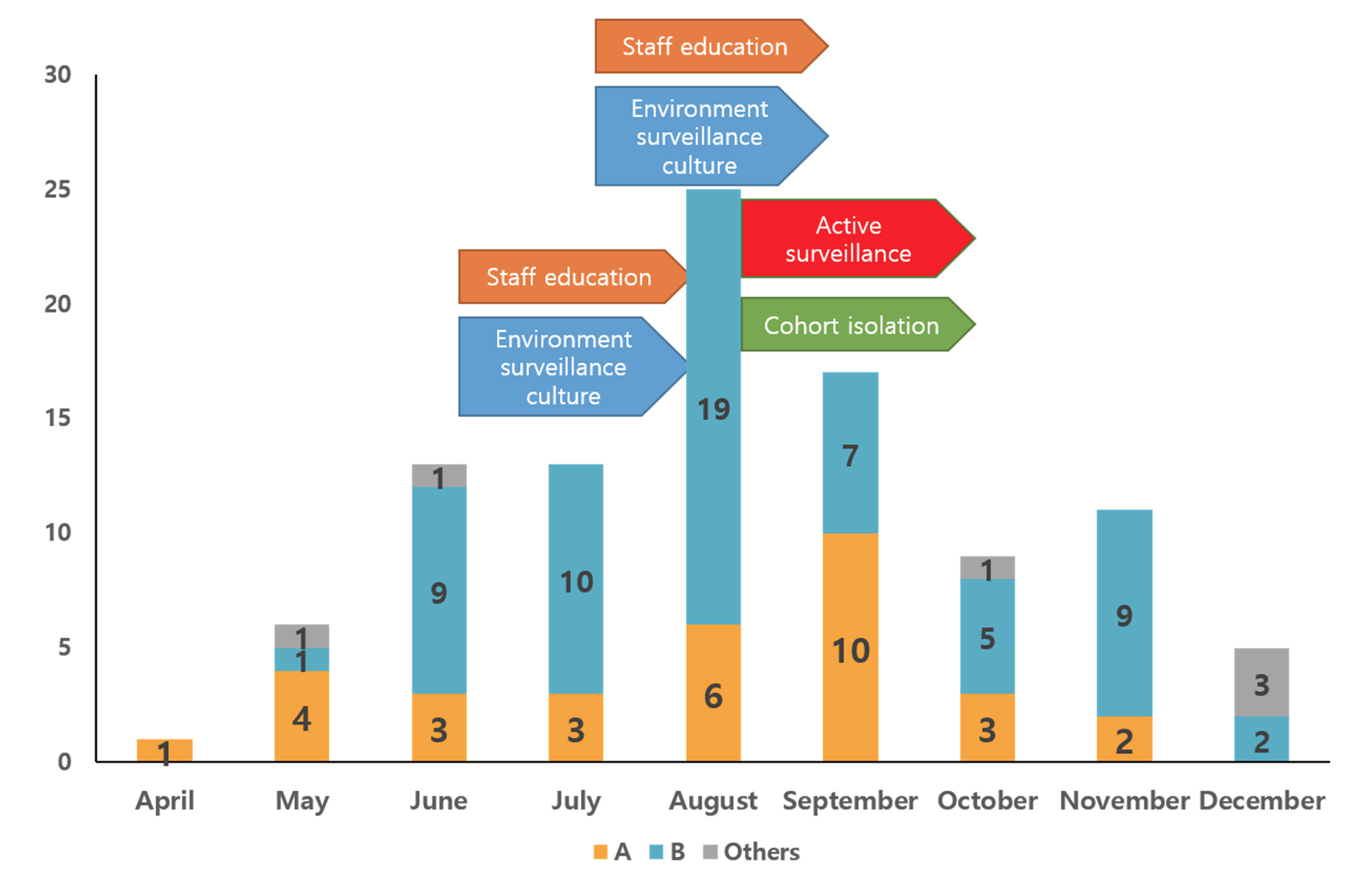
Table 3
Antimicrobial susceptibility results of KPC-producing K. pneumoniae isolates obtained by Phoenix system
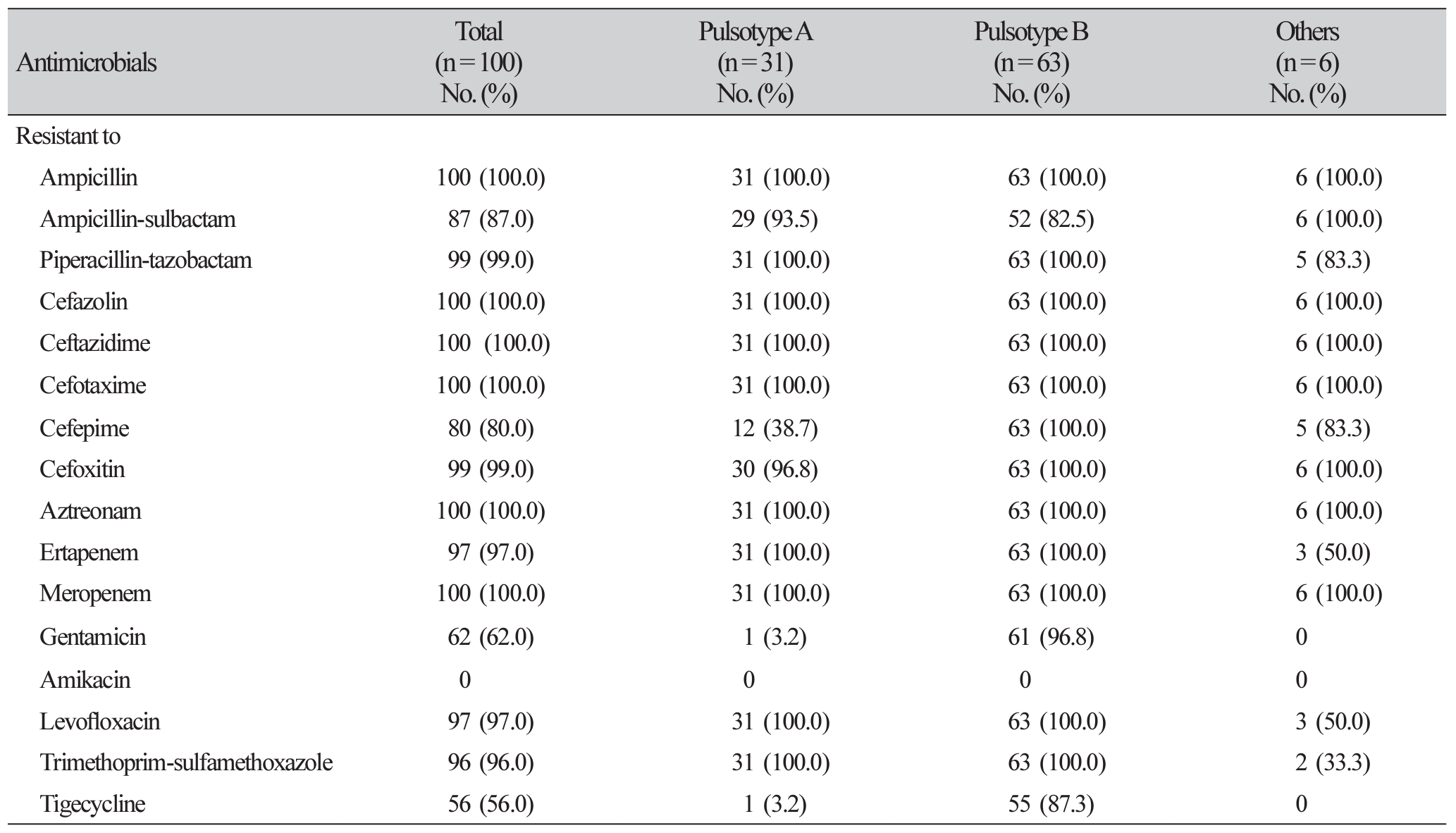
Fig. 3.
Representative results of disk diffusion method for antimicrobial susceptibility testing in KPC-2 producing K. pneumoniae isolates exhibiting pulsotype A (ST395). (A) shows results of zone diameter for 12 antibiotics on MH 150 Ø agar, (B) shows results of zone diameter for remaining 6 antibiotics on MH 90 Ø agar, and (C) is results of antimicrobial susceptibility. R, resistant; I, intermediate; S, susceptible.
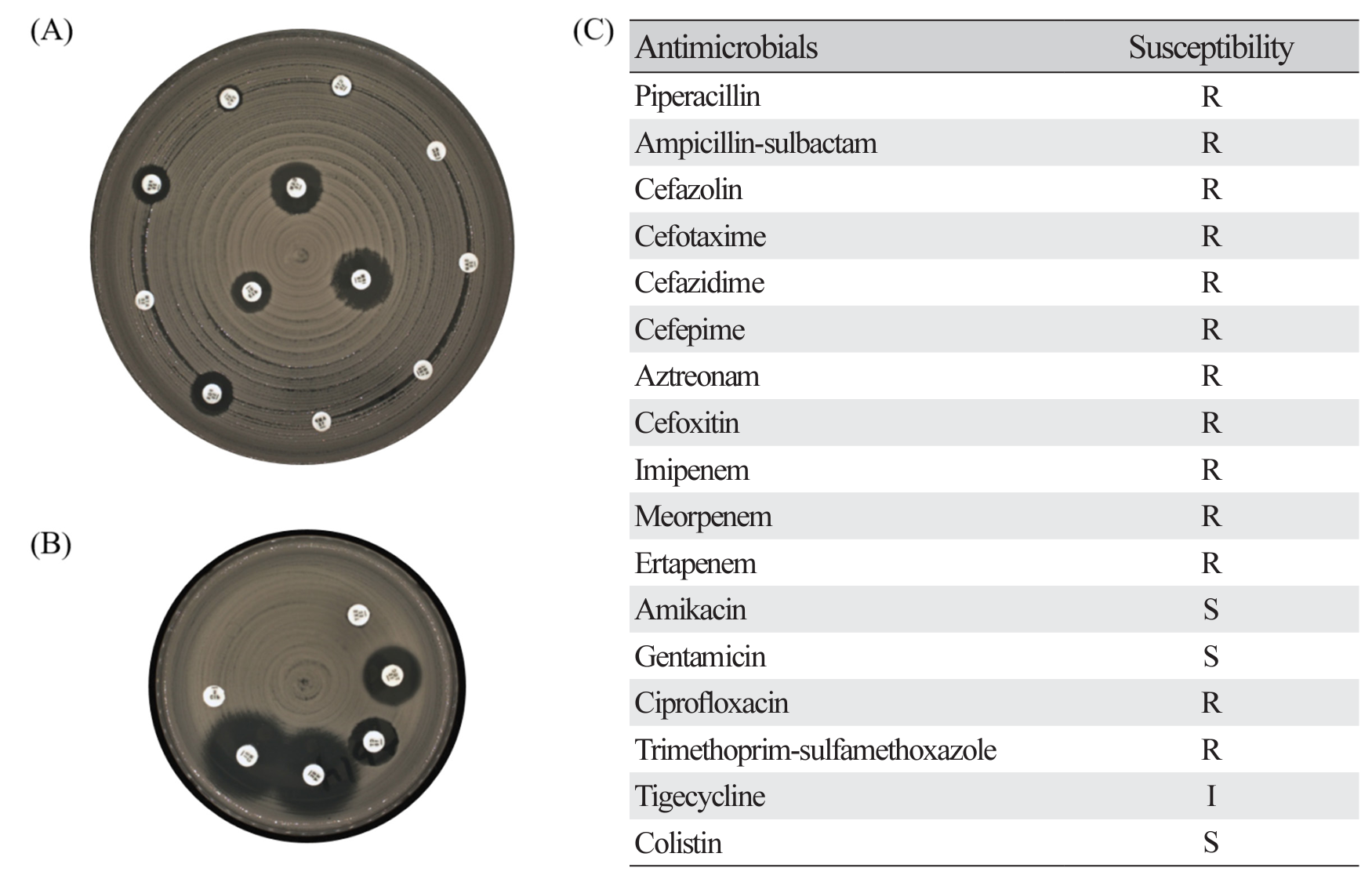
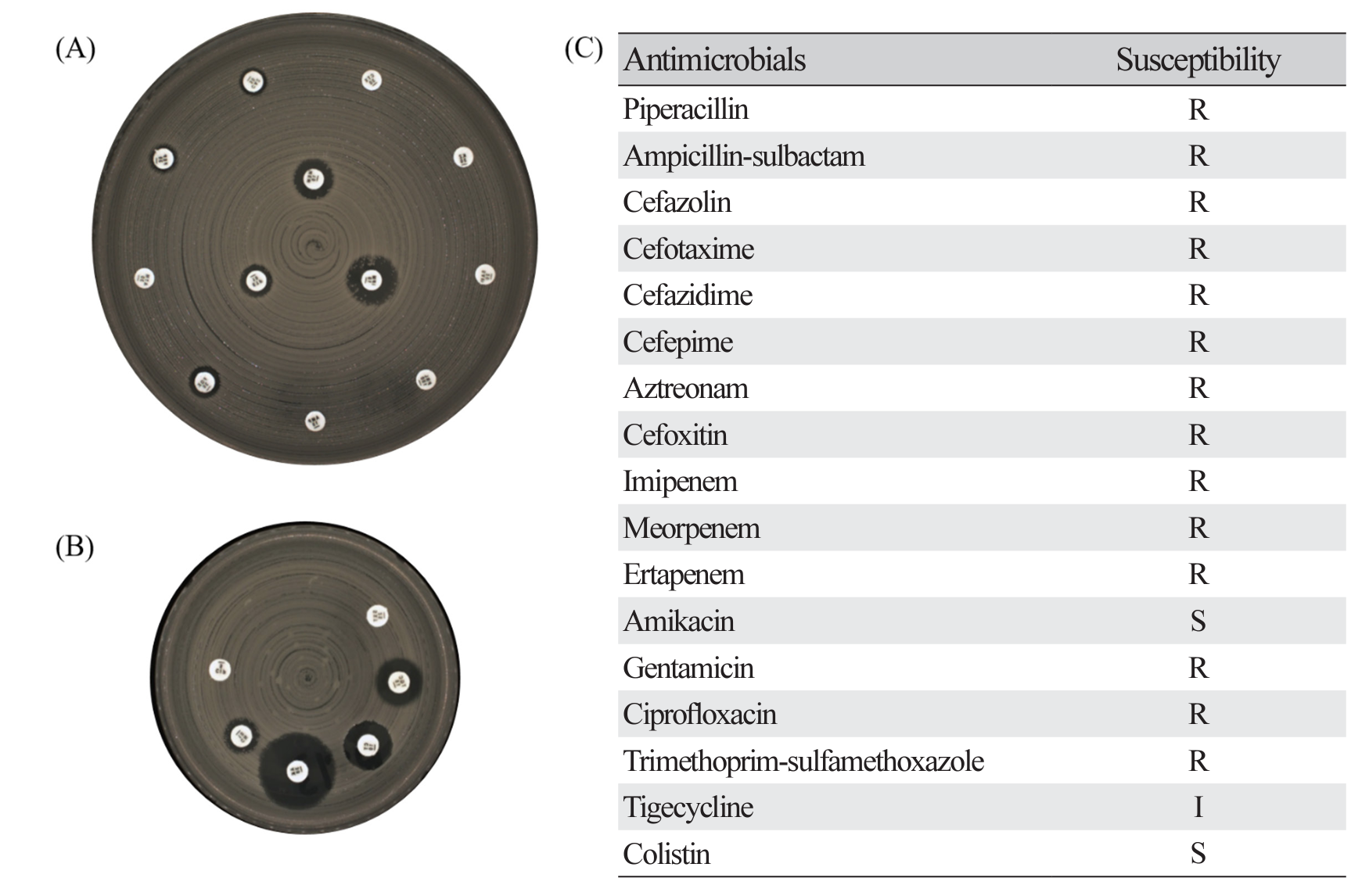
Fig. 4.
Representative results of disk diffusion method for antimicrobial susceptibility testing in KPC-2 producing K. pneumoniae isolates exhibiting pulsotype B (ST307). (A) shows results of zone diameter for 12 antibiotics on MH 150 Ø agar, (B) shows results of zone diameter for remaining 6 antibiotics on MH 90 Ø agar, and (C) is results of antimicrobial susceptibility. R, resistant; I, intermediate; S, susceptible.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download