This article has been
cited by other articles in ScienceCentral.
Abstract
Subepithelial tumors in the upper gastrointestinal (GI) tract are often detected during nationwide endoscopic gastric cancer screening in Korea. Most GI lipomas are asymptomatic and do not necessitate further treatment. However, large tumors may lead to complications such as bowel obstruction, intussusception, and bleeding. These GI lipomas require endoscopic or surgical resection. On radiological examination, GI lipomas typically manifest as hypodense lesions with similar density to that of fat tissue. White-light endoscopy generally reveals a yellowish subepithelial tumor exhibiting a positive cushion sign, while endoscopic ultrasonography shows a homogeneous hypoechoic mass within the third layer of the GI tract. We present the case of an 81-year-old woman with symptomatic duodenal lipoma following endoscopic resection.
Go to :

Keywords: Duodenum, Lipoma, Gastrointestinal hemorrhage, Endoscopic mucosal resection
Introduction
Subepithelial tumors (SETs) in the upper gastrointestinal (GI) tract are often detected during nationwide endoscopic gastric cancer screening in Korea. These GI SETs vary from benign lesions, such as leiomyomas, to malignant ones, including GI stromal tumors and neuroendocrine tumors. Most SETs are asymptomatic and of minimal clinical significance [
1]. One type, GI lipomas, constitute approximately 4% of all GI tumors. The colon is the most common site of these lipomas, with only about 4% found in the duodenum [
2]. Since most GI lipomas are asymptomatic, they typically do not require further treatment. However, large lipomas may lead to complications such as bowel obstruction, intussusception, and bleeding [
3]. These symptomatic GI lipomas necessitate endoscopic or surgical removal. In this report, we present a case of duodenal lipoma that was incidentally diagnosed due to upper GI bleeding, an atypical symptom of GI lipoma.
Go to :

Case presentation
Ethics statement
Informed consent for publication was obtained from the patient.
Case
An 81-year-old woman with a history of osteoporosis and hypertension was referred to the emergency room of Pusan National University Hospital after experiencing melena for 1 day. Upon admission, her vital signs were normal. Electrocardiography and chest X-ray showed no abnormalities, and the patient’s abdomen was soft and non-tender.
A peripheral blood test revealed a hemoglobin level of 9.1 mg/dL, mean corpuscular volume of 80.2 fL, leukocyte count of 6,630/mm3, eosinophil count of 13/mm3, and platelet count of 204,000/mm3. Liver and renal function tests indicated an aspartate aminotransferase/alanine aminotransferase ratio of 18/10, alkaline phosphatase level of 38 IU/L, albumin level of 3.8 g/dL, total bilirubin level of 0.63 mg/dL, blood urea nitrogen level of 25.2 mg/dL, and blood creatinine level of 0.73 mg/dL. Serum electrolyte levels were within normal limits. Prothrombin time was measured at 11.3 seconds, and activated partial thromboplastin time was determined to be 19 seconds.
Initial CT scans revealed a 3-cm hypodense mass lesion, consistent with fat density, in the transverse portion of the duodenum (
Fig. 1). Esophagogastroduodenoscopy showed a 3-cm SET in the third portion of the duodenum, with an active ulcer at its apex. Upon retraction of the duodenal lipoma, another ulceration with active bleeding was discovered on the opposite side of the lesion (
Fig. 2). Consequently, immediate coagulation therapy was initiated. The patient reported that, while she had undergone endoscopic evaluation as part of Korea’s nationwide cancer screening program, she had not been told that she had a duodenal subepithelial lesion (SEL).
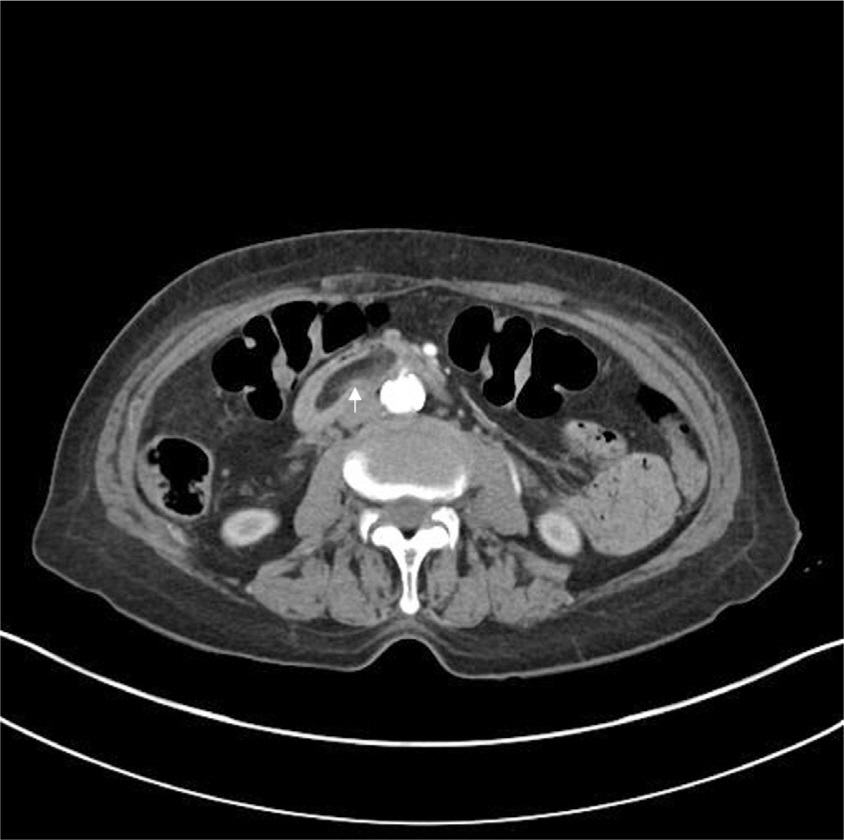 | Fig. 1.Abdominal CT revealed a hypodense mass in the third portion of the duodenum (indicated by the white arrow).
|
 | Fig. 2.Endoscopic findings of duodenal lipoma. (A) A 3-cm duodenal lipoma, presenting as a polypoid mass. (B) Multiple ulcerations were observed on the tip of the lesion. (C) A bleeding focus was identified on an ulcer located on the lipoma. (D) Endoscopic ultrasonography revealed a homogeneous hyperechoic mass (indicated by the white arrow). The muscle layer remained intact. The lesion appeared stretched, causing the fold to overlap; consequently, multiple mucosal and submucosal layers were visible beneath the duodenal lipoma (indicated by the black arrow).
|
Endoscopic ultrasonography was performed 2 weeks after achieving symptom control. This assessment revealed a hyperechoic lesion in the third layer of the duodenal wall (
Fig. 2). The patient then underwent endoscopic mucosal resection (EMR) with an endoloop (that is, endoloop-assisted polypectomy) to mitigate the risks of bleeding and perforation. Following EMR, the defect was sealed with additional endoclips to address concerns regarding the potential for delayed perforation (
Fig. 3). No postoperative complications were observed.
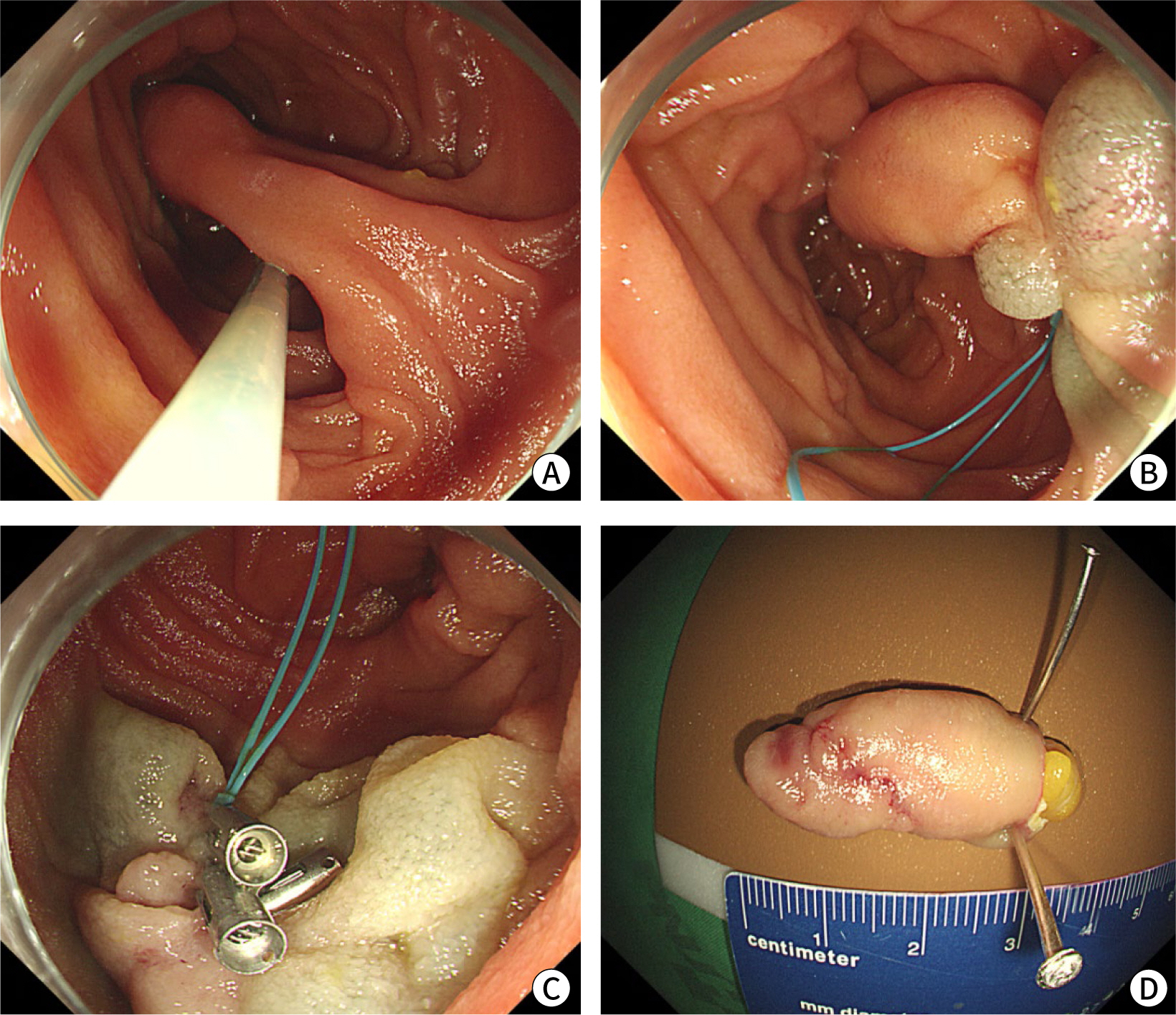 | Fig. 3.Endoscopic resection of the duodenal lipoma. (A,B) Submucosal injection and an endoloop were applied to prevent bleeding and perforation. (C) Following polypectomy, the mucosal defect was sealed with endoclips. (D) The resected specimen measured approximately 3.0 cm, and “naked” fat was noted at the resection margin.
|
After EMR, tissue exhibiting the “naked fat” sign was macroscopically identified at the resection margin. Pathological examination revealed a polypoid mass lesion, measuring 3.0×2.0×1.3 cm and covered by duodenal mucosa. Microscopically, the tumor consisted of mature adipocytes within the submucosal layer, consistent with duodenal lipoma (
Fig. 4). Following the procedure, the patient was discharged from the hospital without complications. Two weeks later, during a follow-up appointment at the outpatient clinic, she reported no further GI bleeding. However, the patient declined follow-up endoscopy due to her advanced age and the long distance between her residence and the hospital.
 | Fig. 4.Microscopic finding showing submucosal lipoma with ulceration. (A) Hematoxylin and eosin (H&E) stain, ×5 magnification. (B) High-power view reveals mature adipocytes, indicating lipoma (H&E stain, ×200 magnification).
|
Go to :

Discussion
Duodenal lipomas are rare benign lesions that originate from the submucosal adipose tissue of the GI tract. The most common site for lipomas within the GI tract is the large intestine, accounting for 64% of these lesions. In contrast, only 4% are found in the duodenum [
2].
Most GI lipomas are asymptomatic and are often discovered incidentally during endoscopic or radiological examination. The duodenal lipoma in the present case was similarly found by chance due to GI bleeding. Despite undergoing endoscopy every 2 years as part of a national cancer screening program, the patient had never been informed of having a duodenal SEL. This is likely because the SEL was located in the third portion of the duodenum, which is not typically examined during national cancer screening endoscopy. Various modalities are available for the diagnosis of lipoma, including CT and endoscopic ultrasound. On CT, GI lipomas present as low-density lesions that correspond to the fat density of the submucosal layer of the GI tract [
4]. On esophagogastroduodenoscopy, GI lipomas appear as yellowish, solitary SELs that exhibit a cushion sign when compressed with endoscopic forceps. On endoscopic ultrasonography, GI lipomas are revealed as homogenous, hyperechoic lesions situated in the third layer—or submucosal layer—of the GI tract [
5].
Typically, GI lipomas are asymptomatic and do not necessitate further treatment [
6,
7]. However, larger lipomas may cause symptoms such as intussusception, bowel obstruction, and GI bleeding due to ulceration, as observed in our case. One proposed mechanism for ulceration in GI lipomas is the mechanical irritation and pressure exerted by the lipoma on the mucosa of the GI tract. As the lipoma grows, it can continuously press against the duodenal lining, leading to mucosal irritation and eventually ulceration [
8]. Symptomatic duodenal lipomas should be removed via endoscopy or surgery [
7]. Additionally, growing GI lipomas should also be resected due to the potential for symptom development and the risk of associated malignancy [
9,
10]. Since most GI lipomas are benign SETs, endoscopic resection may be the preferred treatment when feasible [
3]. However, endoscopic resection of duodenal lipomas can result in complications, such as bleeding and perforation. Using an endoloop-assisted endoscopic resection technique, as in our case, can minimize the risks of hemorrhage and perforation following the procedure. In the present case, we administered a submucosal injection before applying the endoloop. This approach has both advantages and drawbacks. It creates a cushion between the lesion and the muscularis propria layer, reducing the risk of perforation. However, this technique also carries a risk of bleeding from the injection site and potential anatomical distortion, which can complicate the placement of the endoloop. Therefore, the decision to use submucosal fluid injection before endoloop application should be made based on the characteristics of the lipoma and the experience of the endoscopist.
To date, fewer than 30 cases of duodenal lipoma with hemorrhage have been reported [
7]. To our knowledge, this is the third case report of a duodenal lipoma with hemorrhage in Korea that was successfully treated through endoscopic resection without complications. Previously reported cases of duodenal lipoma with hemorrhage in Korea were located in the bulb or the second portion of the duodenum [
7,
11,
12]. Our case is unique among these in that the lipoma was located in the third part of the duodenum.
Duodenal lipomas are rare benign neoplasms of the GI tract. Although most such lipomas are asymptomatic, some larger tumors can be associated with complications, including bleeding. For symptomatic lipomas, endoscopic resection may be the optimal treatment option.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download