Abstract
Purpose
Methods
Results
Conclusion
Notes
Fund/Grant Support: This study was supported by the Basic Science Research Program grant (No. NRF-2022R1F1A107273712) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning of Korea, and also by the Biomedical Research Institute Fund from Gyeongsang National University Hospital (No. GNUHBRIF-2021-0004).
References
Fig. 1
CPNE1 expression increased in CRC specimens. (A) CPNE1 mRNA is upregulated in CRC tissues (compared to normal tissues, P < 0.001). (B) The graph depicting overall survival rate of CRC patients (n = 585). Curves were stratified based on the mean CPNE1 expression level values for all patients. (C) CPNE1 expression in human CRC cells was detected by western blotting. (D) A total of 263 formalin-fixed, paraffin-embedded CRC tissues were subjected to evaluation of CPNE1 expression using immunohistochemistry (IHC). Representative specimens demonstrating a range of CPNE1 expression levels in CRC by IHC. CPNE1 expression was graded according to the intensity of IHC staining: 0, absent staining; 1, weak staining; 2, moderate staining; and 3, strong staining. CPNE1, copine 1; CRC, colorectal cancer; mRNA, messenger RNA. ****P < 0.001 vs. normal group; ###P < 0.01 vs. CRC group.
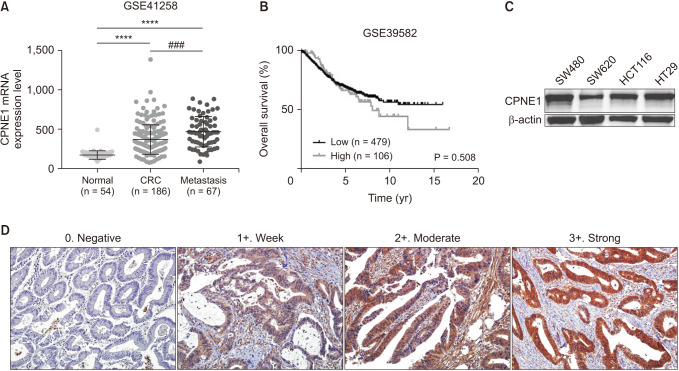
Fig. 2
CPNE1 silencing inhibits cell proliferation and colony formation in CRC cells. (A) Each cell line was divided into the following 2 groups: sh-NC and sh-CPNE1. (B, C) Knockdown of CPNE1 represses CRC cell proliferation. The Cell Counting Kit-8 assay of cell proliferation in CRC cell lines; cell viability was determined at 24, 48, and 72 hours. (D) Representative images and quantitative clonogenic analysis of plate colony formation in CRC cells. (E) Effect of CPNE1 knockdown on protein levels of cyclin A, cyclin B1, and cyclin E. All experiments were performed in triplicate. Bar charts showing the clonogenic growth of cells. CPNE1, copine 1; CRC, colorectal cancer; sh-NC, negative control cells; sh-CPNE1, CPNE1-silenced cells. **P < 0.01 and ***P < 0.001 vs. sh-NC.
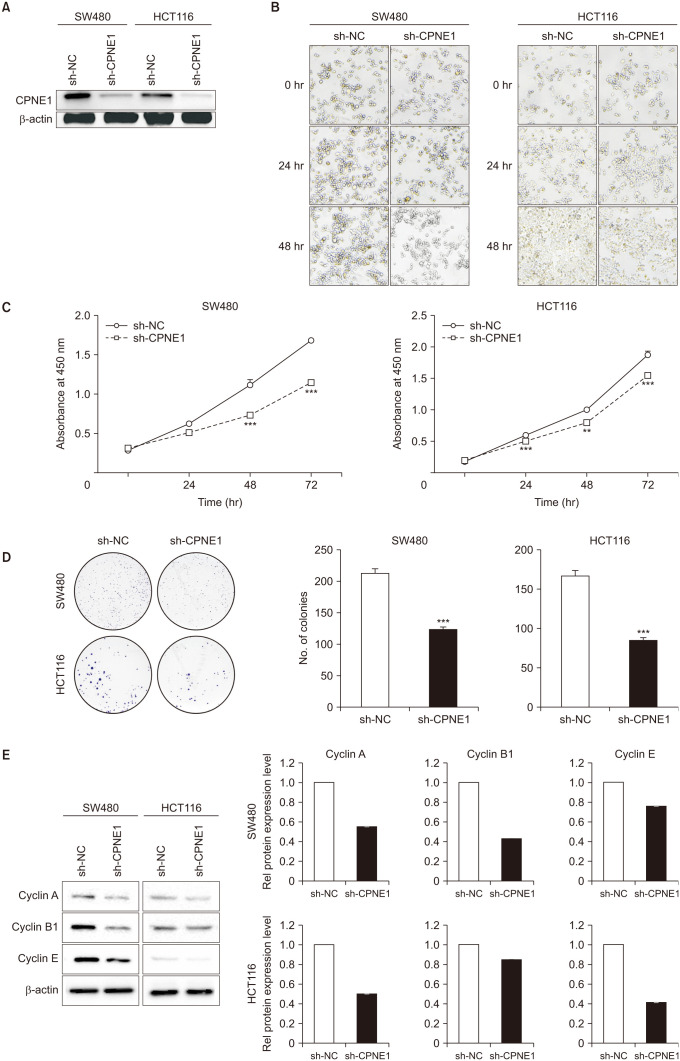
Fig. 3
Silencing of CPNE1 inhibits the gap closure and invasive abilities of CRC cells. (A) Effects of CPNE1 silencing on the gap closure assay of CRC cells. The gap closure assay showed that the speed at which cells migrated toward the scratch was lower in CPNE1-silenced cells than in control cells. (B) Effects of CPNE1 inhibition on the invasion of CRC cells. Assays were performed on a Transwell plate. (magnification, ×200). (C) Western blot was used to determine the protein contents of vimentin, Slug, and Snail in different cells. CPNE1, copine 1; CRC, colorectal cancer; sh-NC, negative control cells; sh-CPNE1, CPNE1-silenced cells. *P < 0.05 and ***P < 0.001 vs. sh-NC.
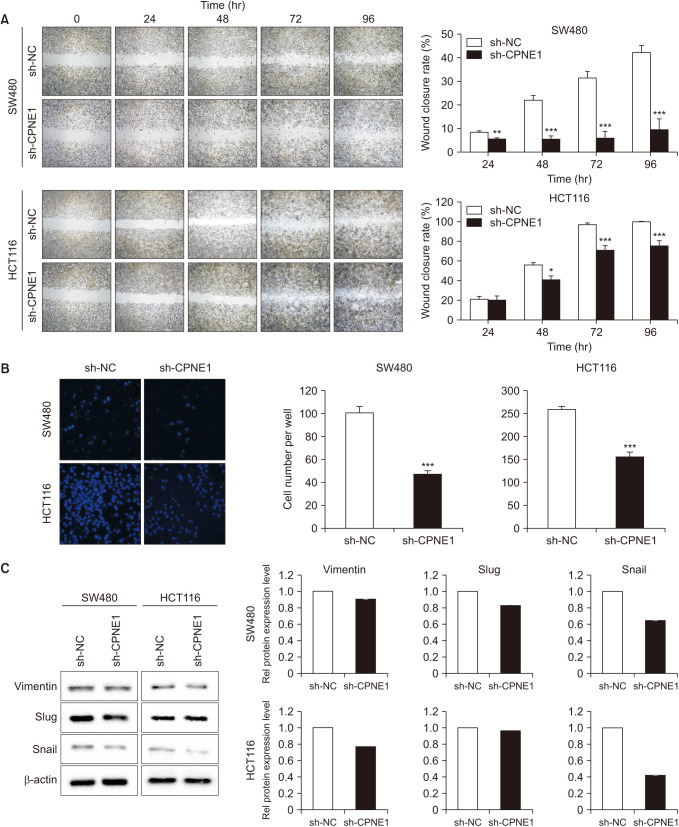
Fig. 4
Knockdown of CPNE1 inhibits tumor growth in the mouse xenograft model. HCT116 cells were subcutaneously injected into the left flank of athymic nude mice. (A) Macroscopic appearance of tumors in mice at the experimental endpoint. Images of representative excised tumors from each group. (B) Tumor volumes were averaged for each experimental group and time point over the course of the study. (C) The final tumor weights of the isolated tumors were measured for each group. (D) H&E staining, CPNE1, and Ki-67 immunostaining of tumor tissue from nude mice xenografts. More than 95% of tumor cells showed a positive nuclear immunoreaction for Ki-67. CPNE1, copine 1; sh-NC, negative control cells; sh-CPNE1, CPNE1-silenced cells. *P < 0.05 and ***P < 0.001 vs. sh-NC.
Table 1
The correlation of CPNE1 expression (ICH) with clinicopathological parameters
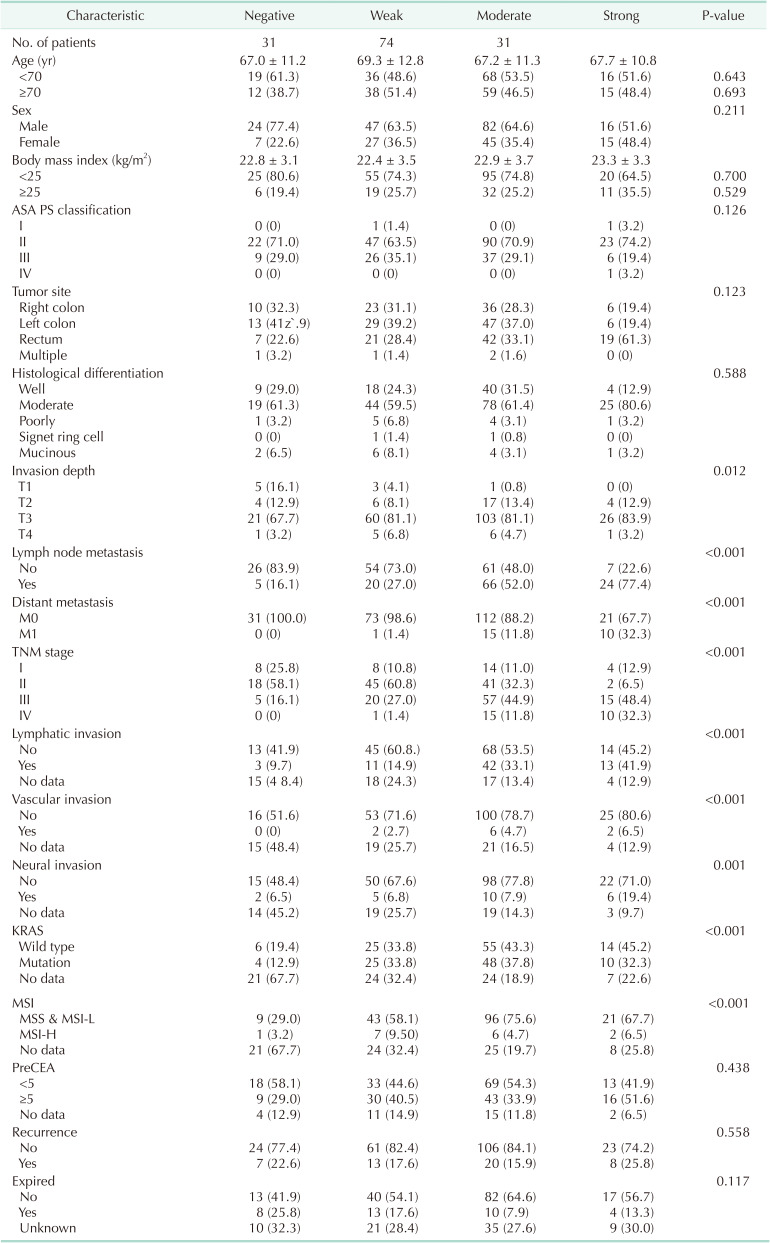
Values are presented as number only, mean ± standard deviation, or number (%).
ASA, American Society of Anesthesiologists; PS, physical status; KRAS, Kristen rat sarcoma viral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; MSI, microsatellite instability; MSS, microsatellite stability; MSI-L, MSI-low; MSI-H, MSI-high.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download