Abstract
We report a patient with whole neuroaxis dissemination of a sporadic supratentorial hemangioblastoma (HB) for more than 15 years. A 68-year-old female patient presented with severe radiating pain in the right leg. Gadolinium-enhanced lumbar spine MRI showed an intradural mass (2.5 cm in diameter) at the L4 level. The patient had been severely disabled for 22 years after a previous intraventricular brain tumor resection. At that time, the diagnosis was angioblastic meningioma, which was thought to be incorrect. At 14 years after the brain surgery, gamma knife radiosurgery was performed three times for newly developed or recurred supratentorial and infratentorial tumors in the cerebrospinal fluid pathway. The patient underwent lumbar spinal surgery, and a gross total removal of the mass was performed, which confirmed the histopathological diagnosis of HB. We reexamined the old histopathological specimen of the intraventricular tumor from 20 years ago and changed the diagnosis from angioblastic meningioma to supratentorial HB. Six months after spinal surgery, the patient underwent a second spinal surgery and brain surgery, and the histopathological diagnosis was HB following both surgeries, which was the same following the first spinal surgery. Here, we report a sporadic supratentorial HB patient who showed cranial and spinal disseminations for more than two decades along with a literature review.
Hemangioblastoma (HB) is a rare central nervous system (CNS) tumor commonly found in the cerebellum, brain stem, and spinal cord [1]. It is generally regarded as a benign, slow-growing, non-metastasizing neoplasm [12]. Sporadic HB is usually solitary and accounts for 70% of cases. In contrast, HB with manifestations of von Hippel-Lindau (VHL) disease is often multifocal and accounts for 30% of cases [1]. Sporadic HB occasionally shows local recurrences; however, dissemination recurrence is extremely rare [13]. Here, we report a patient with sporadic supratentorial HB, who exhibited whole neuroaxis dissemination for more than two decades.
A 68-year-old female patient presented with severe radiating pain in the right leg. Gadolinium (Gd)-enhanced lumbar spine MRI showed an intradural mass (2.5 cm in diameter) at the L4 level (Fig. 1A). Additional whole-spine MRI showed multiple small masses at the cervical and thoracic spines (Fig. 1B).
The patient underwent spinal surgery and a total removal of the intradural mass (July 2022). During surgery, it was found that the mass was related to neither the dura matter nor the adjacent nerve rootlets. However, interestingly, the final histological diagnosis was HB. Histopathologically, the tumor exhibited analogous histological features, showing irregularly formed vessels interspersed with large stromal cells exhibiting hyperchromatic nuclei (Fig. 1C). Notably, the tumor cells exhibited limited immunopositivity for alpha-inhibin (Fig. 1D) but demonstrated robust immunopositivity for epidermal growth factor receptor (EGFR) (Fig. 1E).
The postoperative clinical course was uneventful, and the previous radiating pain disappeared. Evaluation for VHL disease, including systemic radiological and ophthalmological examination, showed negative results.
In December 2000 (22 years ago), the patient had undergone craniotomy and a subtotal removal of a large solid tumor in the third ventricle extending to the lateral ventricles (Fig. 2). As per the medical records, a profound bleeding episode occurred during surgery, and the pathological diagnosis was angioblastic meningioma with hemorrhage. Immunohistochemistry revealed positive vimentin and factor VIII staining and negative HMB45 staining. However, the findings were limited by the extent of the available information. Clinically, after the first brain tumor resection, the patient was severely disabled due to left hemiplegia, and the Karnofsky performance score (KPS) was 60.
At 14 years after the brain surgery, Gd-enhanced axial brain MRIs (April 2014) showed two newly developed tumors in the cerebellomedullary cistern (Fig. 3A) and fourth ventricle (Fig. 3B). The first gamma knife radiosurgery (GKRS) was performed for them; the tumor volumes were 0.57 cm3 and 0.19 cm3, respectively, and the prescription radiation doses were 13.0 Gy and 13.5 Gy, respectively (April 2014). After 5 years, the second GKRS was performed for three newly developed tumors, which were located in the cerebellopontine cistern, ambient cistern, and lateral ventricular wall. Two-fraction GKRS was performed with a prescription dose of 2×7.6 Gy (December 2019). Two years later, the third GKRS was performed for a newly developed tumor near the right optic nerve in the basal cistern. The tumor volume and the prescription radiation dose were 0.71 cm3 and 12 Gy, respectively (July 2021). Until this point, we managed this patient as a recurred and disseminated ventricular meningioma patient.
The original tissue specimen in 2000 was reevaluated following two instances of recurrence. Microscopic examination at low magnification revealed irregularly dilated blood vessels and extensive hemorrhage (Fig. 4A). Upon closer inspection at high magnification, vacuolated stromal cells characterized by large hyperchromatic nuclei and an eosinophilic foamy cytoplasm were identified amidst well-developed blood vessels (Fig. 4B). Based on a biopsy review, the presence of the primary tumor was consistent with the histological diagnosis of HB.
Six months after the first spinal surgery (February 2023), follow-up whole-spine MRI showed the progression of all spinal tumors, and the tumor at the T9 level demonstrated a severe mass effect on the spinal cord (Fig. 5A). The patient underwent a second spinal surgery and gross total removal with thoracic midline myelotomy. The location of the tumor was in the central canal of the thoracic spinal cord, and the histopathological diagnosis was HB, which was the same as in the first spinal surgery. The postoperative course was uneventful, and the patient’s neurological status remained unchanged.
Three months after the second spinal surgery (May 2023), the patient underwent suboccipital craniotomy due to the progression of the tumor in the right cerebellopontine angle (Fig. 5B). The tumor severely compressed the brain stem, which was subtotally removed.
In the subsequent recurrence, there were prominent tumor cells characterized by a clear cytoplasm and delicate fibrovascular septae (Fig. 5C). Immunohistochemical analysis revealed positive staining for alpha-inhibin (Fig. 5D) and EGFR (Fig. 5E). This was the fourth diagnosis of HB for this patient.
The dissemination of sporadic HB is very rare, and less than 20 patients have ever been reported [14567891011121314]. Most of them had a solid cerebellar HB as a primary tumor, and more than three-fourths of cases showed no association with VHL disease [23481415161718192021]. However, thus far, there has been no report of the dissemination of a supratentorial HB.
Two decades ago, the patient was diagnosed with an angioblastic meningioma. However, the diagnosis may be incorrect considering that the origin was the third ventricle. We reviewed the histopathological specimen from the first surgery in 2000 and concluded that the diagnosis was supratentorial HB. The incorrect diagnosis may be explained as follows: 1) the immunohistochemical findings of this tumor could be the same as those of HB; 2) at the time of pathological examination, due to blood clots caused by hemorrhage, accurate diagnosis could be difficult; and 3) when diagnosing the tumor, both the ventricular origin of the tumor and the rarity of the supratentorial HB could not be considered [2223].
Interestingly, there have been several reports of sporadic HB dissemination over an extended period [13]. Bains et al. [1] reported a patient with disseminated CNS HB, and the interval to dissemination was 12 years. They also reviewed previously reported cases, and the interval to dissemination varied from 7 months to 30 years. Kim et al. [3] reported a similar case of disseminated HB into the cauda equina after the removal of a solitary cerebellar HB after a 10-year duration. They suggested that the disseminated HB is an extremely unusual type of recurrence, which is known as hemangioblastomatosis [38141516171819]. They also described the pathophysiology of hemangioblastomatosis as the spillage and spread of tumor cells through the cerebrospinal fluid (CSF) space, which may be an origin of hemangioblastomatosis in patients with a genetic predisposition. They noted that no case of de novo disseminated HB without previous surgery has been reported. Most of the previously reported cases showed multiple mass lesions in the infratentorial area and spinal cord, and the rarity of supratentorial lesions might reflect CSF flow and the effect of gravity, thus supporting the hypothesis of tumor spread through the CSF pathway [3].
Similarly, the case presented here showed a medical history of disseminated sporadic HB, which was disseminated from the supratentorial area to the posterior fossa and finally to the spinal cord via the CSF system over 20 years. We investigated reported cases of disseminated sporadic HB with a duration of 10 years or more (Table 1).
The weak point of this report might be the initial diagnosis (ventricular meningioma) in the old medical records, which we believe was an incorrect diagnosis. There have been reports on the difficulty of differential diagnosis between supratentorial HB and angioblastic meningioma [2223]. Therefore, there may be a high possibility of misdiagnosis as ventricular meningioma. Kim et al. [22] reported a VHL patient with meningeal supratentorial HB mimicking angioblastic meningioma. Crivelli et al. [23] also reported a rare case of solid supratentorial HB. They suggested that the difference between angioblastic meningioma and HB is controversial because of the nonspecific radiological findings of these supratentorial uncommon tumors [23]. Therefore, differential diagnosis between supratentorial HB and angioblastic meningioma might be relatively difficult.
In the management of disseminated HB, there is currently no optimal treatment for preventing the progression of dissemination [12]. Chung et al. [2] emphasized that surgeons should be cautious of tumor cell spillage during the initial surgical management of these tumors, and continuous follow-up should be performed for patients, including those who underwent complete removal of the primary HB.
In conclusion, we presented a patient with whole neuroaxis dissemination of a sporadic supratentorial HB with a brief literature review. The involvement of a pathomechanism is highly likely. This report describes the dissemination of a supratentorial HB, which has not been reported previously. Notably, the dissemination from the supratentorial area to the spine took more than 20 years in the presented case. Therefore, physicians should be aware of the possibility of a long latent period for the dissemination of sporadic HB in the CNS after surgery.
Notes
Ethics Statement: This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital with a waiver of informed consent (CNUHH-2023-252).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Bains SJ, Niehusmann PF, Meling TR, Saxhaug C, Zuchner M, Brandal P. Disseminated central nervous system hemangioblastoma in a patient with no clinical or genetic evidence of von Hippel-Lindau disease-a case report and literature review. Acta Neurochir (Wien). 2019; 161:343–349. PMID: 30652202.

2. Chung SY, Jeun SS, Park JH. Disseminated hemangioblastoma of the central nervous system without von Hippel-Lindau disease. Brain Tumor Res Treat. 2014; 2:96–101. PMID: 25408933.

3. Kim HR, Suh YL, Kim JW, Lee JI. Disseminated hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: a case report. J Korean Med Sci. 2009; 24:755–759. PMID: 19654966.

4. Amelot A, Bouazza S, Polivka M, George B, Bresson D. Sporadically second localization of cerebellar hemangioblastoma in sella turcica mimicking a meningioma with no associated von Hippel-Lindau disease. Br J Neurosurg. 2015; 29:589–591. PMID: 25817084.

5. Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995; 194:629–642. PMID: 7862955.

6. Girmens JF, Erginay A, Massin P, Scigalla P, Gaudric A, Richard S. Treatment of von Hippel-Lindau retinal hemangioblastoma by the vascular endothelial growth factor receptor inhibitor SU5416 is more effective for associated macular edema than for hemangioblastomas. Am J Ophthalmol. 2003; 136:194–196. PMID: 12834696.

7. Hasselblatt M, Jeibmann A, Gerss J, Behrens C, Rama B, Wassmann H, et al. Cellular and reticular variants of haemangioblastoma revisited: a clinicopathologic study of 88 cases. Neuropathol Appl Neurobiol. 2005; 31:618–622. PMID: 16281910.

8. Kato M, Ohe N, Okumura A, Shinoda J, Nomura A, Shuin T, et al. Hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: a case report. J Neurooncol. 2005; 72:267–270. PMID: 15937651.

9. Lee JY, Dong SM, Park WS, Yoo NJ, Kim CS, Jang JJ, et al. Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer Res. 1998; 58:504–508. PMID: 9458097.
10. Omar AI. Bevacizumab for the treatment of surgically unresectable cervical cord hemangioblastoma: a case report. J Med Case Rep. 2012; 6:238. PMID: 22883663.

11. Raghavan R, Krumerman J, Rushing EJ, White CL 3rd, Chason DP, Watson ML, et al. Recurrent (nonfamilial) hemangioblastomas involving spinal nerve roots: case report. Neurosurgery. 2000; 47:1443–1448. PMID: 11126917.

12. Ramachandran R, Lee HS, Matthews B, Shatzel A, Tihan T. Intradural extramedullary leptomeningeal hemangioblastomatosis and paraneoplastic limbic encephalitis diagnosed at autopsy: an unlikely pair. Arch Pathol Lab Med. 2008; 132:104–108. PMID: 18181660.

13. Takayanagi S, Mukasa A, Tanaka S, Nomura M, Omata M, Yanagisawa S, et al. Differences in genetic and epigenetic alterations between von Hippel-Lindau disease-related and sporadic hemangioblastomas of the central nervous system. Neuro Oncol. 2017; 19:1228–1236. PMID: 28379443.

14. Tohyama T, Kubo O, Kusano R, Miura N, Himuro H. [A case of hemangioblastoma with subarachnoid dissemination]. No Shinkei Geka. 1990; 18:83–88. Japanese. PMID: 2304611.
15. Bakshi R, Mechtler LL, Patel MJ, Lindsay BD, Messinger S, Gibbons KJ. Spinal leptomeningeal hemangioblastomatosis in von Hippel-Lindau disease: magnetic resonance and pathological findings. J Neuroimaging. 1997; 7:242–244. PMID: 9344008.

16. Mohan J, Brownell B, Oppenheimer DR. Malignant spread of haemangioblastoma: report on two cases. J Neurol Neurosurg Psychiatry. 1976; 39:515–525. PMID: 1084914.

17. Reyns N, Assaker R, Louis E, Lejeune JP. Leptomeningeal hemangioblastomatosis in a case of von Hippel-Lindau disease: case report. Neurosurgery. 2003; 52:1212–1215. discussion 1215-6. PMID: 12699568.

18. Weil RJ, Vortmeyer AO, Zhuang Z, Pack SD, Theodore N, Erickson RK, et al. Clinical and molecular analysis of disseminated hemangioblastomatosis of the central nervous system in patients without von Hippel-Lindau disease. Report of four cases. J Neurosurg. 2002; 96:775–787. PMID: 11990821.

19. Hanse MC, Vincent A, van den Bent MJ. Hemangioblastomatosis in a patient with von Hippel-Lindau disease. J Neurooncol. 2007; 82:163–164. PMID: 17256106.

20. Akimoto J, Fukuhara H, Suda T, Nagai K, Hashimoto R, Michihiro K. Disseminated cerebellar hemangioblastoma in two patients without von Hippel-Lindau disease. Surg Neurol Int. 2014; 5:145. PMID: 25324974.

21. Seystahl K, Weller M, Bozinov O, Reimann R, Rushing E. Neuropathological characteristics of progression after prolonged response to bevacizumab in multifocal hemangioblastoma. Oncol Res Treat. 2014; 37:209–212. PMID: 24732646.

22. Kim H, Park IS, Jo KW. Meningeal supratentorial hemangioblastoma in a patient with von Hippel-Lindau disease mimicking angioblastic menigioma. J Korean Neurosurg Soc. 2013; 54:415–419. PMID: 24379949.

23. Crivelli G, Dario A, Cerati M, Dorizzi A. Solid supratentorial haemangioblastoma. Case report. J Neurosurg Sci. 1992; 36:161–166. PMID: 1484303.
Fig. 1
Gadolinium (Gd)-enhanced sagittal lumbar spine MRI (July 2022) (A) of an intradural mass (2.5 cm in diameter) at the L4 level with well-homogeneous enhancement, which occupied the whole spinal canal at this level. Gd-enhanced whole-spine MRI of other masses at the cervical and thoracic spines (B). The tumor showed histological features with irregularly formed vessels interspersed with large stromal cells (C; H&E, ×100). The tumor cells were slightly positive for alpha-inhibin (D; immunohistochemistry, ×200) but strongly positive for EGFR (E; ×200).
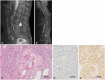
Fig. 2
Gadolinium-enhanced axial brain MRI (December 2000) of an intraventricular mass (4 cm in diameter) at the third and lateral ventricles.
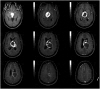
Fig. 3
Gadolinium-enhanced axial brain MRIs (April 2014) of the tumors in the cerebellomedullary cistern (A) and fourth ventricle (B).
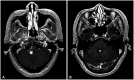
Fig. 4
The tumor exhibited irregularly dilated blood vessels and extensive hemorrhage at low magnification (A; H&E staining, original magnification ×40). At high magnification, the tumor had vacuolated stromal cells characterized by large hyperchromatic nuclei and an eosinophilic foamy cytoplasm (B; H&E, ×200).
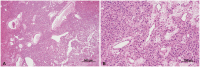
Fig. 5
Gadolinium (Gd)-enhanced sagittal spine MRI (January 2023) of a progressed 2 cm-sized vividly enhancing intramedullary mass at the T9 level (A), and Gd-enhanced axial brain MRI (May 2023) of the tumor in the right cerebellopontine angle (B). Histopathologically, the second recurrent brain tumor was composed of plump tumor cells characterized by a clear cytoplasm (C; H&E, ×100) with immunopositivity for alpha-inhibin (D; ×200) and EGFR (E; ×200).
Table 1
Reported disseminated sporadic hemangioblastoma cases with a long interval (10 years or more)
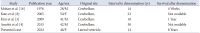
| Study | Publication year | Age/sex | Original site | Interval to dissemination (yr) | Survival after dissemination |
|---|---|---|---|---|---|
| Mohan et al. [16] | 1976 | 28/M | Cerebellum | 14 | 4 Weeks |
| Kato et al. [8] | 2005 | 50/F | Cerebellum | 22 | Not available |
| Kim et al. [3] | 2009 | 41/M | Cerebellum | 10 | 1 Year |
| Amelot et al. [4] | 2015 | 42/M | Cerebellum | 30 | Not available |
| Presented case | 2024 | 46/F | Lateral ventricle | 14 | 8 Years |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download