1. Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin lymphoma. Lancet. 2021; 398:1518–1527. PMID:
33493434.

2. Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol. 2019; 184:45–59. PMID:
30407610.

3. Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018; 68:116–132. PMID:
29194581.

4. Grimm S, Chamberlain M. Hodgkin’s lymphoma: a review of neurologic complications. Adv Hematol. 2011; 2011:624578. PMID:
20975772.

5. Kaji FA, Martinez-Calle N, Sovani V, Fox CP. Rare central nervous system lymphomas. Br J Haematol. 2022; 197:662–678. PMID:
35292959.

6. Re D, Fuchs M, Schober T, Engert A, Diehl V. CNS involvement in Hodgkin’s lymphoma. J Clin Oncol. 2007; 25:3182.

7. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017; 35:2410–2418. PMID:
28640701.

8. Phillips EH, Fox CP, Cwynarski K. Primary CNS lymphoma. Curr Hematol Malig Rep. 2014; 9:243–253. PMID:
24969265.

9. Gerstner ER, Abrey LE, Schiff D, Ferreri AJ, Lister A, Montoto S, et al. CNS Hodgkin lymphoma. Blood. 2008; 112:1658–1661. PMID:
18591379.

10. Hochberg FH, Baehring JM, Hochberg EP. Primary CNS lymphoma. Nat Clin Pract Neurol. 2007; 3:24–35. PMID:
17205072.

11. Camilleri-Broët S, Martin A, Moreau A, Angonin R, Hénin D, Gontier MF, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Am J Clin Pathol. 1998; 110:607–612. PMID:
9802345.

12. Nagashima K, Mori S, Yoshimasu N, Takahashi K. Primary Hodgkin’s disease of the falx cerebri. Acta Neuropathol. 1980; 51:161–163. PMID:
7435148.

13. Bender BL, Mayernik DG. Hodgkin’s disease presenting with isolated craniospinal involvement. Cancer. 1986; 58:1745–1748. PMID:
3756795.

14. Doorly TP, Farrell MA, Phillips J. Primary intracerebral Hodgkin’s lymphoma. J Neurol Neurosurg Psychiatry. 1987; 50:1048–1050. PMID:
3655810.

15. Ashby MA, Barber PC, Holmes AE, Freer CE, Collins RD. Primary intracranial Hodgkin’s disease. A case report and discussion. Am J Surg Pathol. 1988; 12:294–299. PMID:
3354756.
16. Sickler GK, Hanson SK, Hsu SM, Papasozomenos SC. Primary intracerebral Hodgkin’s disease: a case report. Clin Neuropathol. 1990; 9:143–147. PMID:
2364594.
17. Clark WC, Callihan T, Schwartzberg L, Fontanesi J. Primary intracranial Hodgkin’s lymphoma without dural attachment: case report. J Neurosurg. 1992; 76:692–695. PMID:
1545264.
18. Deckert-Schlüter M, Marek J, Setlík M, Marková J, Pakos E, Fischer R, et al. Primary manifestation of Hodgkin’s disease in the central nervous system. Virchows Arch. 1998; 432:477–481. PMID:
9645450.

19. Klein R, Müllges W, Bendszus M, Woydt M, Kreipe H, Roggendorf W. Primary intracerebral Hodgkin’s disease: report of a case with Epstein-Barr virus association and review of the literature. Am J Surg Pathol. 1999; 23:477–481. PMID:
10199479.
20. Biagi J, MacKenzie RG, Lim MS, Sapp M, Berinstein N. Primary Hodgkin’s disease of the CNS in an immunocompetent patient: a case study and review of the literature. Neuro Oncol. 2000; 2:239–243. PMID:
11265233.

21. Herrlinger U, Klingel K, Meyermann R, Kandolf R, Kaiserling E, Kortmann RD, et al. Central nervous system Hodgkin’s lymphoma without systemic manifestation: case report and review of the literature. Acta Neuropathol. 2000; 99:709–714. PMID:
10867808.

22. Johnson MD, Kinney MC, Scheithauer BW, Briley RJ, Hamilton K, McPherson WF, et al. Primary intracerebral Hodgkin’s disease mimicking meningioma: case report. Neurosurgery. 2000; 47:454–456. discussion 456-7. PMID:
10942021.

23. Figueroa BE, Brown JR, Nascimento A, Fisher DC, Tuli S. Unusual sites of Hodgkin’s lymphoma: case 2. Hodgkin’s lymphoma of the CNS masquerading as meningioma. J Clin Oncol. 2004; 22:4228–4230. PMID:
15483035.
24. Hirmiz K, Foyle A, Wilke D, Burrell S, Brownstone R, Ago C, et al. Intracranial presentation of systemic Hodgkin’s disease. Leuk Lymphoma. 2004; 45:1667–1671. PMID:
15370222.

25. de Castro AF, Júnior AS, de Lins e Horta H, Neuenschwander LC, Fonseca RP, Lima SS, et al. Primary intracerebral Hodgkin lymphoma. Br J Haematol. 2007; 138:562. PMID:
17686050.

26. Hwang CY, Song YJ, Kim DC, Choi SS, Choi YM, Kim KU. Primary cerebellar Hodgkin’s lymphoma. J Korean Neurosurg Soc. 2007; 42:149–152.
27. Morawa E, Ragam A, Sirota R, Nabhan C. Hodgkin’s lymphoma involving the CNS. J Clin Oncol. 2007; 25:1437–1438. PMID:
17416864.

28. Subklewe M, Anagnostopoulos I. Radiologic and pathologic features of a posttransplantation primary central nervous system lymphoma demonstrating Epstein-Barr virus-positive Hodgkin lymphoma. Clin Lymphoma Myeloma. 2007; 7:535–537. PMID:
18021471.

29. Apollonsky N, Edelman M, Johnson A, Bhuiya T, Karayalcin G. Intracerebral presentation of Hodgkin disease mimicking meningioma in a young woman: case presentation with literature review. J Pediatr Hematol Oncol. 2008; 30:369–372. PMID:
18458571.

30. Almhanna K, Wongchaowart N, Sweetenham J. Intracerebral Hodgkin’s lymphoma in a patient with chronic lymphocytic leukemia/small lymphocytic lymphoma: a case report and literature review. Cancer Invest. 2009; 27:215–220. PMID:
19235595.

31. Foo WC, Desjardins A, Cummings TJ. Primary intracerebral Hodgkin lymphoma with recurrence. Clin Neuropathol. 2011; 30:75–79. PMID:
21329616.

32. Torgerson S, Olteanu H, Tinguely M, Fenske TS. Central nervous system Hodgkin lymphoma: case report and review of the literature. J Neurooncol. 2011; 102:329–334. PMID:
20676729.

33. Gessi M, Kuchelmeister K, Kellner U, Ritter M, Morgner A, Urbach H, et al. Unusual clinico-pathological features in primary Hodgkin’s lymphomas of the central nervous system. Acta Neurochir (Wien). 2013; 155:19–24. PMID:
23139103.

34. Kresak JL, Nguyen J, Wong K, Davis R. Primary Hodgkin lymphoma of the central nervous system: two case reports and review of the literature. Neuropathology. 2013; 33:658–662. PMID:
23530967.

35. Henkenberens C, Franzke A, Raab P, Oschlies I, Klapper W, Christiansen H. Primary EBV-positive Hodgkin’s lymphoma of the CNS under azathioprine treatment: case report and review of the literature. Strahlenther Onkol. 2014; 190:847–852. PMID:
24823896.

36. Martinez DL, Gujrati M, Geoffroy F, Tsung AJ. Isolated CNS Hodgkin’s lymphoma: implications for tissue diagnosis. CNS Oncol. 2014; 3:383–387. PMID:
25438809.

37. Sharaf N, Lobo B, Lee J, Prayson RA. Primary Hodgkin lymphoma of the central nervous system. J Clin Neurosci. 2014; 21:1271–1273. PMID:
24589557.

38. van Blydenstein SA, Patel M, Philip V, Lakha A, Pather S, Westgarth-Taylor T, et al. Classical Hodgkin lymphoma involving the central nervous system (brain) - an unusual presentation. Clin Case Rep. 2014; 2:88–92. PMID:
25356257.

39. Shivane A, Smith ME, Lewis D, Berei T. A rare case of primary intracerebral Hodgkin lymphoma. Clin Neuropathol. 2016; 35:389–392. PMID:
27719749.

40. Alfaseh A, Rajeh MN, Hamed G. Primary central nervous system Hodgkin lymphoma: a case discussion and a hypothesis on the etiology. Avicenna J Med. 2019; 9:28–31. PMID:
30697523.

41. Azriel A, Towner JE, Gaillard F, Box G, Rogers T, Morokoff A. Solitary intraventricular Hodgkin lymphoma post-transplant lymphoproliferative disease (HL-PTLD): case report. J Clin Neurosci. 2019; 69:269–272. PMID:
31451379.

42. Riccioni L, Cremonini AM, Gessaroli M. Primary intracranial Hodgkin’s lymphoma after a blunt trauma: a case report. J Neurosci Neurol Disord. 2020; 4:79–83.

43. Szczepanek D, Szumiło J, Stoma F, Szymczyk A, Jarosz B, Szczepanek A, et al. A case report of a female patient with Hodgkin lymphoma localized in the central nervous system and with concomitant pulmonary lymphomatoid granulomatosis. Front Neurol. 2020; 11:963. PMID:
33013640.

44. Ahmed S, Irfan B, Raza M, Haider G. Atypical involvement of central nervous system in classic Hodgkin lymphoma: a case report. J Med Case Rep. 2021; 15:532. PMID:
34711281.

45. Fu H, Shi S, Chen L, Xu B, Huang W, Chen Y, et al. Primary central nervous system Hodgkin’s lymphoma: a case report. J Int Med Res. 2021; 49:300060521999533. PMID:
33874776.

46. Kanagalingam T, Velker V, Pejhan S, Zhang Q, Mangel J, Young S. Isolated Hodgkin lymphoma of the intracranial dura: a case report and review of the literature. Clin Case Rep. 2023; 11:e7562. PMID:
37361649.

47. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022; 20:167–192. PMID:
35130500.
48. Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Armand P, Bello CM, et al. Hodgkin lymphoma, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020; 18:755–781. PMID:
32502987.
49. Cheah CY, Bröckelmann PJ, Chihara D, Moskowitz AJ, Engert A, Jerkeman M, et al. Clinical characteristics and outcomes of patients with Hodgkin lymphoma with central nervous system involvement: an international multicenter collaboration. Am J Hematol. 2016; 91:894–899. PMID:
27222367.

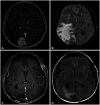
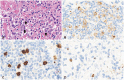





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download