Abstract
Angiocentric glioma (AG) is an extremely rare tumor that often develops in adolescents. Awake surgery for AG occurring in the eloquent area has not been reported to date. We report a case involving a right-handed 15-year-old boy with AG. He presented with a first-time generalized tonic-clonic seizure and was rushed to the local hospital. CT of the head indicated a left frontal low-density mass with no calcification. He was subsequently referred to our hospital. Comparison with a CT scan obtained two years prior due to mild head trauma indicated that the lesion showed a trend toward enlargement. The lesion was located in the anterior and lateral portions of the primary motor cortex, and MRI showed homogenous hypointensity on T1-weighted and hyperintensity on T2-weighted images. Contrast-enhanced MRI showed a linear contrast effect. The patient underwent awake surgery with successful intraoperative brain mapping and total resection, and brain function was preserved. Pathological analysis revealed AG. He returned to his normal life and has shown no recurrence without additional treatment for 2 years. Thus, awake surgery for complete tumor resection while preserving brain function is effective and safe even in adolescents with AGs.
Angiocentric glioma (AG) was first reported in 2005, and more than 100 cases of AG have been reported so far [1]. In the 2016 World Health Organization Classification of Tumors of the Central Nervous System, AG was newly classified under “other gliomas.” During awake surgery for lower-grade gliomas, including astrocytomas and oligodendrogliomas, intraoperative electrical stimulation mapping is necessary to avoid permanent neurologic deficits and preserve brain function in and near the eloquent area [2]. In surgery for lower grade glioma, awake surgery is widely used to maximize removal and preserve brain function. However, no previous case reports have described the removal of AGs by awake surgery. Here, we present the first successful case of awake craniotomy for AGs in the eloquent area in an adolescent patient, which was performed using intraoperative electrical stimulation mapping (IESM) for preserving language function.
A 15-year-old right-handed boy presented with a first-time generalized tonic-clonic seizure. CT of the head showed a left frontal low-density mass with no calcification. In comparison with a CT examination performed two years prior due to mild head trauma, the lesion showed a trend toward enlargement. The lesion was located in the anterior and lateral portions of the primary motor cortex, and was homogenously hypointense on T1-weighted images and hyperintense on T2-weighted images. Contrast-enhanced MRI showed a linear contrast effect in the posterior part of the lesion (Fig. 1). The differential diagnosis included dysembryoplastic neuroepithelial tumors and gliomas. Since the tumor was symptomatic and enlarging, surgical resection was recommended. To achieve total resection while preserving essential functions, awake surgery using IESM was planned.
The patient underwent awake surgery with IESM to identify and preserve the cortical and subcortical eloquent structures. This method, including the electrical parameters and the intraoperative clinical tasks, has been extensively described previously [345]. The tumor was delineated using ultrasonography and a neuro-navigation system (Brain Lab, Munich, Germany). After opening the dura mater, the patient was woken up. With cortical mapping at an intensity of 3.4 mA, cessation of counting was elicited in the primary motor cortex of the face, and a phonemic paraphasia was elicited in the supramarginal gyrus. No other speech disturbances were induced by cortical stimulation on the surface of the tumor (tumor boundary: A–E) (Fig. 2A).
After completion of cortical mapping, tumor resection was initiated. We confirmed language function through picture naming. First, we resected the surface of the tumor while preserving the central artery running along its surface. Second, we carefully resected the tumor with cavitron ultrasonic surgical aspirator, and cessation of counting was reproducibly elicited at the superior part of the tumor cavity by subcortical stimulation (Fig. 2B). At the final stage of the awake condition, the patient could perform counting and picture-naming without any problems. The postoperative functional outcome was assessed by the same speech therapist using the same neurological and language examinations as those used preoperatively. The postoperative course was uneventful. A postoperative MRI assessment was performed the day after surgery to evaluate the extent of resection, which revealed gross total resection of the lesion (Fig. 3).
Histopathological examination showed bipolar spindle cells arranged in an angiocentric pattern. Immunohistochemical analysis showed positive results for glial fibrillary acidic protein and S100 and negative results for epithelial membrane antigen, CD34, and isocitrate dehydrogenase 1 R132H. The Ki-67 immunolabelling index value was 2.4% (Fig. 4). No findings suggestive of high-grade glioma, including mitosis, necrosis, and microvascular proliferation, were observed. The final diagnosis was AG, WHO grade I. The patient resumed his normal life and has shown no recurrence without any additional treatment in 2 years.
AGs were first reported in 2005 by two different independent study groups, and were accepted as a newly identified WHO grade-I tumor in the 2007 WHO classification of tumors of the central nervous system [678]. AGs are also recognized among “other gliomas” in the 2021 WHO classification of tumors of the central nervous system [9]. They are rare neoplasms, and to the best of our knowledge, more than 100 cases have been reported till date. They typically present in children and young adults (mean age, 17 years) with intractable partial seizures and equally affect patients of both sexes. Refractory epilepsy is the leading presentation, and is often drug-resistant [10]. The tumors are predominantly supratentorial and often seen superficially, showing a stalk-like extension to the adjacent lateral ventricle.
Surgery for AG is curative when gross total resection is achieved. In some cases with partial resection, the patients died or experienced recurrence [10]. In general, tumor removal is widely performed using motor and somatosensory evoked potentials in brain tumors that require preservation of motor function. Since brain tumors occur in and near the eloquent area, including motor, somatosensory, and language area, awake surgery with intraoperative functional brain mapping is effective to avoid permanent neurologic deficits and preserving brain function [24]. However, awake surgery in adolescents has been poorly reported. Lohkamp et al. [11] reported that awake surgery is beneficial even in pediatric cases in terms of efficient tumor resection as well as preservation of neurological function. The importance of patient motivation and family cooperation for awake surgery in pediatric patients has been reported [12]. A review by O’Leary et al. [13] also reported that although awake surgery in children and adolescents is generally performed without problems, some patients may show postoperative panic disorder, highlighting the importance of preoperative and postoperative psychological assessments. Although the patient in the present study was 15 years old, his understanding of the disease and motivation were very high, and he underwent awake surgery. Two years have passed since the surgery, and he has resumed going to high school without any psychological problems.
Thus, we present a case of successful awake surgery for AG. AG must be considered in the differential diagnosis of pediatric and adult patients presenting with brain tumor-related seizures. Awake surgery to preserve brain function is a treatment option for AGs arising in the eloquent area in adolescents.
Notes
Author Contributions:
Conceptualization: Yuma Yano, Ryosuke Matsuda.
Data curation: Ryosuke Matsuda, Fumi Okada, Maiko Takeda.
Formal analysis: Yuma Yano, Ryosuke Matsuda.
Methodology: Ryosuke Matsuda.
Supervision: Ichiro Nakagawa.
Writing—original draft: Yuma Yano, Ryosuke Matsuda.
Writing—review & editing: all authors.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Han G, Zhang J, Ma Y, Gui Q, Yin S. Clinical characteristics, treatment and prognosis of angiocentric glioma. Oncol Lett. 2020; 20:1641–1648. PMID: 32724405.

2. Fernández Coello A, Moritz-Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg. 2013; 119:1380–1394. PMID: 24053503.

3. Matsuda R, Moritz-Gasser S, Duvaux S, Fernández Coello A, Martinoni M, Duffau H. The persistent crucial role of the left hemisphere for language in left-handers with a left low grade glioma: a stimulation mapping study. Acta Neurochir (Wien). 2014; 156:661–670. discussion 670. PMID: 24452594.

4. Matsuda R, Coello AF, De Benedictis A, Martinoni M, Duffau H. Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: surgical technique and functional results: clinical article. J Neurosurg. 2012; 117:1076–1081. PMID: 23039148.

5. Matsuda R, Tamura K, Nishimura F, Nakagawa I, Motoyama Y. Subcortical calculation mapping during parietal glioma surgery in the dominant hemisphere: a case report. World Neurosurg. 2019; 121:205–210. PMID: 30326305.

6. Wang M, Tihan T, Rojiani AM, Bodhireddy SR, Prayson RA, Iacuone JJ, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol. 2005; 64:875–881. PMID: 16215459.

7. Lellouch-Tubiana A, Boddaert N, Bourgeois M, Fohlen M, Jouvet A, Delalande O, et al. Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol. 2005; 15:281–286. PMID: 16389940.

8. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.

9. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23:1231–1251. PMID: 34185076.

10. Chaudhari JP, Kothari KS, Pandya TP, Goel NA. Angiocentric glioma: report of a rare case presenting with psychosis. Asian J Neurosurg. 2018; 13:1186–1192. PMID: 30459891.

11. Lohkamp LN, Beuriat PA, Desmurget M, Cristofori I, Szathmari A, Huguet L, et al. Awake brain surgery in children-a single-center experience. Childs Nerv Syst. 2020; 36:967–974. PMID: 32055975.

12. Collée E, Satoer D, Wegener Sleeswijk B, Klimek M, Smits M, Van Veelen ML, et al. Language improvement after awake craniotomy in a 12-year-old child: illustrative case. J Neurosurg Case Lessons. 2022; 3:CASE2293. PMID: 35733631.

13. O’Leary KD, Philippopoulos AJ, Koslofsky A, Ahmed Y. How often do awake craniotomies in children and adolescents lead to panic and worry? Childs Nerv Syst. 2023; 08. 23. DOI: 10.1007/s00381-023-06117-6. [Epub].

Fig. 1
Preoperative MRI images. MRI of the brain showing a left frontal lesion on T2-weighted axial and coronal images (A and B), and fluid-attenuated inversion recovery images (C). Enhanced MRI showing a linear contrast effect in the posterior part of the lesion (D: yellow arrow).
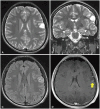
Fig. 2
Intraoperative images. A: Intraoperative photograph of the surface of the exposed operative field. The tumor boundaries identified using ultrasonography and neuro-navigation are marked by alphabets (A–E). The eloquent cortical sites identified using electrical stimulations are marked by numbers as follows: 1: primary motor cortex of the face, and 2: phonemic paraphasia. B: Intraoperative photograph after resection. The eloquent sites of the white matter identified using electrical stimulations are marked by numbers as follows: 47–49: facial dysfunction (pyramidal tract).
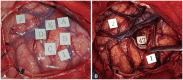
Fig. 3
Postoperative MRI images. T2-weighted axial (A) and coronal (B) images obtained the day after surgery showed gross total removal.

Fig. 4
Histopathological images. Histopathological examination showed that tumor cells were oriented around the capillaries (A and B: hematoxylin-eosin staining, ×200). These cells were positive for glial fibrillary acidic protein (×200) (C), while the Ki-67 immunolabelling index (×200) value was 2.4% (D).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download