This article has been
cited by other articles in ScienceCentral.
Abstract
Differential diagnosis of focal brainstem lesions detected on MRI is challenging, especially in young children. Formerly, brainstem gliomas were classified mainly based on MRI features and location. However, since 2016, the World Health Organization’s brainstem lesion classification requires tissue biopsy to reveal molecular characteristics. Although modern techniques of stereotactic or navigation-guided biopsy ensure accurate biopsy of the lesion with safety, biopsy of brainstem lesions is still generally not performed. Here, we report a focal brainstem lesion mimicking brainstem glioma in a 9-year-old girl. Initial MRI, MR spectroscopy, and 11C-methionine positron emission tomography (PET) features suggested low-grade glioma or diffuse intrinsic pontine glioma. However, repeated MR spectroscopy, perfusion MRI, and 18fluorodeoxyglucose PET findings suggested that it was more likely a non-tumorous lesion. As the patient presented not with a neurological manifestation but with precocious puberty, the attending oncologist chose to observe with regular follow-up MRI. The pontine lesion with high signal intensity on T2-weighted MRI regressed from the 6-month follow-up and became invisible on the 1.5-year follow-up MRI. We reviewed brainstem glioma–mimicking lesions in the literature and discussed the key points of differential diagnosis.
Keywords: Glioma, Brainstem, Diffuse intrinsic pontine glioma, Brainstem tumors, Case reports
INTRODUCTION
Brainstem gliomas, which make up 10%–20% of pediatric central nervous system tumors, are classified into two different types—focal brainstem gliomas and diffuse intrinsic pontine gliomas (DIPGs)—largely based on MRI findings [
1]. Focal brainstem gliomas are usually benign tumors of World Health Organization (WHO) grade I–II, whereas DIPGs are almost always highly malignant and fatal [
123]. The prototypical features of focal brainstem gliomas on brain MRI are well-defined borders, lack of surrounding edema, iso- or hypointensity on T1-weighted images, hyperintensity on T2-weighted images, and homogeneous contrast enhancement. By contrast, DIPGs are infiltrative diffuse pontine tumors, typically presenting with brainstem expansion on imaging with partial contrast enhancement [
4]. Since 2016, the WHO classification of brainstem tumors requires tissue biopsy to reveal molecular characteristics [
5]. In this classification, many DIPGs are newly referred to as diffuse midline glioma (DMG), H3 K27M-mutant. Although modern techniques of stereotactic or navigation-guided biopsy ensure accurate and safe biopsy of the lesion, biopsy of brainstem lesions is still not generally performed due to the possibility of critical side effects [
67].
Most sporadic brainstem gliomas exhibit neurological symptoms and signs, such as isolated cranial nerve deficits, neck stiffness, pain, and contralateral hemiparesis [
3]. However, some of them occur asymptomatically, especially among neurofibromatosis type I patients [
8]. Thus, it is challenging for neurosurgeons or oncologists to perform the biopsy of a brainstem lesion, especially when the patient presents with minimal or no symptoms.
We report a case of a 9-year-old girl with no neurological symptoms who presented with a brainstem glioma–mimicking lesion on MRI, which regressed spontaneously during years of follow-up.
CASE REPORT
A 9-year-old girl presented to a local university hospital for a precocious puberty examination due to low height (25th percentile). She did not have any neurological symptoms. Gonadotropin-releasing hormone (GnRH) stimulation test results were consistent with central precocious puberty. Next, brain MRI was performed. It revealed a 12×9×10-mm well-defined, lobulated, contoured, T1 low-intensity and T2 high-intensity intra-axial lesion with equivocal gadolinium enhancement, diffusion restriction, mild choline (Cho) elevation (Cho/creatine [Cr] ratio of 1.68) with N-acetylasparate (NAA) suppression (Cho/NAA ratio of 0.82), and
11C-methionine positron emission tomography (PET) uptake at the pons, suggesting a tumorous lesion or toxic/metabolic lesion (
Fig. 1).
The patient visited our outpatient clinic for a second opinion. A routine laboratory examination, including a hormone test, revealed no abnormal findings. On physical examination, she had breast budding but did not show any other secondary sexual characteristics. The attending pediatrician and radiation oncologist brought the case to the neuro-oncology board meeting. As the brainstem lesion had no mass effect unlikely to DIPG, brainstem tumor was not a perfect diagnosis explaining the MR findings. Tumor-mimicking demyelinating lesion is one of the possible diagnoses, but no further workup such as cerebrospinal fluid examination was done since parents were reluctant of further exams because she had neither corresponding clinical history nor symptoms and signs. The board members suggested observation and follow-up MR spectroscopy with perfusion MRI as she had no symptoms or signs related to the brainstem lesion.
One and a half months after the initial MRI, MR spectroscopy with perfusion MRI and
18fluorodeoxyglucose (FDG) PET were conducted for further evaluation. T2-weighted MRI showed no definite change in the pontine high-signal lesion, and MR spectroscopy revealed no choline elevation (Cho/Cr ratio of 1.13) or NAA reduction (Cho/NAA ratio of 0.56) (
Fig. 2A and B, respectively). Perfusion MRI showed no definite relative cerebral blood volume increase (
Fig. 2C), and
18FDG PET showed no hypermetabolic lesion (
Fig. 2D).
The attending physician did not start any type of treatment as the patient had no symptoms from the lesion, although it had a small possibility of being tumorous, and chemo-/radiation therapy for brainstem lesions could cause serious adverse events for a child. A leuprolide acetate (a GnRH agonist) injection was done 3 months after the initial MRI.
Follow-up MRI 6 months after the initial study showed a decrease in the extent of the lesion on T2-weighted imaging (
Fig. 3A); on a follow-up MRI 1.5 years after the initial visit, the lesion was almost invisible (
Fig. 3B), and it remained invisible on the most recent follow-up 6.5 years after the initial evaluation (
Fig. 3C).
DISCUSSION
Differential diagnosis of brainstem lesions in children is challenging as pediatric brainstem tumors are among the most common lesions [
9]. DIPG or DMG, one the most common brainstem tumors, shows typical radiographic features on MRI of infiltrating the majority of the pons, hypo/iso-intensity on T1-weighted imaging, hyperintensity on T2-weighted imaging, and, frequently, ventral involvement of the pons with encasement of the basilar artery. By contrast, focal brainstem lesions pose a particular diagnostic concern by including not only tumors but also inflammatory mimics. Fortunately, focal brainstem tumors are usually low-grade gliomas, such as tectal midbrain and cervicomedullary gliomas.
The tumor-like lesions that occur in the brain are diverse and can result from infection, hemorrhage, stroke, demyelination, phakomatoses (neurocutaneous syndromes), malformations, vasculitis, and metabolic disorders [
10]. Among these, demyelinating lesions, including those from acute disseminated encephalomyelitis and multiple sclerosis, are relatively frequent and share some MRI characteristics with tumorous lesions [
11]. However, those diseases can be differentiated by a preceding history of infection, multiple bilaterality of the lesions, or the patient’s age at initial presentation. In some cases, demyelinating disease manifests as a solitary lesion similar to a tumor, called a tumefactive demyelinating lesion. These lesions exhibit decreased cerebral blood volume in perfusion MRI and elevation of Cho, lactate, and even glutamate-glutamine peaks in MR spectroscopy [
12]. They usually appear as hypometabolic lesions in
18FDG and
11C-methionine PET, but some appear as hypermetabolic lesions [
13]. In this case, the lesion had
11C-methionine PET uptake but did not show hyper uptake in
18FDG PET 1.5 months after initial imaging. PET uptake suggests malignancy in many cases, but reports have also shown uptake in tumefactive demyelinating lesions [
13]. The mechanism of uptake differs for
18FDG and
11C-methionine as methionine uptake is increased by elevated methionine metabolism and active methionine transport, whereas FDG uptake is increased by high glycolytic metabolism [
14].
In this case, MR spectroscopy results were different in the initial imaging and follow-up imaging 1.5 months later in our institution. The initial imaging showed mild Cho elevation (Cho/Cr ratio of 1.68) with NAA suppression (Cho/NAA ratio of 0.82), whereas follow-up imaging showed no Cho elevation (Cho/Cr ratio of 1.13) or NAA reduction (Cho/NAA ratio of 0.56). The difference in MR spectroscopy is hard to explain, but we suggest that not only the time interval but also different MR sequences for analysis according to the hospital or device could contribute to the difference.
Since the 2016 WHO central nervous system tumor classification update, molecular characteristics have been included as a crucial factor in tumor classification, and most DIPGs have fallen into the category of DMG with H3 K27M mutations [
5]. In this case, the focal nature of the lesion suggested that it was unlikely to be DIPG or DMG, which is one of the main reasons for the biopsy being deferred.
The optimal treatment for brainstem gliomas is various according to the malignancy grade, the patient’s performance, etc. [
1516]. Currently, radiation followed by chemotherapy could be recommended if malignant brainstem glioma is suspected. However, one study showed that in low-grade tumors, upfront observation alone did not result in inferior overall survival or progression-free survival compared with the chemotherapy or radiation therapy group [
17].
The limitations of our case are as follows. First, no further workup, such as a cerebrospinal fluid exam or examination of serum markers related to demyelinating diseases, was performed. Second, no biopsy was performed; thus, the final diagnosis remains unknown.
Upon the experience of this case, we cordially suggest that if children is asymptomatic with tumor-like brainstem lesion on MRI, upfront observation should be considered primarily.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Green AL, Kieran MW. Pediatric brainstem gliomas: new understanding leads to potential new treatments for two very different tumors. Curr Oncol Rep. 2015; 17:436. PMID:
25702179.

2. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020; 22(12 Suppl 2):iv1–iv96. PMID:
33123732.

3. Ozair A, Khan E, Bhat V, Faruqi A, Nanda A. Pediatric brain tumors: from modern classification system to current principles of management. Turner SG, editor. Central nervous system tumors. London: IntechOpen;2021. p. 9–36.
4. Leach JL, Roebker J, Schafer A, Baugh J, Chaney B, Fuller C, et al. MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the international DIPG registry. Neuro Oncol. 2020; 22:1647–1657. PMID:
32506137.

5. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID:
27157931.

6. Pincus DW, Richter EO, Yachnis AT, Bennett J, Bhatti MT, Smith A. Brainstem stereotactic biopsy sampling in children. J Neurosurg. 2006; 104(2 Suppl):108–114. PMID:
16506498.

7. Pérez-Gómez JL, Rodríguez-Alvarez CA, Marhx-Bracho A, Rueda-Franco F. Stereotactic biopsy for brainstem tumors in pediatric patients. Childs Nerv Syst. 2010; 26:29–34. PMID:
19784659.
8. Mahdi J, Shah AC, Sato A, Morris SM, McKinstry RC, Listernick R, et al. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology. 2017; 88:1584–1589. PMID:
28330960.

9. Culleton S, McKenna B, Dixon L, Taranath A, Oztekin O, Prasad C, et al. Imaging pitfalls in paediatric posterior fossa neoplastic and non-neoplastic lesions. Clin Radiol. 2021; 76:391.e19–391.e31.

10. Huisman TA. Tumor-like lesions of the brain. Cancer Imaging. 2009; 9:S10–S13. PMID:
19965288.

11. Go JL, Acharya J, Rajamohan AG. Is it or is it not? Brain tumor mimics. Semin Roentgenol. 2018; 53:62–76. PMID:
29405957.

12. Cianfoni A, Niku S, Imbesi SG. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am J Neuroradiol. 2007; 28:272–277. PMID:
17296993.
13. Tomura N, Saginoya T, Kaneko C. 18F-fluorodeoxy glucose and 11C-methionine accumulation in demyelinating lesions. World J Nucl Med. 2022; 21:261–266. PMID:
36398309.

14. Kawase Y, Yamamoto Y, Kameyama R, Kawai N, Kudomi N, Nishiyama Y. Comparison of 11C-methionine PET and 18F-FDG PET in patients with primary central nervous system lymphoma. Mol Imaging Biol. 2011; 13:1284–1289. PMID:
21042866.

15. Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009; 3:259–269. PMID:
19338403.

16. Gwak HS, Park HJ. Developing chemotherapy for diffuse pontine intrinsic gliomas (DIPG). Crit Rev Oncol Hematol. 2017; 120:111–119. PMID:
29198324.
17. Fried I, Hawkins C, Scheinemann K, Tsangaris E, Hesselson L, Bartels U, et al. Favorable outcome with conservative treatment for children with low grade brainstem tumors. Pediatr Blood Cancer. 2012; 58:556–560. PMID:
21618421.

Fig. 1
Initial MRI revealed an intra-axial T1 low-intensity (A) and T2 high-intensity (B) lesion with no enhancing lesion (C) at the upper pons. Diffusion-weighted imaging (D), magnetic resonance spectroscopy (E), and 11C-methionine positron emission tomography (F) findings showed a suspicion of glioma.
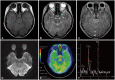
Fig. 2
Repeated neuroimaging study 1.5 months after the initial examination revealed no definite change in the T2 high-intensity lesion (A), neither an elevated choline peak nor a suppressed N-acetylaspartate peak on magnetic resonance spectroscopy (B), no hyperperfusion on perfusion MRI (C), and no hyper uptake on 18fluorodeoxyglucose positron emission tomography compared with normal brain parenchyma (D).
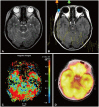
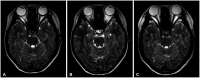
Fig. 3
Gradual regression of the T2 high-intensity lesion was noticed on the 6-month follow-up MRI (A). The lesion became invisible on the 1.5-year follow-up MRI (B) and remained regressed on the recent 6.5-year follow-up MRI (C).






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download