Abstract
This review provides an overview of the current state of pediatric brain tumor imaging, emphasizing the role of various imaging sequences and highlighting the advantages of standardizing protocols for pediatric brain tumor imaging in diagnosis and treatment response evaluation. Basic anatomical sequences such as pre- and post-contrast 3D T1-weighted, T2-weighted, fluid-attenuated inversion recovery, T2*-weighted, and diffusion-weighted imaging (DWI), are fundamental for assessing tumor location, extent, and characteristics. Advanced techniques like DWI, diffusion tensor imaging, perfusion imaging, magnetic resonance spectroscopy, and functional MRI offer insights into cellularity, vascularity, metabolism, and function. To enhance consistency and quality, standardized protocols for pediatric brain tumor imaging have been recommended by expert groups. Special considerations for pediatric patients, including the minimization of anesthesia exposure and gadolinium contrast agent usage, are essential to ensure patient safety and comfort. Staying up-to-date with diagnostic imaging techniques can contribute to improved communication, outcomes, and patient care in the field of pediatric neurooncology.
Brain tumors are the most common solid tumors in pediatric patients, and radiological examinations play a fundamental role in diagnosing and evaluating treatment response in pediatric brain tumor patients. MRI is primarily used in evaluation of pediatric brain tumors because it provides excellent soft tissue contrast. The role of basic anatomical MR imaging includes determining the tumor location, extent of tumor involvement, assessment of tumor burden, presurgical planning, monitoring treatment response, and detecting post-surgical or post-treatment changes. In addition to basic anatomical imaging, advanced imaging techniques such as diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), perfusion imaging, magnetic resonance spectroscopy (MRS), and functional MRI (fMRI) offer valuable insights into cellularity, diffusivity, vascular characteristics, metabolic activity, and functional aspects. This review aims to provide an overview of basics of pediatric tumor imaging. The published guidelines for pediatric brain tumor imaging will be reviewed and recommended basic MR imaging sequences will be presented. Additionally, advanced imaging techniques which can be performed will be discussed.
CT serves a vital role in the emergency setting and may be the initial modality for imaging pediatric brain tumor due to its speed and availability. CT can identify the presence and location of the mass, as well as secondary changes, including obstructive hydrocephalus. Also, some specific features like solid and cystic components, cellularity, hemorrhage, and calcifications can be demonstrated on CT. However, when there is suspicion of a brain tumor based on CT, MRI should be performed afterward, since MRI provides more comprehensive information about tumor characteristics. You can use either a 1.5-T or a 3.0-T scanner, but the 3.0-T scanner offers improved signal-to-noise ratio and enhanced speed.
In adult brain tumor cases, the Response Assessment in Neuro-Oncology (RANO) working group has developed imaging guidelines that are in use. However, applying RANO criteria directly to pediatric cases is challenging due to several specific differences. Pediatric brain tumors are more commonly low-grade compared to adults, and contrast enhancement may be less pronounced or irregular. Moreover, the volume of the tumor may remain unchanged while the degree of contrast enhancement varies, limiting the application of the 2D measurement methods based on RANO criteria. Efforts have been made to address these challenges, leading to the publication of recommendations by the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group in 2018 for medulloblastoma and leptomeningeal seeding tumor [1]. Subsequent recommendations have been published for pediatric low-grade glioma [2], pediatric high-grade glioma [3], diffuse intrinsic pontine glioma in 2020 [4], pediatric intracranial ependymoma in 2022 [5], and pediatric craniopharyngioma in 2023 [6], all of which include imaging guidelines. However, MRI protocols for brain tumor imaging can vary among institutions, depending on variable factors, including the patient’s status, the specific tumor type, and the MRI system being used. These variations can result in differences in image quality, making it challenging to compare results and interpret findings consistently. Standardizing MRI protocols for the evaluation of central nervous system (CNS) tumors can offer significant advantages. It enhances the consistency and quality of oncologic evaluations, promotes uniformity in clinical trials, and facilitates comparisons of treatment outcomes among institutions. By ensuring that all patients receive the same high-quality imaging evaluation, standardized protocols contribute to improved patient care and the advancement of brain tumor research and treatment. To address this issue, in November 2022, the Children’s Oncology Group (COG) Diagnostic Imaging Committee, the Society for Pediatric Radiology (SPR) Oncology Committee, and the American Society of Pediatric Neuroradiology (ASPNR) jointly published a Brain Tumor Imaging White Paper [7]. This document synthesizes previously published literature, RAPNO imaging guidelines, and expert opinions to standardize pediatric brain tumor imaging. The white paper includes minimal recommendations and optional imaging sequences that can be implemented on most MRI systems and are suitable for both high and low-grade tumors.
In general, the minimal recommended sequences for a pediatric brain tumor assessment include pre- and post-contrast 3D T1-weighted (3D T1), T2-weighted (T2), fluid-attenuated inversion recovery (FLAIR), hemorrhage sensitive sequence such as susceptibility-weighted imaging (SWI) and DWI.
The T1 sequence is a basic anatomic imaging sequence. Ideally, it is acquired in 3D format, but 2D sequence can be used when 3D is not available. Certain substances such as fat, blood products, and mineralization appear T1 hyperintense and can aid in tissue characterization (Fig. 1). One important consideration in the pediatric brain tumor imaging is to always look for the posterior pituitary gland on T1-weighted imaging which is due to vasopressin storage. The absence of the expected T1 bright signal of the posterior pituitary, even without thickening of the pituitary stalk, can indicate infiltration by a tumor, especially germinomas and Langerhans cell histiocytosis [8]. Therefore, it must be followed up with imaging (Fig. 2).
The T2 sequence is also an anatomic imaging sequence sensitive to the detection of tumors, evaluation of tumor margin, and measurements. The T2 sequence also reflects tumor cellularity, presence of cystic changes, necrosis, and areas of edema (Fig. 3). T2-FLAIR sequence is sequence in which T2 imaging is combined FLAIR technique to suppress the signal from fluid such as cerebrospinal fluid (CSF). This suppression causes the CSF to appear dark on FLAIR images, enhancing the visibility of brain lesions especially those near the areas containing CSF. In IDH-mutated, 1p/19q non-codeleted low-grade gliomas in adults, a distinct imaging pattern is observed on T2-FLAIR sequences [9]. These tumors appear homogeneously hyperintense on T2 images but relatively hypointense on FLAIR images, except for a peripheral hyperintense rim which is referred to as “T2-FLAIR mismatch sign” (Fig. 4). In pediatrics, the “T2-FLAIR mismatch sign” may be observed in other tumors including DNET and astrocytoma but absent in KIAA1549-BRAF–fused and BRAF p.V600E–mutated pediatric low-grade gliomas [10].
Gadolinium contrast agents are commonly used for diagnosis and assess treatment response in wide range of CNS disorders including stroke, demyelination, infection, and tumors. When gadolinium-based contrast media is administered intravenously, contrast media passes through the cerebral circulation. In normal circumstances, the blood-brain barrier (BBB) prevents leaking into surrounding tissues but when the BBB is disrupted contrast media extravasates from the blood vessels into the interstitial space. This leads to increased signal intensity in the area of BBB disruption referred to as “contrast enhancement” [11]. In the case of diffuse gliomas, there is generally a direct relationship between the extent of contrast enhancement on imaging and the grade of the tumor. However, in children, low-grade gliomas are more frequent accounting for approximately 40% consisting of diverse groups of tumors. And some low-grade tumors may show avid enhancement and some high-grade tumors show no or minimal enhancement on post-contrast T1 sequence (Fig. 5). And to further complicate, enhancement can change, even in the absence of treatment and without variation in the volume of the tumors (Fig. 6). Contrast enhancement can also be affected by technical factors including difference in imaging techniques or timing of imaging after administration of gadolinium [12], which can lead to misinterpretation of enhancement on interval imaging.
When performing contrast-enhanced images, it is necessary to perform a 3D acquisition with reconstructions in all three anatomical planes to reduce the risk of missing small lesions (Fig. 7). Typically, images are acquired in the “sagittal” plane and then reconstructed into both axial and coronal planes. Post-contrast T2-FLAIR sequence is sensitive for detecting meningeal pathologies including leptomeningeal metastasis and 3D is preferred over 2D if available (Fig. 8).
SWI is highly sensitive for magnetic susceptibility effect caused by blood products or mineralization. It allows detection of hemorrhage, vascular structures and identification of calcium within tumors which can aid in tumor grading. Calcium deposits and blood vessels exhibit different phase patterns due to their differing magnetic susceptibilities allowing differentiation between calcium and blood possible (Fig. 9).
DWI maps the motion of water molecules in tissue with generated apparent diffusion coefficient (ADC) map. In general, tumor cellularity increases with tumor proliferation resulting in restricted diffusivity of water molecules [13]. When water diffusion is restricted, it results in low ADC values, which can be indicative of highly cellular tumor, whereas in low-grade tumors, ADC values are higher (Fig. 10). It also helps differential diagnosis with tumor like lesions, with epidermoid cyst or abscesses which show restricted diffusion.
Additional MRI sequences include post-contrast 3D T1 or 3D T2 sequences for neurosurgical navigation, and advanced MRI sequence, including DTI for surgical guidance, 3D heavily T2-weighted sequences, task-based fMRI, and MRS [7]. One thing to note is that MR perfusion and MRS are useful when performed both at baseline and follow-up since a one-point-in-time assessment of perfusion and MRS can be misleading and cannot aid in problem-solving.
Post-contrast 3D T1 or 3D T2 sequence for surgical navigation or stereotactic radiosurgery planning can be obtained with markers or a head frame. Obtaining these images during the initial imaging session eliminates the need for additional imaging sessions, anesthesia, and contrast agent administration, critical for pediatric patients.
The use of 3D heavily T2-weighted sequences has been growing including tumor imaging [14]. These sequences enable the identification of small non-enhancing residual tumors and leptomeningeal metastases due to their high resolution and assist in distinguishing between intra-axial and extra-axial tumors by allowing visualization of CSF cleft (Fig. 11). Additionally, 3D heavily T2-weighted sequence gives guidance to endoscopic surgery and allows visualization of surgical defects, membranes, or adhesions.
DTI is an advanced technique of diffusion imaging that provides insights into diffusion within tissues. DTI combines information about the rate and direction of water molecule movement. Commonly used metrics are mean diffusivity and fractional anisotropy. Tumors in the brain have the potential to infiltrate, destroy, or displace the surrounding white matter tracts, which are critical for various brain functions. DTI can provide critical information for presurgical planning and neuronavigation (Fig. 12).
fMRI is a technique that indirectly measures neuronal activity in the brain by assessing changes in blood oxygenation and blood flow in response to neural activity referred to as blood oxygen level-dependent (BOLD) signal difference. In fMRI, when a brain region is more active, it consumes more oxygen leading to increase in the blood flow resulting in increased BOLD signal in that region. This allows noninvasive spatial mapping of eloquent cortex during the performance of specific tasks, therefore helpful for achieving effective tumor resection while minimizing post-surgical neurological deficits.
Angiogenesis is a key factor for tumor cell proliferation, resulting in abnormally large, tortuous, highly permeable vessels [11]. Perfusion is a marker of angiogenesis and aids in understanding tumor characteristics. The most widely used method is dynamic susceptibility contrast perfusion (DSC), based on the detection of signal loss on T2*-weighted sequence with the gadolinium contrast injection. This allows derivation of relative cerebral blood volume (rCBV) and other perfusion parameters. Elevated rCBV is observed in tumors and indicative of angiogenesis and has been positively correlated to glioma grade (Fig. 13) [15]. Arterial spin labeling (ASL), on the other hand, does not require IV injection because arterial blood water is used as an endogenous tracer to evaluate cerebral blood flow. ASL offers the advantage of 3D whole brain coverage and is particularly promising in pediatric patients due to its non-invasive nature, obviating the need for IV, large bore needle, and contrast agent use. However, ASL is limited by a relatively low signal-to-noise ratio. It is crucial to consider the perfusion trends and make comparisons with baseline and previous exams when interpreting perfusion imaging. Isolated assessment of perfusion can be misleading.
MRS is a non-invasive technique to provide information on specific metabolites within the brain (Table 1). MRS can be obtained using a single-voxel technique or multivoxel technique. In general, MRS of brain tumors demonstrate increased choline (Cho) and decreased N-acetylaspartate (NAA) levels (Fig. 14), reflecting increased cellular turnover and the loss of normal neuronal markers [16]. When analyzing MRS, ratios are used rather than the absolute heights of individual metabolite peaks.
In Table 2, we have summarized key MRI sequences for pediatric brain tumor imaging.
Both treatment-related changes and tumor progression can present similarly on standard imaging, showing enlargement and new contrast enhancement, which poses a diagnostic challenge. Recurrent tumors are characterized by increased vascular proliferation, cellularity, and neoangiogenesis, while radiation necrosis is characterized by necrosis, an inflammatory response, and vascular endothelial proliferation, leading to enhanced BBB permeability and increased contrast enhancement and edema. Parameters such as rCBV, Cho/Cr, and Cho/NAA ratios are higher, and ADC is decreased in recurrent tumors compared to radiation necrosis [1617]. Multiparametric MRI may enhance diagnostic accuracy in differentiating tumor progression and from treatment-related change [18].
The optimal timing for MRI evaluation to assess residual tumor after surgery is typically within the first 72 hours following the surgical procedure. This initial post-surgery MRI helps in identifying any remaining tumor tissue and evaluating the extent of resection, but in some cases, post-surgical changes can make it difficult to distinguish residual tumor from these changes. In such situations, a second MRI should be done 2–3 weeks after the surgery to allow some of the acute postoperative changes to resolve.
Assessment of spinal leptomeningeal metastasis is integral to pediatric brain tumor staging, along with brain imaging for certain brain tumors to assess for CNS dissemination. When imaging the pediatric spine, one of the major challenges is dealing with CSF flow-related artifacts, particularly when evaluating conditions like leptomeningeal metastases. Utilizing 3D sequences can offer better control over CSF pulsation artifacts. The use of DWI can significantly enhances sensitivity when detecting leptomeningeal metastasis, especially in tumors with high cellularity, such as embryonal tumors and germ cell tumors. Also, use of 3D myelographic T2-weighted images enable detection of small metastasis which might be missed on enhanced T1 mages (Fig. 15) [1].
The U.S. Food and Drug Administration warning issued in December 2016 regarding the use of repeated and lengthy general anesthesia and sedation in children under the age of 3 years highlighted concerns about potential neurotoxicity [19]. Minimizing the frequency and duration of anesthesia or sedation procedures is in line with these safety considerations. Pediatric patients may find undergoing anesthesia or sedation multiple times distressing. By completing both brain and spine imaging in a single session, you reduce the need for repeated anesthesia, making the overall experience less challenging for the child and their family.
Imaging of the spine can be performed immediately following brain MRI. Enhanced T1 acquired first, as necessary, followed by T2. This approach reduces the frequency of sedation and the dose of anesthetic agent, as well as minimizes the use of gadolinium, even for non-sedated children. Combining brain and spine imaging during the same imaging session is more efficient, as it minimizes the need for multiple anesthesia or sedation sessions for pediatric patients. This approach can significantly reduce the frequency of sedation and the dose of anesthetic agents required, which is particularly important for the safety and comfort of young children.
Gadolinium-based contrast agents are commonly used to enhance the visibility of certain structures during MRI scans. However, concerns about the potential risks associated with repeated gadolinium exposure, particularly in children, have arisen. By acquiring both brain and spine imaging together during a single session, you can avoid the need for additional gadolinium administration for spinal imaging, reducing the cumulative exposure to these contrast agents.
The current standard clinical practice for assessing tumor response involves using 2D or 3D measurements on the single cross section that best shows tumor extent. However, these measurements are prone to interrater variability, especially for tumors that are diffuse or irregular in shape [20]. In a recent study comparing volumetric and 2D tumor assessments, inconsistent responses were noted in 20% of 70 pediatric low-grade gliomas [21]. There is ongoing research into alternative methods for evaluating treatment response including targeted therapy, with volumetric assessment showing promise [22]. Further research is required to determine its clinical usefulness for various tumor types and to establish methods for integration into routine clinical practice.
The evolving landscape of pediatric brain tumors underscores the importance of staying up to date with the latest research and diagnostic techniques. Standardizing neuroimaging protocols helps ensure consistent and high-quality care for pediatric patients, especially those who may receive care from multiple institutions. Utilizing additional advanced imaging techniques with combining multiple parameters may enhance diagnostic accuracy. Optimizing imaging sessions to minimize sedation/anesthesia and contrast agent exposure can contribute to improved patient outcomes and safety.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
1. Warren KE, Vezina G, Poussaint TY, Warmuth-Metz M, Chamberlain MC, Packer RJ, et al. Response assessment in medulloblastoma and leptomeningeal seeding tumors: recommendations from the Response Assessment in Pediatric Neuro-Oncology Committee. Neuro Oncol. 2018; 20:13–23. PMID: 28449033.

2. Fangusaro J, Witt O, Hernáiz Driever P, Bag AK, de Blank P, Kadom N, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2020; 21:e305–e316. PMID: 32502457.

3. Erker C, Tamrazi B, Poussaint TY, Mueller S, Mata-Mbemba D, Franceschi E, et al. Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2020; 21:e317–e329. PMID: 32502458.

4. Cooney TM, Cohen KJ, Guimaraes CV, Dhall G, Leach J, Massimino M, et al. Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2020; 21:e330–e336. PMID: 32502459.

5. Lindsay HB, Massimino M, Avula S, Stivaros S, Grundy R, Metrock K, et al. Response assessment in paediatric intracranial ependymoma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2022; 23:e393–e401. PMID: 35901835.

6. Hoffman LM, Jaimes C, Mankad K, Mirsky DM, Tamrazi B, Tinkle CL, et al. Response assessment in pediatric craniopharyngioma: recommendations from the response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Neuro Oncol. 2023; 25:224–233. PMID: 36124689.

7. Jaju A, Li Y, Dahmoush H, Gottardo NG, Laughlin S, Mirsky D, et al. Imaging of pediatric brain tumors: a COG diagnostic imaging committee/SPR oncology committee/ASPNR white paper. Pediatr Blood Cancer. 2023; 70(Suppl 4):e30147. PMID: 36519599.
8. Kim DY, Kim PH, Jung AY, Choi JH, Cho YA, Yoon HM, et al. Neoplastic etiology and natural course of pituitary stalk thickening. J Clin Endocrinol Metab. 2022; 107:563–574. PMID: 34614160.

9. Do YA, Cho SJ, Choi BS, Baik SH, Bae YJ, Sunwoo L, et al. Predictive accuracy of T2-FLAIR mismatch sign for the IDH-mutant, 1p/19q noncodeleted low-grade glioma: an updated systematic review and meta-analysis. Neurooncol Adv. 2022; 4:vdac010. PMID: 35198981.

10. Wagner MW, Nobre L, Namdar K, Khalvati F, Tabori U, Hawkins C, et al. T2-FLAIR mismatch sign in pediatric low-grade glioma. AJNR Am J Neuroradiol. 2023; 44:841–845. PMID: 37348970.

11. Farnsworth RH, Lackmann M, Achen MG, Stacker SA. Vascular remodeling in cancer. Oncogene. 2014; 33:3496–3505. PMID: 23912450.

12. D’Arco F, Culleton S, De Cocker LJL, Mankad K, Davila J, Tamrazi B. Current concepts in radiologic assessment of pediatric brain tumors during treatment, part 1. Pediatr Radiol. 2018; 48:1833–1843. PMID: 29980859.

13. Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017; 81:397–415. PMID: 28486641.

14. Cavallaro M, Coglitore A, Tessitore A, Galletta K, Frosina L, Cuffari A, et al. Three-dimensional constructive interference in steady state (3D CISS) imaging and clinical applications in brain pathology. Biomedicines. 2022; 10:2997. PMID: 36428564.

15. Pollice S, Capuano M, Scarabino T. Magnetic resonance technique. Scarabino T, Pollice S, editors. Imaging gliomas after treatment: a case-based atlas. Cham: Springer;2020. p. 41–45.
16. Tamrazi B, Mankad K, Nelson M, D’Arco F. Current concepts and challenges in the radiologic assessment of brain tumors in children: part 2. Pediatr Radiol. 2018; 48:1844–1860. PMID: 30215111.

17. Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One. 2016; 11:e0141438. PMID: 26741961.

18. Nael K, Bauer AH, Hormigo A, Lemole M, Germano IM, Puig J, et al. Multiparametric MRI for differentiation of radiation necrosis from recurrent tumor in patients with treated glioblastoma. AJR Am J Roentgenol. 2018; 210:18–23. PMID: 28952810.

19. Artunduaga M, Liu CA, Morin CE, Serai SD, Udayasankar U, Greer MC, et al. Safety challenges related to the use of sedation and general anesthesia in pediatric patients undergoing magnetic resonance imaging examinations. Pediatr Radiol. 2021; 51:724–735. PMID: 33860861.

20. Tsai JW, Choi JJ, Ouaalam H, Murillo EA, Yeo KK, Vogelzang J, et al. Integrated response analysis of pediatric low-grade gliomas during and after targeted therapy treatment. Neurooncol Adv. 2023; 5:vdac182. PMID: 36926246.

21. D’Arco F, O’Hare P, Dashti F, Lassaletta A, Loka T, Tabori U, et al. Volumetric assessment of tumor size changes in pediatric low-grade gliomas: feasibility and comparison with linear measurements. Neuroradiology. 2018; 60:427–436. PMID: 29383433.

22. Ramakrishnan D, von Reppert M, Krycia M, Sala M, Mueller S, Aneja S, et al. Evolution and implementation of radiographic response criteria in neuro-oncology. Neurooncol Adv. 2023; 5:vdad118. PMID: 37860269.

Fig. 1
Variable T1 hyperintensity in brain tumors. A: A newborn’s cranial ultrasound reveals a hyperechoic lesion in the superior vermis (arrow). B: Sagittal T1-weighted image displays a T1 hyperintense lesion (arrow), corresponding to the ultrasound demonstrated hyperechoic lesion, indicative of a lipoma. C: Axial T1-weighted image of a pineal mixed germ cell tumor shows multifocal T1 hyperintensities attributed to its fat components (arrows).
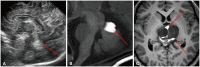
Fig. 2
Loss of expected T1 bright signal in germinoma. A: Unenhanced sagittal T1-weighted image shows the expected T1 bright signal of the posterior pituitary gland (arrow) due to vasopressin storage. B: Unenhanced sagittal T1-weighted image from a 13-year-old boy with diabetes insipidus shows the absence of the T1 bright signal of the posterior pituitary gland (arrow). C: Post-contrast sagittal T1-weighted image exhibits equivocal thickening of the pituitary stalk (arrow). D: A subsequent image 15 months later after the patient was lost to follow-up displays multifocal masses in the suprasellar and pineal regions with extensive ventricular seeding.

Fig. 3
Variable T2-weighted signal in brain tumors. A: Axial T2-weighted image shows a 4th ventricle mass extending through bilateral foramen Luschka (arrows), confirmed as posterior fossa ependymoma. B: Coronal T2-weighted image shows a heterogeneous, T2 hypointense mass in the right cerebellum causing a mass effect and edema, confirmed as medulloblastoma. C: Axial T2-weighted image depicts a T2 hypointense, hemorrhagic mass in the left parietal lobe accompanied by perilesional edema, confirmed as glioblastoma. D: Axial T2-weighted image reveals a large cystic and solid mass in the left cerebral hemisphere, diagnosed as supratentorial ependymoma.
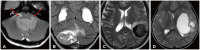
Fig. 4
T2-fluid-attenuated inversion recovery (FLAIR) mismatch in IDH-mutant, 1p/19q non-codeleted astrocytoma. A: Axial T2-weighted image shows a well-demarcated T2 hyperintense mass in the right frontal lobe. B: Axial FLAIR image shows a FLAIR hypointense mass with a surrounding hyperintense rim, representing the “T2-FLAIR mismatch” sign.

Fig. 5
Variable enhancement patterns of pediatric brain tumors. A: Axial enhanced T1-weighted image shows a right thalamic mass with some enhancement, confirmed as pilocytic astrocytoma. B: Axial enhanced T1-weighted image depicts a well-enhancing heterogeneous mass involving the optic pathway, confirmed as pilocytic astrocytoma. C: Axial enhanced T1-weighted image illustrates a solid mass in the right thalamus causing a mass effect and compression of the 3rd ventricle leading to obstructive hydrocephalus. A small internal enhancing focus is discernible, diagnosed as glioblastoma. D: Axial enhanced T1-weighted image demonstrated expansile mass centered in the pons (arrow) with minimal enhancement, diagnosed as diffuse midline glioma, H3K27M altered.

Fig. 6
Optic pathway glioma. A: Axial fluid-attenuated inversion recovery enhanced image displays tortuous thickening of the intraorbital segment of the left optic nerve (arrow) in a neurofibromatosis type 1 patient, suggestive of an optic pathway glioma. B: Enhanced axial T1-weighted image shows diffuse enhancement of the enlarged left optic nerve (arrow). C: Follow-up MRI without treatment shows mildly reduced thickening of the left optic nerve and the disappearance of enhancement (arrow).
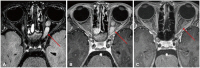
Fig. 7
Enhanced 3D T1-weighted imaging in brain tumors. A: Enhanced 3D sagittal T1-weighted image shows a large posterior fossa mass with some enhancing components. Another enhancing mass in the suprasellar cistern (arrow) is noted due to cerebrospinal fluid dissemination. B: Enhanced 3D sagittal T1-weighted image in a medulloblastoma show a small enhancing nodule (arrow) in superior cerebellum. C: The presence of a small enhancing nodule (arrow) on the enhanced 3D axial T1-weighted image corresponding to the nodule depicted on sagittal image confirms leptomeningeal seeding.
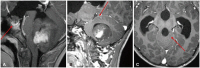
Fig. 8
Utility of post-contrast 3D fluid-attenuated inversion recovery (FLAIR). A: Enhanced axial 3D T1 image displays a posterior fossa mass with central enhancement. B: Enhanced axial 3D FLAIR image offers a better depiction of the large posterior fossa mass with multiple tiny FLAIR-enhancing nodules along the cerebellar folia. C: Enhanced axial T1 image of a patient post-ependymoma resection does not show discrete enhancing lesions in the surgical bed. D: Enhanced axial 3D FLAIR image better demonstrates FLAIR hyperintense nodules (arrows), suggesting tumor recurrence.
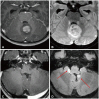
Fig. 9
Susceptibility-weighted imaging (SWI) in medulloblastoma. A: Axial T2-weighted image shows a well-defined mass in the 4th ventricle with multiple internal T2 hypointense foci. B: Axial SWI image shows multiple susceptibility foci corresponding to the T2 hypointense foci. C: Axial phase image shows several hyperintense foci, suggesting calcifications rather than hemorrhages. D: Axial non-enhanced CT images display multiple hyperdense foci, indicating calcifications within the 4th ventricle mass.

Fig. 10
Diffusion-weighted imaging in brain tumors. A: Axial apparent diffusion coefficient (ADC) image shows a reduced ADC value in medulloblastoma (arrow). B: Axial ADC image presents an intermediate ADC value in the posterior fossa ependymoma (arrow). C: Axial ADC image illustrates a high ADC value within the solid component of a posterior fossa cystic mass (arrow), confirmed as pilocytic astrocytoma.
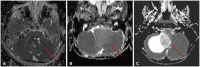
Fig. 11
Utility of 3D heavily T2-weighted sequences. A: Axial T2 preoperative image of posterior fossa ependyma shows a 4th ventricle mass extending to the bilateral foramina of Luschka. B and C: Axial heavily T2-weighted images demonstrate multiple nodular lesions along the right trigeminal nerve (arrow) and right cerebellopontine cistern (dotted arrow) which were less apparent on conventional sequences.
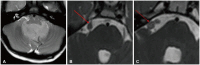
Fig. 12
Diffusion tensor imaging (DTI) in thalamic glioma. A: Axial T2 image depicts a T2 hyperintense mass in the right thalamus (arrow). B: DTI image shows the corticospinal tract (dotted arrow) traversing posteriorly to the right thalamic tumor but within a 2 cm distance.
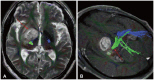
Fig. 13
Perfusion characteristics in brain tumors. A: Enhanced axial T1-weighted image offers a view of a large cystic mass in the posterior fossa with a peripherally strong enhancing nodule (arrow), suggestive of hemangioblastoma. B: Cerebral blood volume (CBV) map from dynamic susceptibility contrast perfusion (DSC) perfusion reveals elevated relative CBV in the solid enhancing nodule (arrow). C: Enhanced axial T1-weighted image illustrates a peripheral rim-enhancing lesion in the left frontal lobe (dotted arrow) with accompanying perilesional edema in a patient who had been resected for a diffuse hemispheric glioma, H3 G34-mutant, WHO grade 4. D: CBV map from DSC perfusion shows elevated relative CBV (dotted arrow), suggesting a tumor. This was surgically confirmed as a recurrence of the diffuse hemispheric glioma, H3 G34-mutant, WHO grade 4.
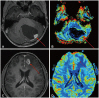
Fig. 14
Magnetic resonance spectroscopy (MRS) in medulloblastoma. A: Axial T2-weighted image shows a large mass in the 4th ventricle with obstructive hydrocephalus. B: The axial apparent diffusion coefficient (ADC) map displays low ADC values, suggesting a highly cellular tumor. C: MRS reveals elevated choline (arrow), decreased NAA (dotted arrow), an elevated Cho/Cr ratio, and elevated lipid peaks. NAA, N-acetylaspartate; Cho, choline; Cr, creatine.
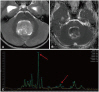
Fig. 15
3D myelographic T2-weighted imaging of the spine in ependymoma. A and B: Sagittal 3D myelographic T2-weighted images show multifocal tiny T2 hypointense seeding nodules (arrows). C-F. Reconstructed axial T2-weighted images show multifocal tiny T2 hypointense seeding nodules (arrows) corresponding to the sagittal images.
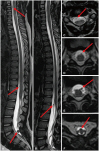
Table 1
Major metabolites on magnetic resonance spectroscopy
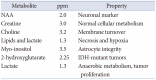
Table 2
Useful MRI sequences for pediatric brain tumor imaging
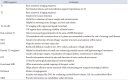
FLAIR, fluid-attenuated inversion recovery; CSF, cerebrospinal fluid; BBB, blood-brain barrier; SWI, susceptibility-weighted imaging; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient; DTI, diffusion tensor imaging; MRS, magnetic resonance spectroscopy; DSC, dynamic susceptibility contrast perfusion; ASL, arterial spin labeling




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download