Abstract
Objective
The Act on Life-Sustaining Treatment (LST) decisions for end-of-life patients has been effective since February 2018. An increasing number of patients and their families want to withhold or withdraw from LST when medical futility is expected. This study aimed to investigate the status of the Act on LST decisions for patients with acute cerebrovascular disease at a single hospital.
Methods
Between January 2017 and December 2021, 227 patients with acute cerebrovascular diseases, including hemorrhagic stroke (n=184) and ischemic stroke (n=43), died at the hospital. The study period was divided into the periods before and after the Act.
Results
The duration of hospitalization decreased after the Act was implemented compared to before (15.9±16.1 vs. 11.2±18.6 days, p=0.127). The rate of obtaining consent for the LST plan tended to increase after the Act (139/183 [76.0%] vs. 27/44 [61.4%], p=0.077). Notably, none of the patients made an LST decision independently. Ventilator withdrawal was more frequently performed after the Act than before (52/183 [28.4%] vs. 0/44 [0%], p<0.001). Conversely, the rate of organ donation decreased after the Act was implemented (5/183 [2.7%] vs. 6/44 [13.6%], p=0.008). Refusal to undergo surgery was more common after the Act was implemented than before (87/149 [58.4%] vs. 15/41 [36.6%], p=0.021) among the 190 patients who required surgery.
Conclusion
After the Act on LST decisions was implemented, the rate of LST withdrawal increased in patients with acute cerebrovascular disease. However, the decision to withdraw LST was made by the patient’s family rather than the patient themselves. After the execution of the Act, we also observed an increased rate of refusal to undergo surgery and a decreased rate of organ donation. The Act on LST decisions may reduce unnecessary treatments that prolong end-of-life processes without a curative effect. However, the widespread application of this law may also reduce beneficial treatments and contribute to a decline in organ donation.
Human dignity must be respected and protected by the Constitution. Humans want to die with dignity at the end of their lives and be respected for their right to decide about death [20,26,47,48]. Life-sustaining treatment (LST), which prolongs the dying process without curative effects, has been discussed for several decades in South Korea [20,26,27,48].
Since the “Boramae Hospital incident” in 1997, discussion on withdrawing LST had not been actively conducted [25,26]. In the Boramae Hospital incident, the Supreme Court convicted the doctors for aiding and abetting a homicide because they ordered the discharge of a patient in a vegetative state after a traumatic brain injury in consideration of the insistent requests of the patient’s wife. After this judgment, South Korean physicians became extremely cautious about discontinuing LST. Social demand for the withdrawal of LST was raised due to the “Granma Kim incident” in 2009 [25,26]. Granma Kim was in a persistent vegetative state from hypoxic brain damage during a lung biopsy, and her family wanted to stop mechanical ventilation. The Supreme Court allowed the withdrawal of mechanical ventilation after much social discussion and debate.
Finally, the National Assembly of the Republic of Korea passed the Hospice and Palliative Care and Life-Sustaining Treatment for patients at the end-of-life in February 2016 (henceforth, “Act on LST decisions”) [32]. The Act on LST decisions was implemented on February 4, 2018, permitting the withholding or withdrawing of cardiopulmonary resuscitation (CPR), mechanical ventilation, hemodialysis, and anticancer treatment. The scope of the Act on LST decisions was further extended on March 28, 2019, and decisions regarding extracorporeal life support (ECLS), transfusion, and inotropic drug use were also included. By October 2022, the number of people who had written advance directives for the LST plan exceeded 1.5 million [31].
Patients with severe cerebrovascular disease are typically unconscious and have difficulty expressing their intentions. In addition, it is characterized by a sudden onset and lack of time to decide on LST [4]. An increasing number of patients and their families want to withhold or withdraw LST when medical futility is expected, especially in cancer patients [19]. However, limited data on the withdrawal of LST in patients with acute cerebrovascular disease are available. This study aimed to investigate the status of LST practices in patients with acute cerebrovascular disease. We also evaluated the problems with the Act on LST decisions for end-of-life patients.
The study protocol was reviewed and approved by the Institutional Review Board of Samsung Changwon Hospital (approval No. SCMC2022-08-004). The requirement for informed consent was waived as the study did not require informed consent.
This study was conducted from January 2017 to December 2021 at our hospital. The inclusion criteria were as follows : 1) patients admitted to the neurosurgery department and 2) patients with acute cerebrovascular disease as the underlying cause of death.
We reviewed patients’ medical records and imaging data. Data included baseline and treatment characteristics. Baseline characteristics included sex, age, stroke type, marital status, and Glasgow coma scale (GCS) score at admission. Treatment characteristics included information about the hospital stay, ventilator care, informed consent for the LST plan, ventilator withdrawal, number of days from writing consent to death, organ donation, surgery recommendations, and the refusal to undergo surgery.
Informed consent for the LST plan included consent for a do-not-resuscitate (DNR) order, advance directives on LST, and the LST plan. The Act on LST decisions became effective on February 4, 2018. The law did not apply to patients who died between January 2017 and when the law took effect. Patients who did not want LST before the Act’s execution, such as CPR, ventilator withdrawal, or hemodialysis, were informed of the DNR document according to the form at the institution.
Stroke type was divided into two groups : hemorrhagic and ischemic. Marital status was categorized as “unmarried” and “married.” The GCS score of each patient was assessed on admission. Patients with a GCS score of <9 were defined as those with severe brain damage. Ventilator withdrawal refers to the discontinuation of ventilator support. The decisions regarding surgery and DNR in patients with severe brain damage were taken by the family. The decision-makers considered the patient’s past wishes and beliefs, as well as the circumstances of the remaining family. The decision to donate organs was made by the family after the declaration of brain death.
The study period was divided into before the Act (January 1, 2017 to February 3, 2018) and after the Act (February 4, 2018 to December 31, 2021). The study period was also divided into before the Act extension (January 1, 2017 to March 27, 2019) and after the Act extension (March 28, 2019 to December 31, 2021) (Fig. 1).
The Act adopted two main concepts : “terminal phase” and “end-of-life process” [32]. The terminal phase is defined as the period in which fundamental recovery is impossible, although aggressive treatment is done and the symptoms worsen gradually. The end-of-life process is when death is imminent with no possibility of revitalization or recovery, and symptoms rapidly worsen despite treatment. Both stages are incurable, but the terminal stage is when the patient has a life expectancy of several weeks or months, and the end-of-life process is when death is expected within days. A patient in the end-of-life stage is one who has been medically determined to be in the end-of-life process by the doctor in charge and a medical specialist in the relevant field. LST refers to medical treatment by CPR, hemodialysis, anticancer drugs, mechanical ventilation, ECLS, transfusion, and inotropic drugs for a patient at the end of life, which only prolongs the process of dying without a curative effect.
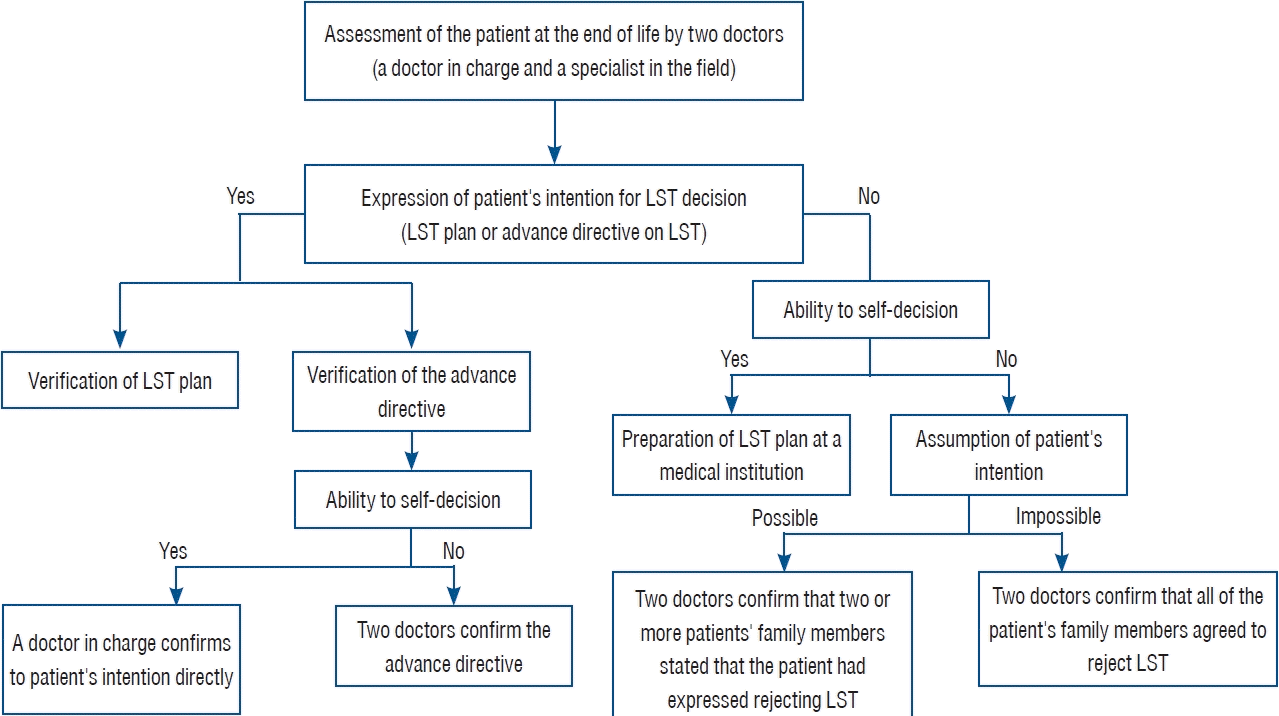
The Act on LST decision mandates a three-step process to terminate LST (Fig. 2). First, two doctors—the doctor in charge and a medical specialist in the relevant field—must determine whether the patient is in the end-of-life process. Second, the patients’ intentions must be verified. If the patient is medically capable of expressing their intentions, an LST plan is completed by the doctor in charge. If a patient is medically incapable of expressing their intentions, the doctor in charge and a medical specialist can verify this intention through statements from two or more members of the patient’s family. If it is difficult to prove the patient’s intentions, all family members must agree unanimously. Third, if both conditions of medical determination—the ongoing treatment offers no further curative effect and the patient wishes for no other treatment—are met, then the doctor in charge and a medical specialist will make the decision and fill out the necessary form.
Data are presented as mean with standard deviation (SD) for continuous variables and as frequencies for categorical variables. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Continuous variables were analyzed using Student’s t-test or the Mann-Whitney U test. Statistical significance was set at p<0.05. Baseline and treatment characteristics were compared before and after the Act, and before and after its extension. Baseline and treatment characteristics were compared between patients who refused surgery and those who underwent surgery. Multivariate logistic regression analysis was used to evaluate factors associated with refusal to undergo surgery. Logistic regression analysis included variables with p<0.20 in the univariate analysis. All statistical analyses were performed using R version 4.0.5. Correlation matrices were displayed using an R language program package.
Table 1 presents the baseline and treatment characteristics of the study population. A total of 227 patients (115 males and 112 females; mean age, 66.2 years; SD, 14.4) were included in this study. Of the 227 patients with acute cerebrovascular disease, 183 (80.6%) had a hemorrhagic stroke, including intracerebral hemorrhage (n=107), aneurysmal subarachnoid hemorrhage (n=70), and arteriovenous malformation rupture (n=6), whereas 44 (19.4%) had an ischemic stroke. Of the total patients, 215 (94.7%) were married. There were 162 patients (71.4%) with GCS scores on admission <9. Ventilator care was provided to 199 patients (87.7%). Of the total 227 patients, 166 (73.1%) provided informed consent for the LST plan. None of the patients made LST decisions by themselves. None of the deceased patients prepared a written advance directive for the LST plan before admission, and the determination of LST was influenced by the family members’ decisions. The mean number of days from obtaining written consent to death was 3.6 days. Of the 166 patients with consent for the LST plan, ventilator withdrawal was performed in 52 patients (31.3%), and the hospitalization days were 12.1±18.2 days. Patients with ventilator withdrawal had significantly shorter hospitalization days than those without ventilator withdrawal (8.8±9.4 vs. 14.2±22.9 days, p=0.031). All patients with ventilator withdrawal died within 1 day. Organ donation was performed in 11 patients (4.8%). Surgery was recommended for 190 patients (83.7%). Of the 190 patients who needed surgery, 82 (46.3%) underwent surgery, and 102 (53.7%) refused surgery.
A comparison of patients before (January 1, 2017 to February 3, 2018) and after the Act (February 4, 2018 to December 31, 2021) is shown in Table 2. Period before the Act had a significantly higher rate of male and younger patients than the period after the Act (29/44 [65.9%] vs. 86/183 [47.0%], p=0.037) (61.2±12.8 vs. 67.4±14.6 years, p=0.010). Hospitalization days tended to be shorter after the Act than before (15.9±16.1 vs. 11.2±18.6 days); however, the statistical significance was not enough (p=0.127). Ventilator care was used more frequently after the Act than before (166/183 [90.7%] vs. 33/44 [75.0%]; p=0.010). Consent for the LST plan tended to be obtained more frequently after the Act than before (139/183 [76.0%] vs. 27/44 [61.4%]); however, the statistical significance was not enough (p=0.077). Ventilator withdrawal was performed more frequently after the Act than before (52/183 [28.4%] vs. 0/44 [0.0%]; p<0.001). Organ donation was performed less frequently after the Act than before (5/183 [2.7%] vs. 6/44 [13.6%], p=0.008). Of the 190 patients who required surgery, 41 were in the period before the Act, and 149 were in the period after the Act. Surgery refusal was more frequently observed after the Act than before (87/149 [58.4%] vs. 15/41 [36.6%], p=0.021). Fig. 3 shows significant changes before and after the Act on LST decisions.
Table 3 compares patients between the Act extension (January 1, 2017 to March 27, 2019) and after the Act extension (March 28, 2019 to December 31, 2021). Consent for the LST plan was more frequently obtained in the period after the Act extension than before (37/127 [29.1%] vs. 15/100 [15.0%], p=0.018). Organ donation tended to be performed less frequently after the Act extension than before (4/127 [3.1%] vs. 7/100 [7.0%]); however, the statistical significance was not enough (p=0.303). Surgery refusal was more frequently observed in the period after the Act extension (65/104 [81.9%] vs. 37/86 [43.0%], p=0.011).
Table 4 compares the baseline and treatment characteristics of the patients who refused and underwent surgery. Patients who underwent surgery had higher rates of male and younger patients than those who refused surgery (54/88 [61.4%] vs. 39/102 [38.2%], p=0.002) and age (61.4±12.8 vs. 73.2±11.1 years, p<0.001). Patients who refused surgery had a higher rate of GCS scores on admission <9 than those who underwent surgery (81/102 [79.4%] vs. 55/88 [62.5%]; p=0.016). Hospitalization days were significantly shorter in patients who refused surgery than in those who underwent surgery (7.9±8.1 vs. 17.7 ±25.1 days, p<0.001). Ventilator care was used significantly less frequently in patients who refused surgery than in those who underwent surgery (82/102 [80.4%] vs. 81/88 [92.0%]; p=0.037). Consent for the LST plan was more frequent in patients who refused surgery than in those who underwent surgery (85/102 [83.3%] vs. 58/88 [65.9%], p=0.009). Organ donation was performed less frequently in patients who refused surgery than in those who underwent surgery (1/102 [1.0%] vs. 7/88 [8.0%], p=0.043).
Multivariate logistic regression analysis of factors affecting refusal of surgery, including sex, age, GCS score on admission <9, consent for LST plan, and the Act on LST decisions showed that age (adjusted odds ratio [OR], 0.920; adjusted 95% confidence interval [CI], 0.850–0.890; p<0.001), consent for LST plan (adjusted OR, 0.460; adjusted 95% CI, 0.210–0.980; p=0.048), and GCS score on admission <9 (adjusted OR, 2.630; adjusted 95% CI, 1.280–5.560; p=0.010) were independently associated with refusal of surgery in patients with acute cerebrovascular disease (Table 5).
In our study, we observed recent trends in LST withdrawal in patients with acute cerebrovascular disease. Previous studies on the Act on LST decisions reported that the rate of writing advance directives for LST and the quality of death improved after the implementation of the Act [6,11,13,47,49]. The rate of patient self-determination regarding LST was higher in cancer patients than in non-cancer patients [25,48]. However, in this study, none of the deceased patients prepared a written advance directive for the LST plan, and the determination of LST was influenced by family members’ decisions. Our results showed that hospital stays tended to be shorter, and ventilator withdrawal was more frequent in the period after the Act’s execution. These results indicated that treatments that prolong the end-of-life process without any therapeutic benefit have been reduced, which is the intent of the Act.
We also found that the refusal of surgery in patients with an acute cerebrovascular disease requiring surgical treatment has increased significantly since the Act on LST decisions was implemented. These findings suggest that patients with acute cerebrovascular disease and their families are more likely to choose to die rather than live without full neurological function. These trends could also indicate that some patients did not receive beneficial and necessary treatments that could have saved their lives. To the best of our knowledge, this is the first study to investigate the effects of LST withdrawal in patients with acute cerebrovascular disease in South Korea.
In the Act on LST decision, it is essential to distinguish the terminal phase and the end-of-life process because the LST decision can be made only in the end-of-life process [32]. The Act can be effectively applied to cancer patients [19,25,48]. If long-term survival is not expected due to recurrence or metastasis, the patient is considered in the terminal phase, and palliative care is provided. Later, as the disease progresses and the patient’s vital signs become unstable, the patient is considered in the end-of-life process, and LST decision can be made. However, applying this Act to patients with acute cerebrovascular disease has different points; they lack decision-making abilities, can deteriorate rapidly, and it is difficult to predict the exact prognosis after initial diagnosis.
Patients with cerebrovascular disease are suffered from the uncertainty about the prognosis after initial diagnosis [12,16]. Physicians use several scales to assess the prognosis of patients with acute stroke patients, including iScore for acute ischemic stroke, ICH score for intracerebral hemorrhage, and Hunt-Hess scale and World Federation of Neurological Surgeon grading for subarachnoid hemorrhage [5,14,37,39]. Previous studies have demonstrated that these prognostication scales are more accurate than physicians’ predictions and help estimate the risk of early stroke mortality and short-term disability [14,38,40]. However, prognostic models have shown limitations in that most have been developed retrospectively and suffer from “withdrawal bias,” and patients may have different perceptions of quality of life [3,9,18]. In addition, physician predictions are imperfect because they can vary widely between physicians and can be optimistic or pessimistic [7,30,33]. A recent study has shown that prognostic accuracy can be improved by making predictions based on clinical data on the fifth day of hospitalization rather than at the time of admission [29]. This is consistent with the recommendation that traumatic brain injury patients should be observed for 72 hours before deciding to withdraw LST [42]. Several studies have shown a strong correlation between decisions to limit therapy and the physician’s prediction that patients will have a poor chance of survival [8]. As a result, patients expected to have a poor prognosis may not receive certain forms of intensive care and neurosurgical interventions. This preemptive judgment can interfere with prognostic prediction. For accurate prediction, physicians should take the necessary time to evaluate a patient’s prognosis, using a combination of their experience and prognostic models tailored to each patient’s specific condition.
The most important aspect when making an LST decision is that it should be based on patients’ wishes and beliefs. Therefore, the Act on LST decisions should be based on patients’ wishes. Previous studies have shown that discussions about LST in patients in the terminal phase usually come too late for patients and that physicians and their family members commonly make LST decisions [15]. This point has improved since the Act’s execution, and self-determination rates have been reported to be up to 30% [36,41]. However, this study, which focused on patients with acute cerebrovascular disease, showed that none of the patients made the LST decision by themselves. These results can be partially explained by the fact that patients with severe cerebrovascular disease are unconscious and are unable to make LST decisions. In addition, because cerebrovascular disease is usually a sudden-onset disease, it is not easy to complete an advance directive for LST. This study suggests that LST decisions for patients with acute cerebrovascular disease may differ from those with other terminal diseases, such as cancer.
Since family members frequently make the LST decision for patients with cerebrovascular disease, they need to decide based on a sufficient understanding of the patient’s condition. A previous study reported that disturbance of consciousness, dysphasia on day 1, and large supratentorial stroke was possible indicators of decisions to withdraw or withhold LST [2]. Families are concerned about poor prognosis and the neurological impairment that will be left in the patient. Although most patients with severe cerebrovascular disease are expected to have poor neurological prognoses, some patients may recover to the point of regaining consciousness [4,10,24]. Surviving a severe stroke means living with a disability. Given a hypothetical scenario of a severe stroke, most people do not favor lifesaving surgery, such as decompressive hemicraniectomy, even if only moderate disability is anticipated [22]. However, most disabled patients following severe stroke would opt to have the surgery again if in that situation [45]. The phenomenon that people with disabilities rate their quality of life higher than people without disabilities is known as the “disability paradox” [1]. Therefore, an environment should be made in which physicians, patients, and families can take the time to discuss and explain the patient’s ability to adapt to physical limitations.
This study found that organ donations have decreased significantly since the implementation of the Act on LST decisions. According to data from the Korean Organ Donation Agency, from 2014 to 2017, an average of 508 per year brain-dead patients’ organs were donated, while from 2018 to 2021, when the law was implemented, an average of 454 brain-dead patients’ organs were donated, a decrease of more than 10% [23,35]. Organ donation screening takes place after a patient has been declared brain dead, and the criteria for determining brain death for organ donation are strict. If a patient with severe brain damage has no consciousness and spontaneous breathing, but some brainstem reflexes remain, they cannot be declared brain dead. LST decision can be made at this step; however, organ donation cannot be made. Organ donation after circulatory death following withdrawal of LST has been practiced in some European countries, the United States, and the United Kingdom, with reports of organ donation after circulatory death rating from 20–38% of total organ donation [28,34]. In South Korea, organ donation after circulatory death following the withdrawal of LST, but not considered to be brain dead, is not permitted. However, there have been recent efforts to implement the possibility of organ donation after circulatory death, specifically following the withdrawal of LST [17,34]. There is a need to discuss organ donation in patients with circulatory death after LST withdrawal.
Comparing the current status of the Act on LST decision for patients with acute cerebrovascular disease in each country could clarify the pros and cons of Korea’s Act on LST decision. However, no country has the specific Act on LST decision regarding patients with acute stroke. Instead, we can compare the Act on LST decision for patients with impaired consciousness between Korea and other countries. The difference between Korea and the United States is whether to accept a proxy decision through an agent [44,46]. In Korea, if it is difficult to prove the patient’s intentions, all family members must agree unanimously [32]. On the other hand, in the United States, the patient can designate a proxy for the advance directives, and not only the patient but also the proxy can participate in preparing the LST decision. Therefore, a feature of domestic laws for unconscious patients without family members is that they cannot make LST decisions. Another feature is that domestic laws distinguish between the terminal phase and endof-life process based on the patient’s remaining life expectancy. Other countries do not distinguish between the two phases but classify them all as terminal phases and apply the law on withdrawing LST [43]. In clinical practice, it is difficult to distinguish when a patient is in the end-of-life process. A recent survey study reported that many physicians working in tertiary hospitals find it challenging to accurately distinguish between the end-of-life process and the terminal phase [21]. In our study, it took about 12 days from hospitalization to death. Applying the law to distinguish between patients with acute cerebrovascular disease, who can die in such a short time, has limitations.
This study has several limitations. First, it had a retrospective design, which may have been subject to selection bias. Second, this study was conducted at a single hospital in South Korea, which may limit the generalizability of the results. Third, this study was based on medical records and lacked detailed records of the family’s decision-making background, including patient wishes, economic situation, and reasons for refusing surgery. Therefore, there may be limitations to understanding the decision-making processes of LST. However, this is meaningful as it is the first study on LST decision-making in patients with cerebrovascular disease. Further studies with larger sample sizes are needed to confirm these results.
After the Act on LST decisions, more patients provided written consent for LST plans. However, in patients with acute cerebrovascular disease, all decisions were made by family members rather than by the patients themselves. We observed an increased rate of refusal to undergo surgery among patients with cerebrovascular disease. The Act on LST decisions may reduce the treatment that only prolongs the end-of-life process without a curative effect; however, widespread application of this law may also reduce beneficial and necessary treatment for patients with cerebrovascular disease and contribute to the decline in organ donation. Further studies with larger sample sizes are needed to confirm these results.
Notes
References
1. Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 48:977–988. 1999.

2. Alonso A, Ebert AD, Dörr D, Buchheidt D, Hennerici MG, Szabo K. Endof-life decisions in acute stroke patients: an observational cohort study. BMC Palliat Care. 15:38. 2016.

3. Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 56:766–772. 2001.

4. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 24:987–993. 1993.

5. Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 34:1717–1722. 2003.

6. Choi Y, Park M, Kang DH, Lee J, Moon JY, Ahn H. The quality of dying and death for patients in intensive care units: a single center pilot study. Acute Crit Care. 34:192–201. 2019.

7. Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 320:469–472. 2000.
8. Cook D, Rocker G, Marshall J, Sjokvist P, Dodek P, Griffith L, et al. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 349:1123–1132. 2003.

9. Creutzfeldt CJ, Holloway RG. Treatment decisions after severe stroke: uncertainty and biases. Stroke. 43:3405–3408. 2012.
10. Farooq S, Shkirkova K, Villablanca P, Sanossian N, Liebeskind DS, Starkman S, et al. National Institutes of Health Stroke scale correlates well with initial intracerebral hemorrhage volume. J Stroke Cerebrovasc Dis. 31:106348. 2022.

11. Friend JM, Alden DL. Improving patient preparedness and confidence in discussing advance directives for end-of-life care with health care providers in the United States and Japan. Med Decis Making. 41:60–73. 2021.

12. Gao L, Zhao CW, Hwang DY. End-of-life care decision-making in stroke. Front Neurol. 12:702833. 2021.

13. Graw JA, Marsch F, Spies CD, Francis RCE. End-of-life decision-making in intensive care ten years after a law on advance directives in Germany. Medicina (Kaunas). 57:930. 2021.

14. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 32:891–897. 2001.
15. Heo DS, Yoo SH, Keam B, Yoo SH, Koh Y. Problems related to the act on decisions on life-sustaining treatment and directions for improvement. J Hosp Palliat Care. 25:1–11. 2022.

16. Holloway RG, Arnold RM, Creutzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 45:1887–1916. 2014.
17. Jeong E, Baik S, Park H, Oh J, Lee Y, Lee JM. First organ donation after circulatory death following withdrawal of life-sustaining treatment in Korea: a case report. J Korean Med Sci. 36:e171. 2021.

18. Kelly AG, Hoskins KD, Holloway RG. Early stroke mortality, patient preferences, and the withdrawal of care bias. Neurology. 79:941–944. 2012.

19. Kim JS, Yoo SH, Choi W, Kim Y, Hong J, Kim MS, et al. Implication of the life-sustaining treatment decisions act on end-of-life care for korean terminal patients. Cancer Res Treat. 52:917–924. 2020.

20. Kim S, Lim A, Jang H, Jeon M. Life-sustaining treatment decision in palliative care based on electronic health records analysis. J Clin Nurs. 32:163–173. 2023.

21. Kim YJ, Lim CM, Shim TS, Hong SB, Huh JW, Oh DK, et al. The influence of new legislation on the withdrawal of life-sustaining treatment on the perceptions and experiences of residents in a tertiary hospital in Korea. Korean J Med Ethics. 23:279–299. 2020.
22. Klein A, Kuehner C, Schwarz S. Attitudes in the general population towards hemi-craniectomy for middle cerebral artery (MCA) infarction. A population-based survey. Neurocrit Care. 16:456–461. 2012.

23. Korean Organ Donation Agency : Donation trends over the years. Available at : https://www.koda1458.kr/info/trend.do.
24. Lahti AM, Nätynki M, Huhtakangas J, Bode M, Juvela S, Ohtonen P, et al. Long-term survival after primary intracerebral hemorrhage: a population-based case-control study spanning a quarter of a century. Eur J Neurol. 28:3663–3669. 2021.

25. Lee HY, Kim HJ, Kwon JH, Baek SK, Won YW, Kim YJ, et al. The situation of life-sustaining treatment one year after enforcement of the act on decisions on life-sustaining treatment for patients at the end-oflife in Korea: data of national agency for management of life-sustaining treatment. Cancer Res Treat. 53:897–907. 2021.

26. Lee SH, Kim JM, Yeo Y, Kim J. The impact of the well-dying law in Korea: comparing clinical characteristics and ICU admissions. Ann Palliat Med. 11:3135–3146. 2022.

27. Lee YJ, Ahn S, Cho JY, Park TY, Yun SY, Kim J, et al. Change in perception of the quality of death in the intensive care unit by healthcare workers associated with the implementation of the “well-dying law”. Intensive Care Med. 48:281–289. 2022.

28. Lomero M, Gardiner D, Coll E, Haase-Kromwijk B, Procaccio F, Immer F, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 33:76–88. 2020.

29. Maas MB, Francis BA, Sangha RS, Lizza BD, Liotta EM, Naidech AM. Refining prognosis for intracerebral hemorrhage by early reassessment. Cerebrovasc Dis. 43:110–116. 2017.

30. Meadow W, Pohlman A, Frain L, Ren Y, Kress JP, Teuteberg W, et al. Power and limitations of daily prognostications of death in the medical intensive care unit. Crit Care Med. 39:474–479. 2011.

31. National Agent for Management of Life-Sustaining Treatment : Monthly Statistics. Available at : https://www.lst.go.kr/comm/monthlyStatistics.do.
32. National Law Information Center : Hospice and Palliative Care and Life-Sustaining Treatment for patients at the end-of-life. Available at : https://law.go.kr/LSW/lsInfoP.do?lsiSeq=180823&viewCls=engLsInfoR&urlMode=engLsInfoR#0000.
33. Navi BB, Kamel H, McCulloch CE, Nakagawa K, Naravetla B, Moheet AM, et al. Accuracy of neurovascular fellows’ prognostication of outcome after subarachnoid hemorrhage. Stroke. 43:702–707. 2012.

34. Park H, Jung ES, Oh JS, Lee YM, Lee JM. Organ donation after controlled circulatory death (Maastricht classification III) following the withdrawal of life-sustaining treatment in Korea: a suggested guideline. Korean J Transplant. 35:71–76. 2021.

35. Park J, Kim CJ. Recent decrease in organ donation from brain-dead potential organ donors in Korea and possible causes. J Korean Med Sci. 35:e94. 2020.

36. Park SY, Lee B, Seon JY, Oh IH. A national study of life-sustaining treatments in South Korea: what factors affect decision-making? Cancer Res Treat. 53:593–600. 2021.

37. Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2:110–118. 2005.

38. Saposnik G, Cote R, Mamdani M, Raptis S, Thorpe KE, Fang J, et al. JURaSSiC: accuracy of clinician vs risk score prediction of ischemic stroke outcomes. Neurology. 81:448–455. 2013.
39. Saposnik G, Kapral MK, Liu Y, Hall R, O’Donnell M, Raptis S, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 123:739–749. 2011.
40. Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, Saver JL, et al. A risk score for in-hospital death in patients admitted with ischemic or hemorrhagic stroke. J Am Heart Assoc. 2:e005207. 2013.

41. Son YJ, Choi J, Ahn JW. Nurses’ perspectives on advance directives before the establishment of the new well-dying law in Korea: a mixed methods study. Appl Nurs Res. 51:151187. 2020.

42. Souter MJ, Blissitt PA, Blosser S, Bonomo J, Greer D, Jichici D, et al. Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management : a position statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 23:4–13. 2015.

43. Tanaka M, Kodama S, Lee I, Huxtable R, Chung Y. Forgoing life-sustaining treatment - a comparative analysis of regulations in Japan, Korea, Taiwan, and England. BMC Med Ethics. 21:99. 2020.

44. Turnbull AE, Ning X, Rao A, Tao JJ, Needham DM. Demonstrating the impact of POLST forms on hospital care requires information not contained in state registries. PLoS One. 14:e0217113. 2019.

45. Vahedi K, Benoist L, Kurtz A, Mateo J, Blanquet A, Rossignol M, et al. Quality of life after decompressive craniectomy for malignant middle cerebral artery infarction. J Neurol Neurosurg Psychiatry. 76:1181–1182. 2005.

46. Vranas KC, Plinke W, Bourne D, Kansagara D, Lee RY, Kross EK, et al. The influence of POLST on treatment intensity at the end of life: a systematic review. J Am Geriatr Soc. 69:3661–3674. 2021.

47. Wang Y, Zhang Y, Hong Y, Zeng P, Hu Z, Xu X, et al. Advance directives and end-of-life care: knowledge and preferences of patients with brain tumours from Anhui, China. BMC Cancer. 21:25. 2021.

Fig. 1.
Implementation of the Act on LST decision during the study period. LST : life-sustaining treatment, DNR : do-not-resuscitate, CPR : cardiopulmonary resuscitation.
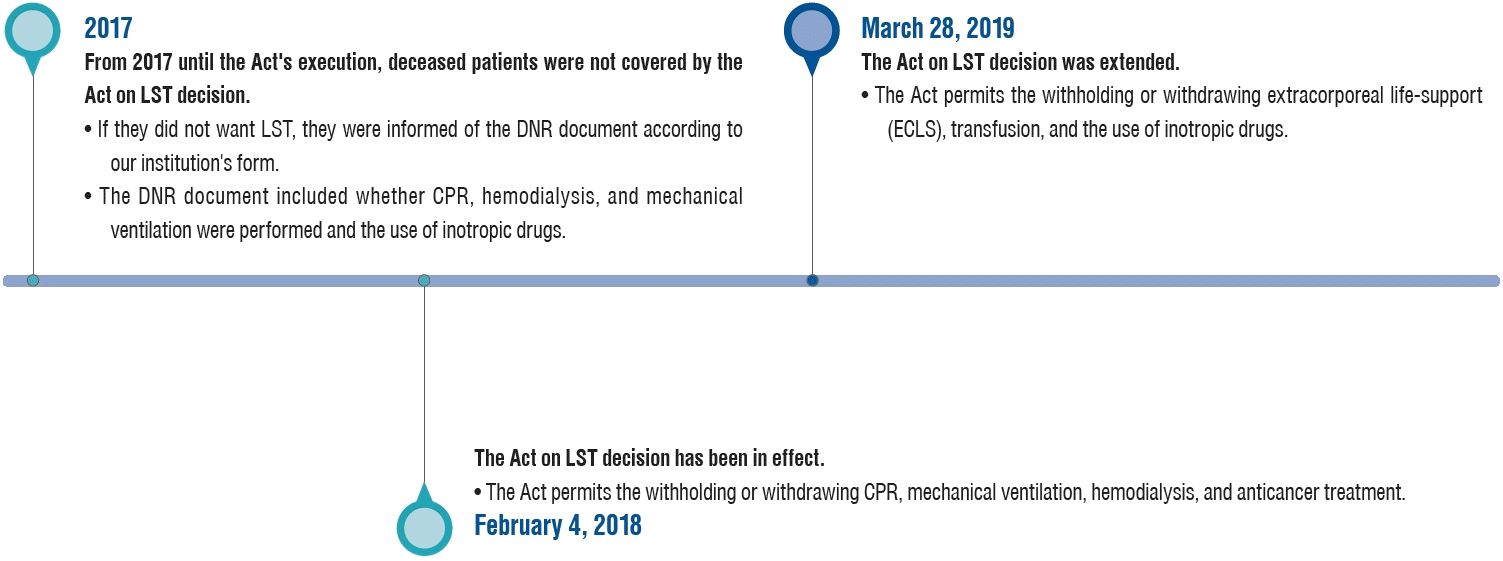
Fig. 3.
Comparison between before and after the Act on LST decision. LST : life-sustaining treatment, DNR : do-not-resuscitate.
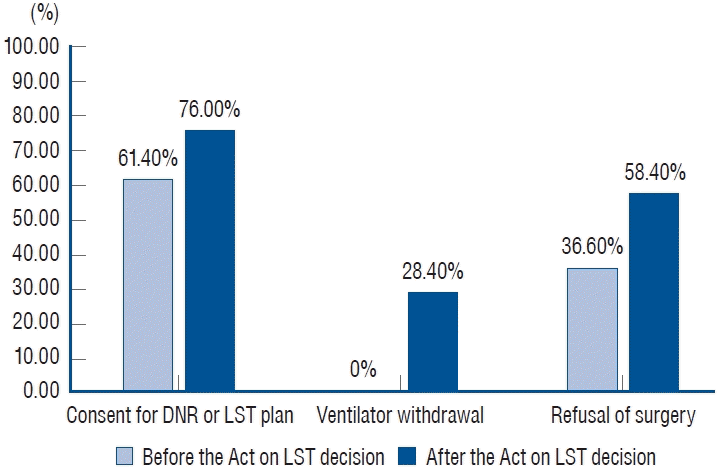
Table 1.
Baseline and treatment characteristics of the deceased patients with acute cerebrovascular disease
Table 2.
Comparison between the period before the Act (January 1, 2017 to February 3, 2018) and after the Act (February 4, 2018 to December 31, 2021)
Table 3.
Comparison of patients before the Act extension (January 1, 2017 to February 27, 2019) and after the Act extension (February 28, 2019 to December 31, 2021)
Table 4.
Comparison of the baseline and treatment characteristics of patients who refused surgery and those who underwent surgery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download