Abstract
The administration of angiotensin type 2 receptor blockers (ARBs) during pregnancy is known to cause ARB fetopathy, including renal insufficiency. We aimed to analyze the outcomes of two patients who survived ARB fetopathy and perform an accompanying literature review. Case 1 was exposed antenatally from a gestational age of 30 weeks to valsartan because of maternal pregnancy-induced hypertension. The patient presented with oliguria immediately after birth, and renal replacement therapy was administered for 24 days. Seven years after birth, renal function was indicative of stage 2 chronic kidney disease (CKD) with impaired urinary concentration. Case 2 had a maternal history of hypertension and transient ischemic attack and was treated with olmesartan until 30 weeks of pregnancy. Renal replacement therapy was performed for 4 days since birth. After 8 years, the patient is with CKD stage 2, with intact tubular function. Recent reports suggest that ARB fetopathy might manifest as renal tubular dysgenesis and nephrogenic diabetes insipidus, in contrast to mild alterations of glomerular filtration. Tubular dysfunction may induce CKD progression and growth retardation. Patients with ARB fetopathy should be monitored until adulthood. The ARB exposure period might be a critical factor in determining the severity and manifestations of fetopathy.
The renin-angiotensin-aldosterone system (RAAS) plays an essential role in the development of fetal kidneys [1]. All components of the RAAS, including angiotensin II receptor type 1 (AT1) and type 2 (AT2), exist in the developing kidneys [1]. Angiotensin II, an active form of angiotensin I converted by angiotensin-converting enzyme, promotes cellular growth through angiotensin II receptors, especially AT1. Administration of RAAS blockers, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), during the second and third trimesters of pregnancy has been reported to cause oligohydramnios, neonatal respiratory failure, renal insufficiency, and skeletal abnormalities, including limb contracture and hypocalvaria [1]. This condition is referred to as an ACEI/ARB fetopathy. Fetal exposure to ACEI/ARB results in decreased glomerular numbers and tubular atrophy, which manifest as a decreased glomerular filtration rate and impaired urinary concentration, respectively [1,2]. Most affected patients show poor survival and progress to end-stage kidney disease [2,3]. However, few studies have examined the long-term renal prognosis of ACEI/ARB fetopathy owing to poor survival rates. The current study aimed to present the long-term outcomes of two patients who survived ACEI/ARB fetopathy, to discuss tubular dysfunction of fetopathy, and to suggest possible factors affecting the clinical course of fetopathy.
A 7-year-old boy was born at a gestational age (GA) of 39 weeks, with a birth weight of 3,360 g (50th–75th percentile). His mother had a history of pregnancy-induced hypertension and valsartan was administered from the second trimester of pregnancy until delivery. Oligohydramnios was observed at 30 weeks of gestation. On the 4th day after birth, the patient was transferred to our center because of persistent anuria and azotemia. At the time of admission, his vital signs were as follows: blood pressure 64/38 mmHg, heart rate 142 beats/min, respiratory rate 52 breaths/min, and body temperature 36.6 °C. A physical examination revealed no remarkable findings. Initial laboratory results showed blood urea nitrogen (BUN) and creatinine (Cr) of 20.5 mg/dL and 4.85 mg/dL, but neither electrolyte imbalance nor acidosis was detected. Urinalysis showed no abnormal findings, while renal parenchymal echogenicity was increased on sonography. However, the patient’s body weight increased over time, azotemia became aggravated (Cr, 5.01 mg/dL), and hyponatremia developed (Na, 129 mmol/L). Peritoneal dialysis was initiated on the 2nd day of admission, and urination was observed on the 4th hospital day (HD). The modality of renal replacement therapy (RRT) was changed to continuous RRT (CRRT) on HD 5 for volume control but returned to peritoneal dialysis after 2 days due to platelet consumption. The RRT was maintained for a total of 24 days. The patient exhibited no other complications, and the last laboratory finding before discharge of HD 38 showed a BUN and Cr of 13.8 mg/dL and 1.71 mg/dL (Fig. 1A). He has been undergoing regular checkups for 7 years.
An 8-year-old boy was born at a GA 36 weeks with a birth weight of 2,560 g (25th–50th percentile). His mother had a history of hypertension, diabetes mellitus, and transient ischemic attack. She was treated with olmesartan until an incidental diagnosis of pregnancy at an estimated GA 30 weeks. A history of oligohydramnios was not available as appropriate antenatal care was not provided. The patient’s Apgar scores were 4 and 8 at 1 and 5 minutes, respectively. Mechanical ventilation and inotropics were applied for meconium aspiration and neonatal hypotension (mean blood pressure 20–30 mmHg). On the 2nd day after birth, pneumothorax was detected. The anuria persisted for 4 days. The patient was transferred to this center 4 days after birth because of anuric acute kidney injury (AKI). The patient’s vital signs and initial laboratory findings upon transfer were as follows: blood pressure 60/52 mmHg, heart rate 145 beats/min, respiratory rate 44 breaths/min, body temperature 36.5 ℃, BUN/Cr 45.2/6.18 mg/dL, Na-K-Cl 126-5.90-81.0 mmol/L, and Ca-P-Uric acid 9.40-7.10-19.3 mg/dL. Blood gas analysis revealed a pH of 7.37, partial pressure of carbon dioxide (pCO2) of 38.7 mmHg, and HCO3 of 22.2 mmol/L. Urinalysis showed no abnormal findings, while renal parenchymal echogenicity was increased on sonography. CRRT was initiated immediately after the admission and was maintained for 4 days. Urination was observed on the 2nd day after admission (Fig. 1B). During hospitalization, the patient was diagnosed with hemorrhagic periventricular leukomalacia and cerebellar hemorrhage. He was discharged on HD 31 and after 8 years of regular follow-up, the patient was transferred to another hospital near his residence.
Table 1 briefly describes the long-term outcomes of the two cases. Both patients maintained favorable growth. For renal functions, their glomerular filtration rate did not recover to normal range which is over 90 mL/min/1.73 m2 even after discharge. Also, ultrasonographic findings were persistently compatible with those of chronic kidney disease (CKD), exhibiting abnormal renal parenchymal echogenicity and poor corticomedullary differentiation. Glycosuria and proteinuria, implying proximal tubular dysfunction, were not detected in either of the patients. However, case 1 was sustained impaired urinary concentration (urine osmolality 101 mOsm/kg) since birth, suggesting distal tubular dysfunction although his growth was within the normal range. Confirmatory tests such as water restriction test for nephrogenic diabetes insipidus (NDI) were not conducted considering the normal aldosterone/renin level. He had started amlodipine use 2 years prior to this report for hypertension without secondary causes. Case 2, on the other hand, exhibited preserved tubular function but neurological sequelae, including delayed development and intellectual disability, associated with perinatal cerebral insult.
Recently, Miura et al. [2] published a paper presenting clinical manifestations of tubular dysfunction in ARB fetopathy. Two patients, after recovering from neonatal AKI, presented with polyuria and polydipsia, and were diagnosed with salt-losing NDI (Table 2). Gang et al. [3] reported a pathologic confirmation of renal tubular dysgenesis (RTD) in ARB fetopathy (Table 2). A kidney biopsy performed during exploratory laparotomy confirmed small and undifferentiated tubules which is compatible with RTD. Exposure to ACEI/ARB decreases the perfusion of fetal kidneys during nephrogenesis and causes pathological changes in the kidneys [4]. This secondary RTD is fatal in affected neonates.
So far, the research presented by Bullo et al. [4] in 2012 is the largest systematic review of ACEI/ARB fetopathy. The 12 cases of ARB fetopathy from the research with at least one available long-term clinical data are listed in Table 2 [2-13]. Among the 12 cases, only five patients were evaluated for renal tubular functions and three out of the five patients had clinical manifestations related to tubular dysfunctions such as growth retardation and renal tubular acidosis (Table 2). On the other hand, glomerular function is relatively followed with attention. This study spotlight the tubular dysfunction derived from ARB fetopathy which leads to growth retardation and deterioration of CKD in affected infants. Pediatric patients who survive ARB fetopathy should be under regular monitoring of tubular function as well as glomerular function.
In 2012, Spaggiari et al. [14] suggested that discontinuation of ACEI/ARB before 34–36 weeks of gestation normalizes the amniotic fluid level and does not cause renal impairment. Correspondingly, Bullo et al. [4] demonstrated that ACEI/ARB fetopathy occurs significantly less frequently in first-trimester exposure to ACEI/ARB when compared with exposure during second and third trimesters or the entire pregnancy. Recently in 2021, Oh et al. [5] compared a case with another previously reported case of ARB fetopathy (Table 2). In case 13, maternal use of telmisartan was discontinued when a 35-week pregnancy was incidentally diagnosed which was 10 days before delivery. Nevertheless, only mild NDI was noted and resolved after 6 months. Another case in comparison was prenatally exposed to candesartan just before delivery, from gestational week of 35 to 36, which resulted in anuria with neonatal hypotension. Their clinical course differed according to the exposure period of ARB.
In this study, case 1 was exposed to ARB during the latter period of pregnancy, whereas case 2 was affected earlier. In the long-term, case 1 demonstrated abnormal urine concentration while case 2 exhibited intact tubular function (Table 1). Although both patients had anuric AKI in the neonatal period, long-term renal complications had more distinct and long-lasting effects in patients with later exposure. Comparably, in previously reported cases, exposure to ARB during period including third trimester of pregnancy causes more severe clinical presentation of fetopathy and poor long-term renal outcomes as shown in Table 2. The following pathophysiology of fetal kidney development also supports these clinical implications: (1) AT1, which is crucial for kidney development, predominates over AT2 in time through kidney development, and (2) ARB is highly selective for AT1. Since kidney development persists until approximately 3 years after birth, neonates and infants are also contraindicated for ACEI/ARB.
In contrast to the second and third trimesters, fetal exposure to ACEI/ARB during the first trimester is often described as relatively safe. However, fetopathy also occurs following first-trimester exposure to ACEI/ARB and it is only the severity that differs [15]. Additionally, according to Quan [1], the use of RAAS blockers especially ACEI, early in pregnancy can cause multisystemic congenital anomalies. Prenatal exposure to ACEI reduces the activation of both AT1 and AT2. This action contributes to early cellular proliferation and growth driven by AT2 which disrupts early embryogenesis. Therefore, maternal ACEI/ARB use should be discontinued immediately after recognizing possible fetopathies. Also, physicians should be aware of the discrete clinical impacts of ACEI and ARB.
This study had several limitations. Since the study was a retrospective observation, limited clinical data were available, and detailed evaluations of tubular dysfunction were not performed. Additional research is needed on the clinical correlation between the affected tubular segments and renal manifestations in relation to the severity-determining factors of ACEI/ARB fetopathy.
Exposure to ACEI/ARB during pregnancy results in evident renal insufficiency, including NDI and RTD. Patients with ACEI/ARB fetopathy should be monitored until adulthood, as tubular dysfunction induces growth retardation and CKD progression. This study emphasizes the potential risk of ACEI/ARB fetopathy and reiterates the importance of physician alertness.
Notes
References
1. Quan A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum Dev. 2006; 82:23–8.

2. Miura K, Sekine T, Iida A, Takahashi K, Igarashi T. Salt-losing nephrogenic diabetes insipidus caused by fetal exposure to angiotensin receptor blocker. Pediatr Nephrol. 2009; 24:1235–8.

3. Gang MH, Lee YW, Chang MY. Secondary renal tubular dysgenesis in a newborn exposed to angiotensin Ⅱ receptor antagonist during gestation. Clin Exp Pediatr. 2021; 64:136–8.

4. Bullo M, Tschumi S, Bucher BS, Bianchetti MG, Simonetti GD. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012; 60:444–50.

5. Oh KT, Namgoong MK, Yoo YM, Lee BK. Angiotensin II receptor blocker fetopathy with persistent pulmonary hypertension, hypocalvaria, nephrogenic diabetes insipidus, transient pseudohypoaldosteronism and polycythemia. Perinatology. 2021; 32:48–53.

6. Serreau R, Luton D, Macher MA, Delezoide AL, Garel C, Jacqz-Aigrain E. Developmental toxicity of the angiotensin II type 1 receptor antagonists during human pregnancy: a report of 10 cases. BJOG. 2005; 112:710–2.

7. Berkane N, Carlier P, Verstraete L, Mathieu E, Heim N, Uzan S. Fetal toxicity of valsartan and possible reversible adverse side effects. Birth Defects Res A Clin Mol Teratol. 2004; 70:547–9.

8. Bos-Thompson MA, Hillaire-Buys D, Muller F, Dechaud H, Mazurier E, Boulot P, et al. Fetal toxic effects of angiotensin II receptor antagonists: case report and follow-up after birth. Ann Pharmacother. 2005; 39:157–61.

9. Schaefer C. Angiotensin II-receptor-antagonists: further evidence of fetotoxicity but not teratogenicity. Birth Defects Res A Clin Mol Teratol. 2003; 67:591–4.

10. Bass JK, Faix RG. Gestational therapy with an angiotensin II receptor antagonist and transient renal failure in a premature infant. Am J Perinatol. 2006; 23:313–8.

11. Simonetti GD, Baumann T, Pachlopnik JM, von Vigier RO, Bianchetti MG. Non-lethal fetal toxicity of the angiotensin receptor blocker candesartan. Pediatr Nephrol. 2006; 21:1329–30.

12. Georgaki-Angelaki E, Stergiou N, Naoum E, Papassotiriou I, Anagnostakou M. Olmesartan medoxomil-induced acute renal failure in a premature newborn following maternal exposure during pregnancy: a case report and review of the literature. NDT Plus. 2009; 2:295–7.

13. Vendemmia M, Garcia-Meric P, Rizzotti A, Boubred F, Lacroze V, Liprandi A, et al. Fetal and neonatal consequences of antenatal exposure to type 1 angiotensin II receptor-antagonists. J Matern Fetal Neonatal Med. 2005; 18:137–40.

Fig. 1.
(A) Case 1 was administered PD for anuric AKI on the 2nd day of hospitalization. Renal replacement therapy, including CRRT, was continued for 24 days. The patient was discharged on hospital day of 38. (B) Case 2 was initiated CRRT for anuric AKI on the first day of hospitalization. CRRT was administered for 4 days. The patient was discharged on hospital day of 31. Cr, creatinine; PD, peritoneal dialysis; CRRT, continuous renal replacement therapy; AKI, acute kidney injury.
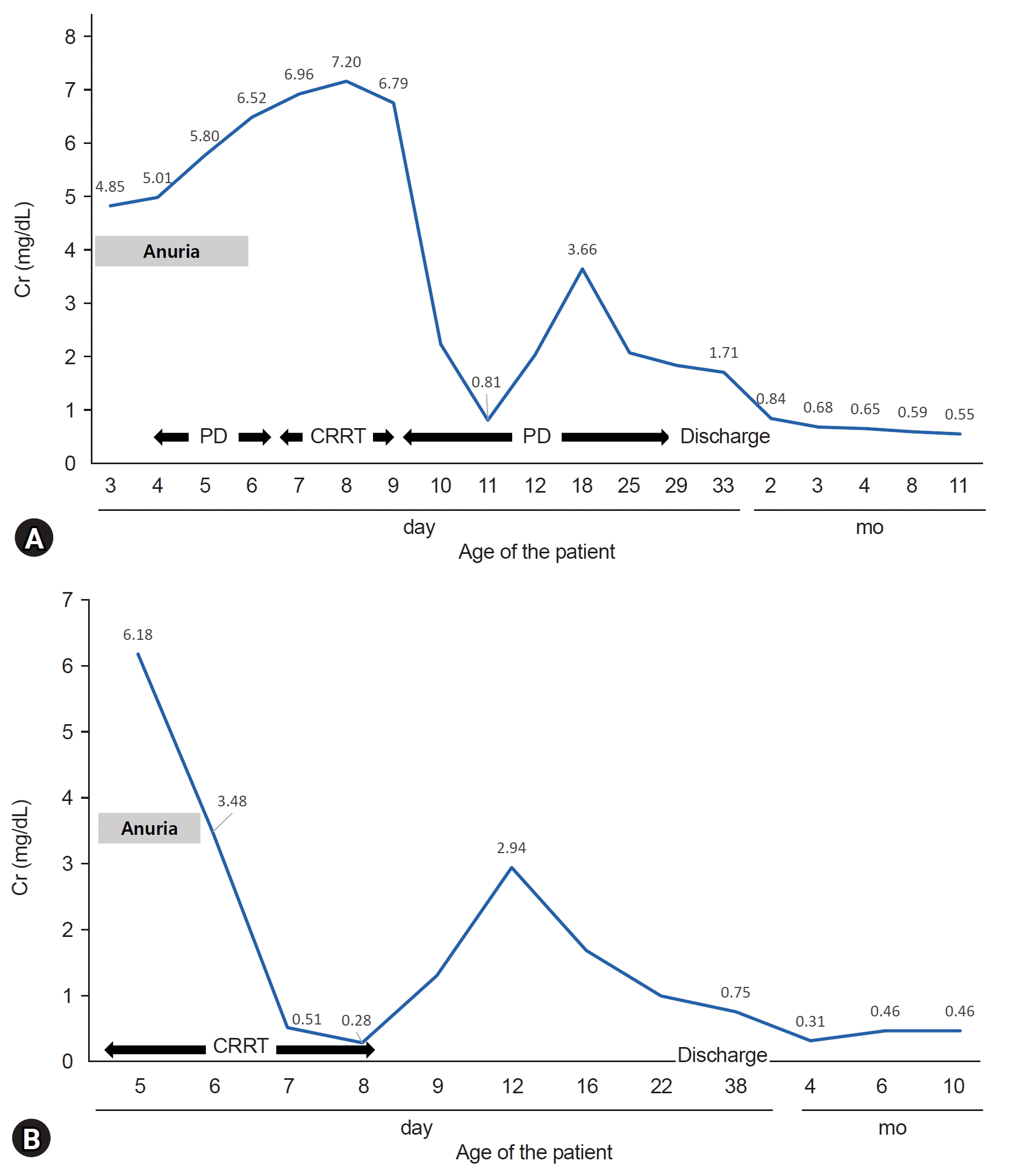
Table 1.
Long-term outcomes of two patients with ARB fetopathy
Table 2.
Characteristics and renal outcomes of previously reported ARB fetopathy comparing with current cases
| Case | ARB exposure | Features of ARB fetopathy | Renal (glomerular/tubular) outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Period (GW) | Oligo-hydramnios | Neonatal hypotension | Respiratory failure | Skeletal involvement | Renal insufficiency | Follow-up period | Glomerular | Tubular | |
| 1 | Valsartan | 30–39 | + | – | – | – | +b) | 7 yr | CKD (stage 2) | Abnormal concentration |
| 2 | Olmesartan | 0–30 | NA | + | + | – | +b) | 8 yr | CKD (stage 2) | Normal |
| 3 [4,6] | Losartan | 0–8a) | + | – | – | – | – | 18 mo | NA | FTT |
| 4 [4,7] | Valsartan | 0–20a) | + | – | – | – | – | 6 mo | Normal | Normal |
| 5 [4,8] | Valsartan | 0–25a) | + | – | – | + | + | 30 mo | CKD (stage 2) | Normal |
| 6 [4,9] | Losartan | 0–27a) | + | – | + | – | – | 3 mo | NA | NA |
| 7 [4,10] | Losartan | 0–29 | + | + | + | + | + | 14 mo | Normal | RTA |
| FTT | ||||||||||
| 8 [4,6] | Candesartan | 0–31 | + | NA | NA | + | + | NA | CKD (NA) | NA |
| 9 [4,11] | Candesartan | 0–31 | + | + | + | + | + | 34 mo | Normal | NA |
| 10 [4,9] | Losartan | 0–31 | + | – | – | + | – | 2 yr | Normal | NA |
| 11 [5] | Candesartan | 0–31 | + | + | + | + | +b) | 6 yr | CKD (stage 2) | NDI |
| 12 [4,12] | Olmesartan | 0–33 | + | – | + | + | + | 1 yr | CKD (stage 2) | NA |
| 13 [4,9] | Valsartan | 0–34 | + | – | + | – | +b) | 1 mo | NA | NA |
| 14 [5] | Telmisartan | 0–35 | – | – | + | + | – | 20 mo | Normal | Transient partial NDI |
| 15 [3] | Olmesartan | 0–36 | + | + | + | – | +b) | 25 day | ESKD | RTD |
| 16 [4,13] | Valsartan | 0–36 | + | + | + | – | + | NA | NA | Abnormal concentration |
| 17 [4,9] | Valsartan | 28–36 | + | NA | + | + | + | 8 mo | CKD (NA) | NA |
| 18 [2] | Candesartan | 33–37 | + | + | + | – | +b) | 2 yr | CKD (stage 3) | NDI |
ARB, angiotensin II receptor blocker; GW, gestational weeks; NA, not available; CKD, chronic kidney disease; FTT, failure to thrive; RTA, renal tubular acidosis; NDI, nephrogenic diabetes insipidus; ESKD, end-stage kidney disease; RTD, renal tubular dysgenesis.
a)Exposure period not including the third trimester, which is GW of 28–40. b)Supported with renal replacement therapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download