1. Kolber MK, Cui Z, Chen CK, Habibollahi P, Kalva SP. Nutcracker syndrome: diagnosis and therapy. Cardiovasc Diagn Ther. 2021; 11:1140–9.

2. Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010; 85:552–9.

3. Miro I, Serrano A, Perez-Ardavin J, March JA, Polo A, Conca MA, et al. Eighteen years of experience with pediatric nutcracker syndrome: the importance of the conservative approach. J Pediatr Urol. 2020; 16:218.
4. Granata A, Distefano G, Sturiale A, Figuera M, Foti PV, Palmucci S, et al. From nutcracker phenomenon to nutcracker syndrome: a pictorial review. Diagnostics (Basel). 2021; 11:101.

5. Agarwal A, Litra F, Barr LL. A rare cause of abdominal and flank pain in children: nutcracker syndrome. Cureus. 2021; 13:e16422.

6. El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutaneous Rev. 1950; 54:257–62.
7. de Schepper A. "Nutcracker" phenomenon of the renal vein and venous pathology of the left kidney. J Belge Radiol. 1972; 55:507–11.
8. Chait A, Matasar KW, Fabian CE, Mellins HZ. Vascular impressions on the ureters. Am J Roentgenol Radium Ther Nucl Med. 1971; 111:729–49.

9. Meyer J, Rother U, Stehr M, Meyer A. Nutcracker syndrome in children: appearance, diagnostics, and treatment: a systematic review. J Pediatr Surg. 2022; 57:716–22.
10. Mallat F, Hmida W, Othmen MB, Mosbah F. Mixed nutcracker syndrome with left renal vein duplication: a severe and exceptional presentation in an 18-year-old boy. Urol Ann. 2015; 7:244–7.

11. Vianello FA, Mazzoni MB, Peeters GG, Fossali EF, Camozzi P, Bianchetti MG, et al. Micro- and macroscopic hematuria caused by renal vein entrapment: systematic review of the literature. Pediatr Nephrol. 2016; 31:175–84.

12. Oteki T, Nagase S, Hirayama A, Sugimoto H, Hirayama K, Hattori K, et al. Nutcracker syndrome associated with severe anemia and mild proteinuria. Clin Nephrol. 2004; 62:62–5.

13. Basaran EG, Yilmaz AC, Gungor O, Tayfur AC, Buyukkaragoz B. Clinical profile and renal ultrasound characteristics of children with nutcracker syndrome in Turkey. Indian Pediatr. 2022; 59:28–30.

14. Reddy DK, Shekar P A. Nutcracker syndrome: a rare but important cause of varicocele in adolescent boys. Urology. 2020; 141:143–6.

15. Shin JI, Lee JS. ACE inhibition in nutcracker syndrome with orthostatic proteinuria: how about a hemodynamic effect? Pediatr Nephrol. 2007; 22:758–60.

16. Alaygut D, Bayram M, Soylu A, Cakmakci H, Turkmen M, Kavukcu S. Clinical course of children with nutcracker syndrome. Urology. 2013; 82:686–90.

17. Mazzoni MB, Kottanatu L, Simonetti GD, Ragazzi M, Bianchetti MG, Fossali EF, et al. Renal vein obstruction and orthostatic proteinuria: a review. Nephrol Dial Transplant. 2011; 26:562–5.

18. Li H, Sun X, Liu G, Zhang Y, Chu J, Deng C, et al. Endovascular stent placement for nutcracker phenomenon. J Xray Sci Technol. 2013; 21:95–102.

19. Jiang Y, Gan Z, Wang Q, Chen Y, Jiang Y. Bibliometric and visual analysis of research on nutcracker syndrome from 1974 to 2021: a systematic review. Medicine (Baltimore). 2022; 101:e29939.

20. Park SJ, Shin JI. Comment on: nutcracker and SMA syndromes: what is the normal SMA angle in children? (Eur J Radiol. 2012;81(August (8)):e854-61). Eur J Radiol. 2013; 82:1034.

21. Beinart C, Sniderman KW, Tamura S, Vaughan ED, Sos TA. Left renal vein to inferior vena cava pressure relationship in humans. J Urol. 1982; 127:1070–1.

22. Park SJ, Lim JW, Cho BS, Yoon TY, Oh JH. Nutcracker syndrome in children with orthostatic proteinuria: diagnosis on the basis of Doppler sonography. J Ultrasound Med. 2002; 21:39–46.
23. Cheon JE, Kim WS, Kim IO, Kim SH, Yeon KM, Ha IS, et al. Nutcracker syndrome in children with gross haematuria: Doppler sonographic evaluation of the left renal vein. Pediatr Radiol. 2006; 36:682–6.

24. Shin JI, Park JM, Lee JS, Kim MJ. Effect of renal Doppler ultrasound on the detection of nutcracker syndrome in children with hematuria. Eur J Pediatr. 2007; 166:399–404.

25. Nishimura Y, Fushiki M, Yoshida M, Nakamura K, Imai M, Ono T, et al. Left renal vein hypertension in patients with left renal bleeding of unknown origin. Radiology. 1986; 160:663–7.

26. Kim SH, Cho SW, Kim HD, Chung JW, Park JH, Han MC. Nutcracker syndrome: diagnosis with Doppler US. Radiology. 1996; 198:93–7.

27. Atasoy D, Cansu A, Bekircavusoglu AF, Ozdogan EB, Ahmetoglu A. The utility of magnetic resonance angiography in children with nutcracker syndrome. Turk J Med Sci. 2021; 51:2396–402.

28. Kim SH. Doppler US and CT diagnosis of nutcracker syndrome. Korean J Radiol. 2019; 20:1627–37.

29. Ahmed K, Sampath R, Khan MS. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg. 2006; 31:410–6.

30. Min KW, Lee OK, Kim MK. Nutcracker syndrome combined with superior mesenteric artery syndrome in a pediatric patient: a case report. Child Kidney Dis. 2018; 22:75–80.

31. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: what is the normal SMA angle in children? Eur J Radiol. 2012; 81:e854–61.

32. Gulleroglu NB, Gulleroglu K, Uslu N, Baskin E. Left renal vein entrapment in postural proteinuria: the diagnostic utility of the aortomesenteric angle. Eur J Pediatr. 2022; 181:3339–43.

33. Fitoz S, Ekim M, Ozcakar ZB, Elhan AH, Yalcinkaya F. Nutcracker syndrome in children: the role of upright position examination and superior mesenteric artery angle measurement in the diagnosis. J Ultrasound Med. 2007; 26:573–80.
34. Li S, Liu Q, Wang J, Pang X, Zhang Y, Cheng Y, et al. Association between left renal vein entrapment and varicocele recurrence: a cohort study in 3042 patients. Sci Rep. 2018; 8:10534.

35. Takahashi Y, Ohta S, Sano A, Kuroda Y, Kaji Y, Matsuki M, et al. Does severe nutcracker phenomenon cause pediatric chronic fatigue? Clin Nephrol. 2000; 53:174–81.
36. Lin TH, Lin CC, Tsai JD. Superior mesenteric artery syndrome and nutcracker syndrome. Pediatr Neonatol. 2020; 61:351–2.

37. Du MC, Chen HY, Zhang YX, Zhang LB. Pica with superior mesenteric artery syndrome and nutcracker syndrome as imaging manifestation: a case report. Asian J Surg. 2022; 45:2038–9.

38. England J, Li N. Superior mesenteric artery syndrome: a review of the literature. J Am Coll Emerg Physicians Open. 2021; 2:e12454.

39. Gungorer V, Ozturk M, Arslan S. A rare cause of recurrent abdominal pain: the coexistence of Wilkie's syndrome and nutcracker syndrome. Arch Argent Pediatr. 2023; 121:e202102373.
40. Ananthan K, Onida S, Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017; 53:886–94.

41. Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999; 135:218–25.

42. Juhn JH, Yoo BW, Lee JS, Kim MJ. A case of nutcracker syndrome associated with orthostatic proteinuria and idiopathic chronic fatigue in a child. J Korean Soc Pediatr Nephrol. 2001; 5:64–8.
43. Shin JI, Lee JS. Can chronic fatigue symptoms associated with nutcracker phenomenon be treated with aspirin? Med Hypotheses. 2007; 69:704–5.

44. Shin JI, Park JM, Shin YH, Lee JS, Kim MJ, Jeong HJ. Nutcracker syndrome combined with IgA nephropathy in a child with recurrent hematuria. Pediatr Int. 2006; 48:324–6.

45. Strul N, Vaessen S, Collard L, Ghuysen MS, Khamis J, Brisbois D, et al. Clinical case of the month: nutcracker syndrome in association with a painful nephrologic disease. Rev Med Liege. 2007; 62:73–6.
46. Shin JI, Lee SM, Park JM, Shin YH, Lee JS, Kim MJ. Doppler ultrasonographic detection of nutcracker syndrome in a young child with intussusception: a case report. Acta Paediatr. 2005; 94:1510–3.

47. Shin JI, Park JM, Shin YH, Lee JS, Kim MJ. Superimposition of nutcracker syndrome in a haematuric child with Henoch-Schönlein purpura. Int J Clin Pract. 2005; 59:1472–5.

48. Rao J, Yang J, Liu Z, Wang L, Liu L, Yin Z, et al. Right retrocaval ureter and left nutcracker syndrome: a case report. Urology. 2008; 71:1226.

49. Altugan FS, Ekim M, Fitoz S, Ozcakar ZB, Burgu B, Yalcinkaya F, et al. Nutcracker syndrome with urolithiasis. J Pediatr Urol. 2010; 6:519–21.

50. Preza Fernandes J, Amorim R, Gomes MJ, Oliveira V, Reis A, Ribeiro-Castro J. Posterior nutcracker syndrome with left renal vein duplication: a rare cause of haematuria in a 12-year-old boy. Case Rep Urol. 2012; 2012:849681.

51. Yavuz S, Ece A, Corapli M, Ilter C, Guven R. Nutcracker syndrome complicating with renal abscess. J Pak Med Assoc. 2016; 66:470–2.
52. Scholbach T. From the nutcracker-phenomenon of the left renal vein to the midline congestion syndrome as a cause of migraine, headache, back and abdominal pain and functional disorders of pelvic organs. Med Hypotheses. 2007; 68:1318–27.

53. Al-Zoubi NA, Al-Ghalayini IF, Al-Okour R. Nutcracker syndrome associated with celiacomesentric trunk anomaly: case report. Int J Nephrol Renovasc Dis. 2017; 10:285–8.

54. Nishio Y, Kawano Y, Hara S. Nutcracker syndrome complicated with intestinal malrotation. BMJ Case Rep. 2019; 12:e231230.

55. Perez-Ardavin J, Serrano Durba A, Miro I, Conca Baena MA, March-Villalba JA, Polo Rodrigo A, et al. Spontaneous spermatic vein thrombosis in pediatric patients: a condition to be considered. Cir Pediatr. 2020; 33:99–101.
56. Chang CT, Hung CC, Ng KK, Yen TH. Nutcracker syndrome and left unilateral haematuria. Nephrol Dial Transplant. 2005; 20:460–1.

57. Zhang H, Li M, Jin W, San P, Xu P, Pan S. The left renal entrapment syndrome: diagnosis and treatment. Ann Vasc Surg. 2007; 21:198–203.

58. Velasquez CA, Saeyeldin A, Zafar MA, Brownstein AJ, Erben Y. A systematic review on management of nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2018; 6:271–8.

59. Shin JI, Park JM, Lee SM, Shin YH, Kim JH, Lee JS, et al. Factors affecting spontaneous resolution of hematuria in childhood nutcracker syndrome. Pediatr Nephrol. 2005; 20:609–13.

60. Reed NR, Kalra M, Bower TC, Vrtiska TJ, Ricotta JJ, Gloviczki P. Left renal vein transposition for nutcracker syndrome. J Vasc Surg. 2009; 49:386–94.

61. Chuang CK, Chu SH, Lai PC. The nutcracker syndrome managed by autotransplantation. J Urol. 1997; 157:1833–4.

62. Xu D, Liu Y, Gao Y, Zhang L, Wang J, Che J, et al. Management of renal nutcracker syndrome by retroperitoneal laparoscopic nephrectomy with ex vivo autograft repair and autotransplantation: a case report and review of the literature. J Med Case Rep. 2009; 3:82.

63. Thompson PN, Darling RC, Chang BB, Shah DM, Leather RP. A case of nutcracker syndrome: treatment by mesoaortic transposition. J Vasc Surg. 1992; 16:663–5.
64. Benrashid E, Turley RS, Mureebe L, Shortell CK. Gonadal vein transposition in the treatment of nutcracker syndrome. J Vasc Surg. 2016; 64:845.

65. Shaper KR, Jackson JE, Williams G. The nutcracker syndrome: an uncommon cause of haematuria. Br J Urol. 1994; 74:144–6.

66. de Macedo GL, Dos Santos MA, Sarris AB, Gomes RZ. Diagnosis and treatment of the nutcracker syndrome: a review of the last 10 years. J Vasc Bras. 2018; 17:220–8.
67. Hao J, Shi H, Xu H, Zhu J, Zhou J, Du T. Ultrasound-assisted microsurgical left spermatic-inferior epigastric vein anastomosis for treating nutcracker syndrome-associated varicocele. Int Urol Nephrol. 2019; 51:1925–32.

68. Amaral J, Honjo O, Hannick JH, Rickard M, Lorenzo AJ. In situ gonadal vein valvulotomy and side-to-side gonado-iliac bypass for the management of nutcracker syndrome in an adolescent with a solitary kidney and absence of pelvic congestion. Urology. 2019; 126:200–3.

69. Ha TS, Lee EJ. ACE inhibition can improve orthostatic proteinuria associated with nutcracker syndrome. Pediatr Nephrol. 2006; 21:1765–8.

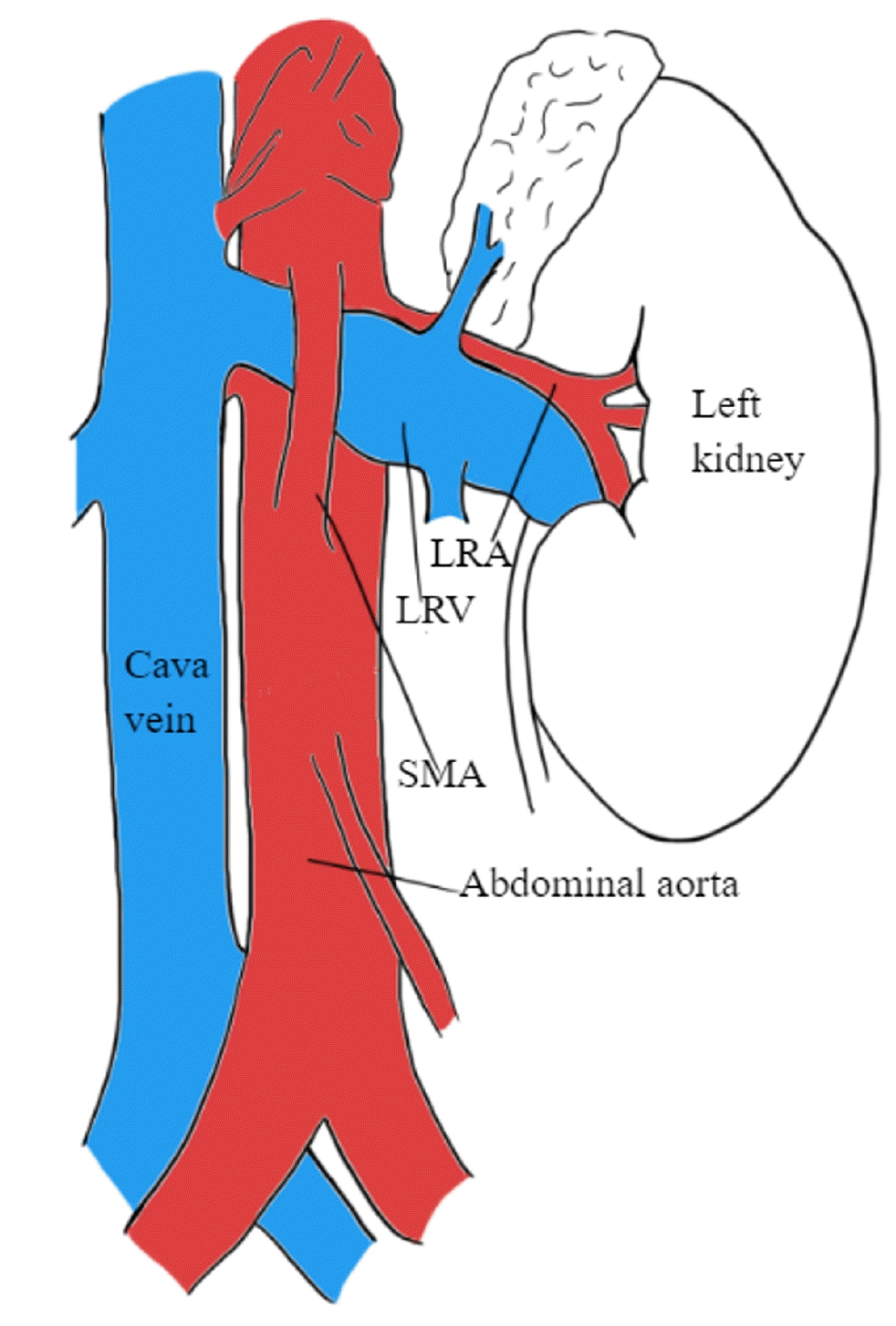
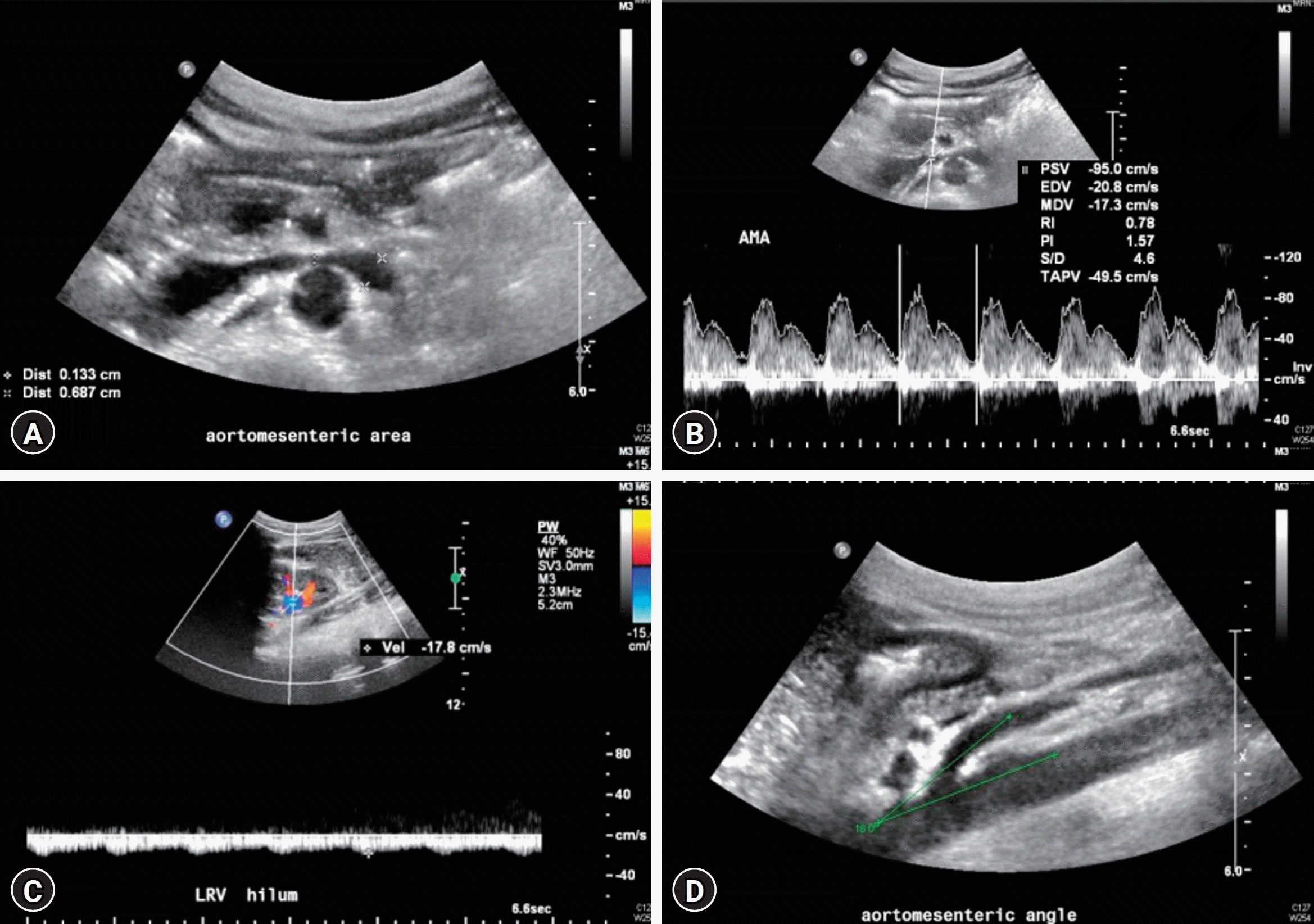
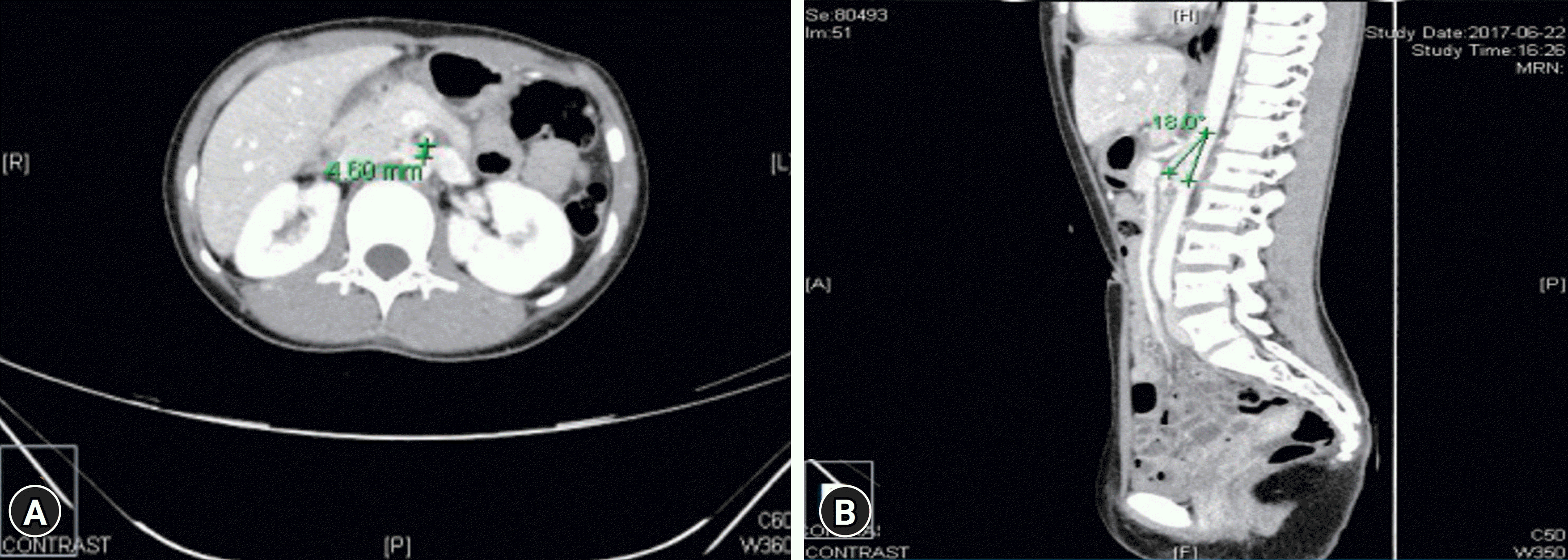




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download