Introduction
The nutcracker phenomenon (NCP), also known as left renal vein (LRV) entrapment, is defined as compression of the LRV between the superior mesenteric artery and abdominal aorta. Nutcracker syndrome (NCS) describes LRV entrapment with clinical manifestations, such as micro- to macroscopic hematuria, proteinuria, flank pain, abdominal pain, and varicocele [
1]. Although the prevalence of NCS is unknown, it is increasingly being diagnosed owing to the development of diagnostic techniques [
2,
3]. Meanwhile, immunoglobulin A (IgA) nephropathy (IgAN) is the most common glomerulonephritis (GN) worldwide and leading cause of chronic kidney disease and end-stage kidney disease. Recurrent hematuria, with or without proteinuria, has also been reported in IgAN [
4]. Although it is a rare presentation, several cases of coexisting NCS and IgAN have been reported [
3,
5-
7]. In a Japanese study, the prevalence of NCP in 146 patients with IgAN was 6.8% [
8]. Despite these reported cases, the relationship between these two diseases has not yet been clarified. Furthermore, it is difficult to diagnose and treat these patients appropriately. In this study, we report two pediatric cases of NCS combined with IgAN to suggest a relationship between the two diseases and emphasize their clinical significance.
Discussion
Herein, we report two cases of NCS combined with biopsy-proven IgAN in pediatric patients. Few studies have described the coexistence of NCS and IgAN with pathological and radiological confirmation, particularly in children. Cases of NCS combined with IgAN can occur and should not be overlooked because of the associated morbidity.
NCS can develop at any age, although mostly in the second and third decades of life [
9]. The clinical manifestations of NCS include hematuria, orthostatic proteinuria, left flank pain, and, although rarely, pelvic congestion symptoms [
1]. Hematuria and proteinuria are generally considered to be caused by elevated LRV pressure, resulting in the rupture of thin-walled collateral veins into the calyceal fornix [
9]. NCS is diagnosed using various tools, such as kidney Doppler ultrasonography, computed tomography, magnetic resonance imaging, and retrograde left renal venography [
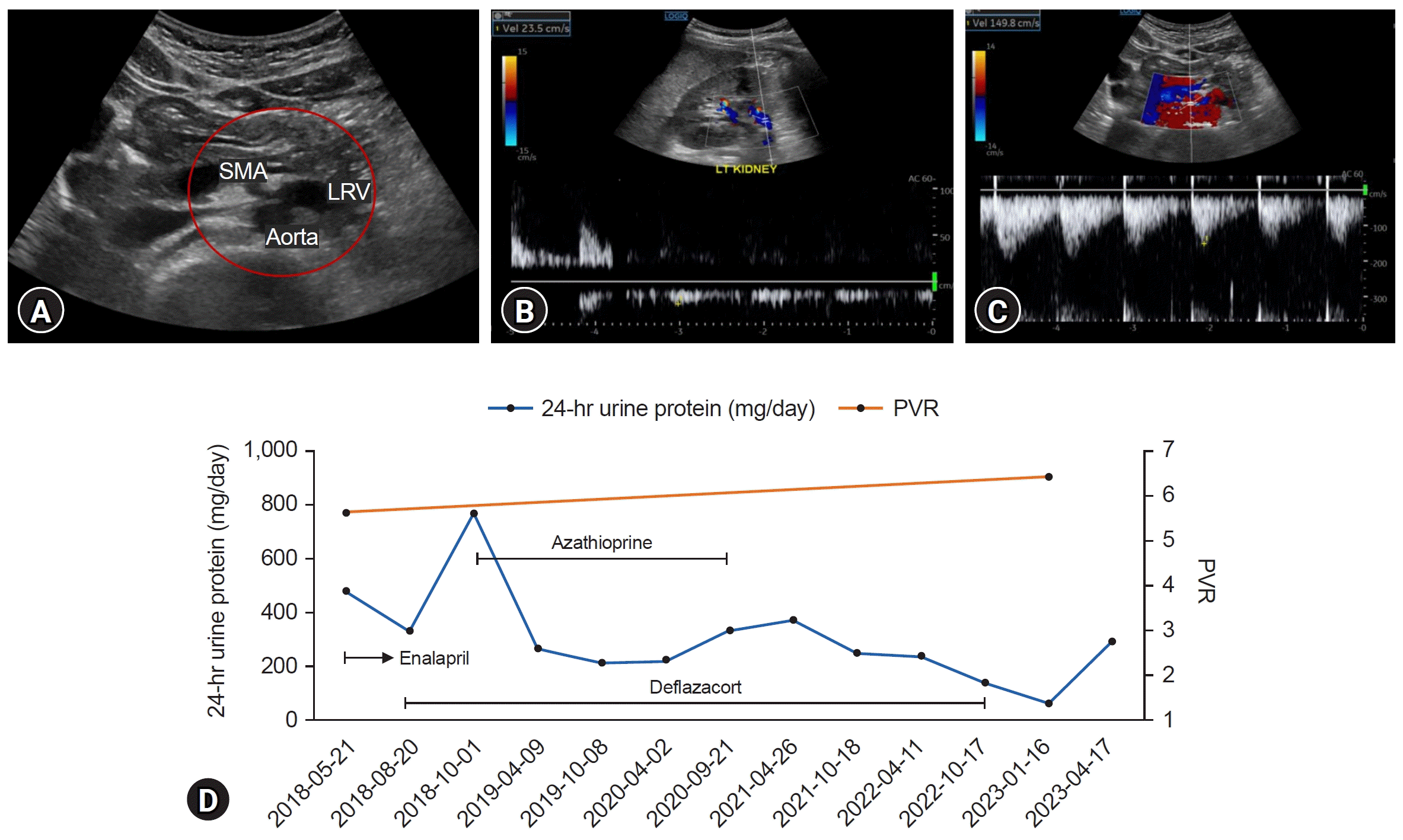
2]. The first imaging tool for suspected NCS was kidney Doppler ultrasonography, with a sensitivity and specificity of 82.3% and 89% to 100%, respectively. However, there are no optimal cutoffs for PVR for diagnosing NCS in children due to the small LRV sampling area and measurement variability according to the patient positioning [
10]. Park et al. [
11] suggested that a cutoff value greater than 4.0 for the PVR should be used as a sonographic criterion to diagnose NCS in children. In our study, two patients were diagnosed with NCS through Doppler ultrasonography with a cutoff value greater than 4.0 for PVR. Meanwhile IgAN, characterized by predominant IgA deposits in glomerular mesangial areas, is the most common form of GN worldwide. Similar to NCS, the clinical features of IgAN can also present as macroscopic hematuria, asymptomatic microscopic hematuria with or without proteinuria, nephrotic syndrome, and, rarely, acute kidney injury [
4]. Although the coexistence of NCS and IgAN is rare, several cases have been reported [
3,
5-
8]; however, a clear correlation is yet to be confirmed. Ozono et al. [
5] reported the case of two young adults with NCP complicated by IgAN. Compared to 10 patients showing only NCP, those with NCP and IgAN showed dysmorphic RBCs, suggesting glomerular hematuria, aggravation of hematuria after upper respiratory infections, persistence of proteinuria, urinary granular casts, and elevation of serum IgA levels. Subsequently, Shin et al. [
3] reported a case of NCS combined with IgAN in a 9-year-old girl with gross hematuria. In a Japanese study [
8], 10 of 146 patients with IgAN showed LRV entrapment, and all patients had dysmorphic urinary RBCs. Recently, a case of coexisting NCP and superior mesenteric artery syndrome in a patient with IgAN was reported [
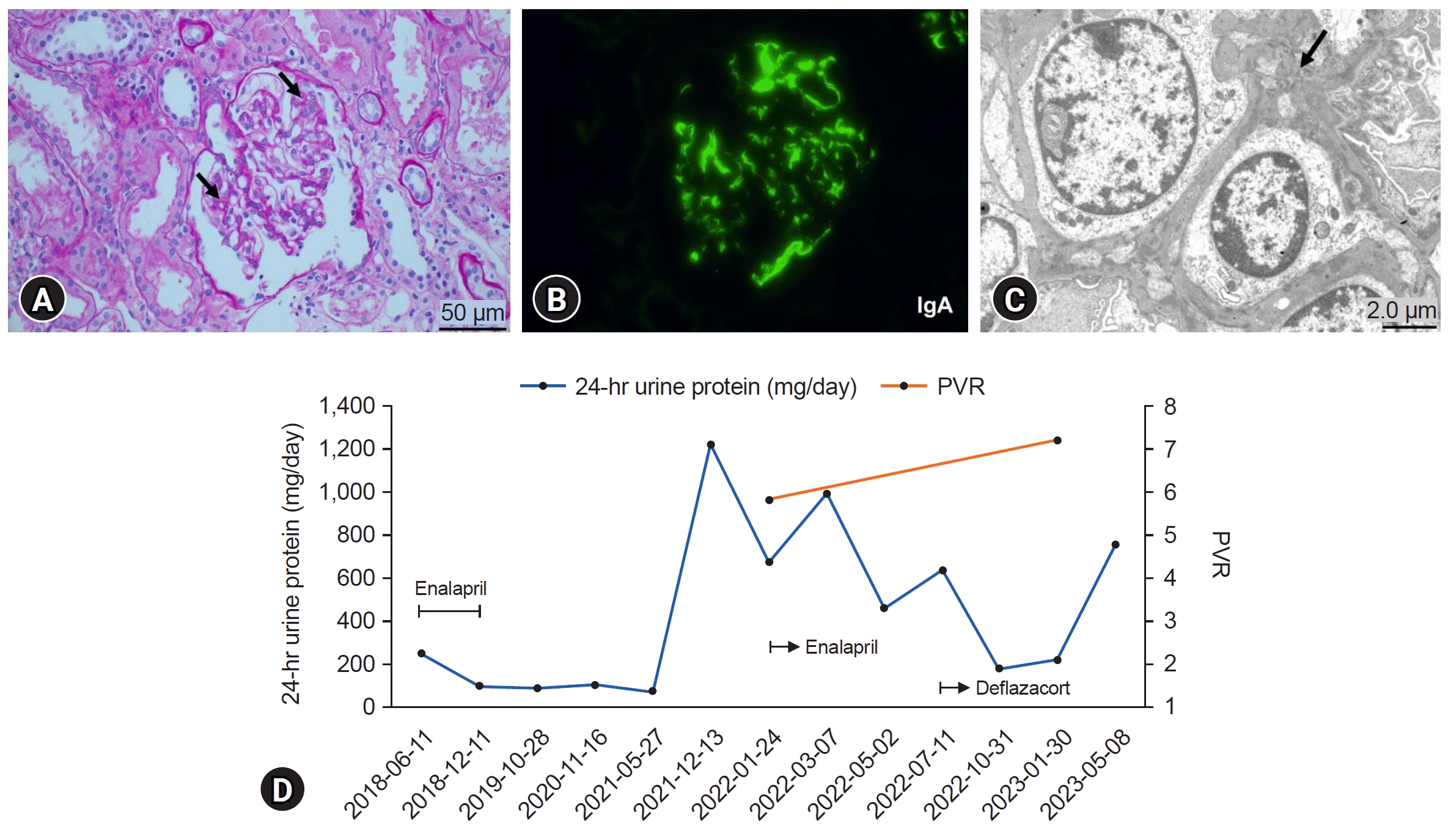
6]. In our study, the two boys were diagnosed with NCS and IgAN. Case 1 had a history of microscopic hematuria 2 years ago, and case 2 had a familial history of thin GBM disease. In case 1, serum IgA levels were elevated in the initial laboratory findings, and urine dysmorphic RBCs were not found. The serum IgA level in case 2 was initially normal but was continuously elevated during the follow-up examination, and dysmorphic RBCs were observed on urinalysis. While case 1 was diagnosed with NCS on initial kidney Doppler ultrasonography, kidney biopsy showed IgAN. In case 2, NCS was confirmed when proteinuria was aggravated 4 years after the initial presentation. The kidney biopsy findings were consistent with IgAN. A causal relationship between NCS and IgAN may exist because of their relatively common combinations.
Several studies have investigated how NCS can induce glomerular changes and are related to IgAN. According to the proposed hypothesis of the IgAN pathogenesis, increased amounts of galactose-deficient IgA1 are recognized as autoantigens by autoantibodies (mostly of the immunoglobulin G subclass) to form pathological immune complexes, some of which are deposited in the glomeruli and induce kidney injury [
4]. Various pathological findings of IgAN are present in addition to IgA deposits in the mesangial areas on immunofluorescence. They are classified according to the Oxford classification as mesangial hypercellularity, endocapillary hypercellularity, segmental glomerulosclerosis, tubular atrophy/interstitial fibrosis, and cellular crescents [
4]. In a study by Ha and Lee [
12], kidney biopsy showed moderate mesangial hypercellularity and increased mesangial matrix in a patient diagnosed with NCS. These authors suggested that the mesangial lesions might have been induced by the angiotensin II (Ang II) effect caused by NCS. Keane and Raij [
13] also found that the accumulation of phlogogenic macromolecules caused by Ang II in the glomerular mesangium may induce mesangial expansion and eventual glomerulosclerosis. Given that vascular endothelial cells actively participate both in innate and adaptive immune responses [
14], LRV entrapment in NCS may cause venous congestion and endothelial activation, contributing to impairment of the immune system. Notably, in a recent large cohort study examining the association between NCP and GN, the prevalence of LRV entrapment was higher in patients with IgAN and IgA vasculitis with nephritis (Henoch-Schönlein purpura nephritis) after adjusting for age, sex, and BMI. Furthermore, glomerular IgA and galactose-deficient IgA1 deposition was more common in patients with lupus nephritis and IgAN-unrelated diseases with LRV entrapment than in those without LRV entrapment [
7].
Kidney biopsy can be an important tool for the definite diagnosis of GN. In the present case, kidney biopsies were performed to diagnose possible combined GN. Since it is difficult to diagnose complicated IgAN in patients with NCS based on clinical and laboratory findings, kidney biopsy should be considered when proteinuria or hematuria is aggravated in patients with NCS. Conversely, kidney Doppler sonography can be performed in patients with an unusual clinical course of GN. While proteinuria with hematuria was initially reduced after immunosuppressant administration in case 1, proteinuria has since been waxing and waning and persisted for a long time relative to the kidney biopsy findings. As NCS persists, prolonged proteinuria can be attributed to both IgAN and NCS. In case 2, NCS was diagnosed when proteinuria was aggravated. Since he was not initially evaluated for the presence of NCS, we could not confirm how NCS contributed to the development and progression of IgAN. However, his proteinuria and hematuria were relatively severe in comparison to the kidney biopsy findings and persisted despite taking immunosuppressants. NCS continued on follow-up ultrasonography. Considering the mechanism by which NCS can influence glomerular changes mentioned earlier, NCS may have affected our patients’ disease courses. Furthermore, Shimada et al. [
15] reported that renal congestion caused tubulointerstitial and glomerular injury in a rat model. In our cases, cystatin C levels were elevated, and considering the renal congestion associated with renal function decline, this observation can be attributed to NCS. Although additional studies are needed, our cases suggest that renal venous congestion due to LRV entrapment could induce proteinuria and hematuria, leading to renal damage.
Conservative care is the first-line treatment of NCS in children. In a systematic review, improvement or complete resolution was observed in nearly 95% of patients treated with a conservative approach over 24 months [
10]. Compared with NCS, the clinical course of IgAN is variable, ranging from an asymptomatic non-progressive disease to a highly aggressive disease. Poor prognosis is correlated with proteinuria ≥1 g/day, sustained hypertension, and severe renal involvement. Angiotensin-converting enzyme inhibitors and Ang II receptor blockers can effectively control proteinuria and hypertension. Combined immunosuppressive therapy can be considered in patients with persistent proteinuria or worsened kidney function [
4]. Since IgAN is a significant cause of chronic kidney disease, and NCS and IgAN have a different treatment and prognosis, it is important to diagnose these two diseases at an early stage and determine appropriate management to improve clinical prognosis in the future.
In summary, our study’s finding of NCS combined with IgAN may be coincidental; however, the relationship between the two disease entities is supported by our patients’ clinical courses and previous studies. Future multicenter studies involving large sample sizes are needed to clarify the relationship between NCS and GN, independent to IgAN. Despite NCS diagnosis, considering the presence of combined GN in case of aggravated proteinuria or hematuria is crucial for appropriate management. Furthermore, clinicians must be aware of combined NCS when the clinical course of GN is uncommon. Likewise, a high suspicion and timely imaging or biopsy are essential for the accurate diagnosis of NCS combined with glomerulopathy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download