Abstract
Refractory status epilepticus (RSE) is defined as the persistence of either clinical or electrographic seizures despite the administration of appropriate doses of an initial benzodiazepine and suitable second-line antiepileptic drugs (AEDs). The Neurocritical Care Society and the American Epilepsy Society have proposed a treatment paradigm for the management of convulsive status epilepticus (CSE). The third-line therapy in refractory CSE may involve general anesthesia using intravenous midazolam, propofol, or other agents, while recent evidence supports the use of ketamine to manage RSE in both adults and children. However, although these treatment strategies are frequently employed in nonconvulsive status epilepticus (NCSE), the efficacy of AEDs and anesthetics in NCSE has not been thoroughly investigated. Recent evidence has demonstrated the safety and efficacy of newer AEDs, including levetiracetam and lacosamide, in the treatment of status epilepticus (SE) and RSE, which also encompasses NCSE. Use of multiple combinations of various intravenous AEDs can also be considered in NCSE before the administration of general anesthesia. In addition, AEDs alone exhibit limited effectiveness in managing SE for new-onset RSE (NORSE) and its subset, febrile infection-related epilepsy syndrome. Therefore, in cases of refractory status, it is imperative to explore treatment options beyond AEDs, including immunotherapy and the incorporation of a ketogenic diet. The present review suggests treatment approaches for RSE based on subgroups, including CSE, NCSE, and NORSE. A discussion of recent clinical studies on AEDs and anesthetics in the management of RSE, as well as proposed treatment methods for NORSE, is also provided.
Refractory status epilepticus (RSE) is characterized by seizures that persist despite administration of first-line treatment with benzodiazepines and second-line options, including “classic” anticonvulsant therapy, such as phenytoin (PHT)/fosphenytoin, valproate (VPA), or levetiracetam (LEV). Typically, managing RSE necessitates the use of anesthetics and continuous electroencephalogram (EEG) monitoring [1,2]. The prevailing guidelines suggest an immediate stepwise intervention, starting with benzodiazepines as the initial monotherapy, and if status epilepticus (SE) persists, second-line drugs should be incorporated sequentially [3]. In experimental models, extended seizures result in the internalization of gamma-aminobutyric acid A (GABAA) receptors, while the concentration of glutamate receptors, particularly an N-methyl-D-aspartate (NMDA) receptor, increases at the synapse [4]. Further, following internalization, GABA receptors undergo reconfiguration, rendering them insensitive to benzodiazepines. Additionally, these receptors are preferentially relocated to extrasynaptic sites. These changes involve alterations in GABAA receptor function and the transmembrane gradient for chloride, both of which diminish the capacity of benzodiazepines to enhance inhibitory synaptic signaling [5]. Hence, resistance to benzodiazepines may also be alleviated by alternative mechanisms, including modifications in other ion channels, including sodium or cholinergic mechanisms [4]. Furthermore, promising candidates among clinically available agents that target NMDA receptors include ketamine and those targeting α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA) receptors, such as perampanel. These receptors appear to be upregulated in SE, and also play a role in the degradation of GABAA receptor-mediated inhibition. Investigations of the early administration of ketamine for treating RSE, often in combination with other drugs, in animal models have yielded promising results [3]. Further, recent research has demonstrated the remarkable neuroprotective effects of ketamine, achieved through the blockade of NMDA receptors, even when administered following the onset of SE [6].
The American Epilepsy Society has proposed practical conclusions and an integrated treatment algorithm for the management of convulsive status epilepticus (CSE) across the age spectrum, from infants through adults [7]. The Neurocritical Care Society has recommended guidelines for CSE based on the literature, utilizing standardized assessment methods from the American Heart Association and the Grading of Recommendations Assessment, Development, and Evaluation system [8]. Nevertheless, no consensus exists on how aggressively to approach nonconvulsive SE (NCSE) during RSE treatment. We have previously attempted intravenous polytherapy with antiepileptic drugs (AEDs); however, this strategy is marred by the controversy regarding when and how to administer anesthetics for refractory NCSE. New-onset RSE (NORSE) and its subset, febrile infection-related epilepsy syndrome (FIRES), is an uncommon and severe condition characterized by the sudden onset of RSE without any identifiable acute or active structural, toxic, or metabolic cause. Thus far, no randomized controlled trials have investigated the management of NORSE, and consensus guidelines are currently lacking. Recently, the International NORSE Consensus Group issued recommendations for managing NORSE, backed by supporting evidence. Therefore, when addressing the management of RSE, distinct treatment algorithms tailored to specific subgroups including CSE, NCSE, and NORSE of RSE must be considered.
This review explored the recent findings related to the administration of anesthetics, including the use of ketamine, which has garnered significant attention in both pediatric and adult cases of RSE. Within specific subgroups of RSE, I emphasized the crucial considerations of when and how to initiate anesthesia in the treatment of NCSE. Additionally, I discussed recent recommendations regarding the implementation of ketogenic diets and immunotherapy in cases of NORSE.
In guidelines established by the American Epilepsy Society and International League Against Epilepsy, SE is defined using two time points, denoted as t1 and t2; t1 represents the duration beyond which seizures are likely to be prolonged, while t2 represents the time beyond which seizures can result in long-term consequences. For tonic-clonic seizures, t1 is set at 5 minutes, and t2 is set at 30 minutes. However, for focal SE or absence status, these specific time points either differ or remain unknown [7,9].
Diagnosing NCSE poses a significant challenge in the medical field. The Salzburg consensus criteria achieves high sensitivity (97.7%) and specificity (90%) by relying on electrographic/electroclinical features. These criteria include EEG evidence of rhythmic epileptiform discharges at a frequency >2.5 Hz, or rhythmic EEG discharges at a frequency ≤2.5 Hz accompanied by spatiotemporal evolution, subtle clinical changes correlating with EEG alterations, or EEG and clinical improvement after intravenous AEDs therapy [10]. The latest standardization of critical care EEG terminology by the American Clinical Neurophysiology Society has integrated the Salzburg criteria, while also mandating the continuous presence of EEG changes indicative of NCSE for a minimum duration of 10 min or 20% of any 60-minute EEG recording [11]. Currently, in the management of NCSE, no consensus exists on how aggressively to pursue treatment. Considering the principle of “Time is brain,” the treatment paradigm for NCSE is similar to that of CSE. Experiments in animal models have yielded substantial evidence to indicate the infliction of neuronal damage during prolonged episodes of NCSE [12,13]. Further, investigations in rat models have shown neuronal loss, sustained immune response, and changes in synaptic proteins crucial for maintaining the excitatory/inhibitory balance [14]. In a clinical study, Cheng confirmed the prior finding that a 30-minute delay in therapy initiation in NCSE was linked to elevated morbidity and mortality [15], Another prospective study indicated that early initiation of treatment leads to good control of NCSE [16].
Nevertheless, in patients with NCSE, the underlying etiology is a critical prognostic factor, and evaluating the sole impact of NCSE on neuronal damage in patients is often challenging [17]. Currently, there is little high-quality evidence to guide the best management practices. Hence, when making decisions regarding the management of RSE, it is imperative to assess the potential risks associated with general anesthesia. Therefore, factors such as the severity of the underlying brain injury, patient’s age, comorbidities, and the overall goals of care must be considered, especially in cases of NCSE.
NORSE is a rare but profoundly devastating condition encompassing a range of diseases and disorders. It is characterized by the sudden onset of uncontrollable seizures, referred to as RSE, without any identifiable acute or active structural, toxic, or metabolic cause [18]. FIRES is regarded as a subset of NORSE rather than a distinct entity, and is characterized by the presence of a preceding febrile infection occurring between 2 weeks and 24 hours before the onset of RSE [19]. Currently, the evidence to guide the treatment of NORSE is primarily sourced from case reports, case series, and limited observational studies, and there are currently no available randomized controlled trials or consensus guidelines for the management of this condition. Recently, Wickstrom et al. [20] developed recommendations for diagnostic approaches, evaluation, and treatment utilizing the Delphi consensus approach, endorsed by the International League Against Epilepsy. According to the recommendations, the disease characteristics for NORSE/FIRES allow for diagnoses at any age, although the patterns of immune activation may vary between different age groups. Differentiating cases that are secondary to identifiable autoimmune encephalitis from cryptogenic NORSE is crucial, as it is likely to assist in determining the appropriate treatment and establishing the prognosis. In terms of acute phase management, the consensus is that treatment of seizures with AEDs and anesthetics during the initial 48 hours should follow a similar approach to the acute treatment of RSE in other conditions.
Current guidelines recommend that the management of NORSE/FIRES patients should be conducted in a tertiary center equipped with the necessary resources and the input of a multidisciplinary team of experts in epileptology, rheumatology, immunology, and intensive care. Additionally, a crucial distinction from the majority of treatment algorithms for RSE involves the incorporation of immunotherapy. The summary of the treatment strategy for SE specific to each subgroup within RSE is presented in Fig. 1.
When treating patients with SE, it is critical to assess airway, breathing, and circulation as a fundamental aspect of care. Respiratory failure or the presence of markers indicating cardiac injury occurs in approximately one-third of SE episodes, and is significantly associated with poor outcomes [21]. Further, the administration of a high dose of AEDs or anesthetics may contribute to respiratory depression, and it is important to note that up to one-third of patients with SE develop neurocardiogenic pulmonary edema, adding to the complexity of managing their condition [22]. Infections can occur as complications in up to 50% of SE cases [23]. Infections in SE cases are linked to a prolonged duration of SE, the requirement for mechanical ventilation, unfavorable discharge outcomes from the hospital, and overall poor recovery [24]. Prolonged seizures can lead to complications such as rhabdomyolysis. Patients experiencing rhabdomyolysis often exhibit additional abnormalities, including hyperkalemia, hyperphosphatemia, and hypocalcemia, particularly in cases involving severe acute kidney injury [25]. As such, intensive medical care management is essential. When treating patients with RSE, it is imperative to take into account the aforementioned systemic complications. In addition, continuous intravenous neuromuscular blocking drugs could mask ongoing seizure activity, making it challenging for clinicians to detect seizures without EEG monitoring. As such, it is prudent to avoid these drugs in such cases. Continuous EEG monitoring is essential when using anesthetics in the intensive care unit (ICU). The justification for tracheal intubation in suspected or confirmed NCSE has not yet been firmly established and requires meticulous evaluation. This assessment must weigh the evident risks associated with prolonged tracheal intubation and ICU stay against the uncertain benefits of therapeutic coma. It is also recommended to explore trials of non-sedative intravenous second-line AEDs before resorting to anesthetic induction in NCSE.
Current continuous intravenous anesthetics’ options include midazolam, propofol, and pentobarbital in the management of RSE or super RSE. Additionally, recent reports have explored the use of ketamine as an alternative agent. However, there are currently no randomized controlled trials to provide guidance on the selection of anesthetic drugs for the treatment of RSE. As such, which anesthetic third-line treatment for RSE offers superior outcomes, minimizes adverse effects, and is most effective in halting seizures in the ICU currently remains unclear. Most of the existing studies lack uniform data collection instruments, making it exceptionally challenging to draw meaningful comparisons between patients [26]. However, one recent retrospective, multicenter, observational cohort study was conducted to compare the efficacy of propofol (median maximum dose: 37 µg/kg/min [interquartile range, IQR: 23–56 µg/kg/min]) and midazolam (median maximum dose: 0.5 mg/kg/hr [IQR: 0.2–2.0 mg/kg/hr]) in the treatment of RSE in the ICU [27]. A favorable outcome was observed in 25 and 21% of patients treated with propofol and midazolam, respectively. The need for vasopressors, and occurrence of lactic acidosis, hyperkalemia, rhabdomyolysis, and hypertriglyceridemia was reported with comparable frequencies in patients treated with both propofol and midazolam.
Ketamine, functioning as an NMDA receptor antagonist, is currently gaining prominence as the most encouraging alternative among anesthetics in the management of RSE. The primary and highly appealing theoretical benefit of ketamine lies in its distinct mechanism of action. Unlike standard anesthetics that act on GABA receptors, ketamine targets an alternative pathway via action on the NMDA receptor. This unique characteristic opens new avenues for the management of RSE [28,29]. Additionally, research on the neuroprotective effects of ketamine has accumulated substantial scientific data, showcasing the potential advantages of this drug [6]. Furthermore, ketamine demonstrates fewer cardiovascular and respiratory side effects compared to those associated with other anesthetics [1]. Conversely, ketamine is associated with numerous drawbacks, including hallucinations and sympathetic adrenergic effects, leading to elevated intracranial pressure [30]. One systematic review, included 244 cases of SE treated with ketamine (starting dose: 0.2 mg/kg/hr [IQR: 0.1–0.5 mg/kg/hr] and continued for 1.6 days [IQR: 0.6–2.9 days]), encompassing 13 case reports and five case series in adults, along with four case reports and three case series in children that were extracted from the PubMed database. The overall rate of success was 74% (153 out of 207 cases) in adults and 73% (27 out of 37 cases) in children [31]. Further, two recent studies have highlighted the use of ketamine for RSE and super RSE. One was a single-center retrospective study of 69 children who received ketamine for RSE [32]. Ketamine infusions were initiated at 1 mg/kg/hr in 66 of 69 patients (96%) and continuous infusion doses were 1–7 mg/kg/hr. The median total duration of ketamine infusion was 85.7 hours (IQR: 49.7–128.0 hours). Seizure termination was notably more successful when ketamine was administered as the initial anesthesia, compared to its success in cases where it was used after midazolam had been attempted first and proved ineffective. Furthermore, the in-hospital adverse effects were considered limited and manageable. This study contributed significant data from neonates and younger children to the global literature, with strong results indicating that ketamine can be utilized effectively and safely in the treatment of RSE in both adults and children. The second study involved a consecutive series of 68 adult patients diagnosed with super RSE, all of whom received ketamine treatment between 2009 and 2018 [33]. The average dose of ketamine infusion was 2.2±1.8 mg/kg/hr, with median duration of 2 days (IQR: 1–4 days). Within the first 24 hours of initiating ketamine treatment, the seizure burden decreased by 50%, and complete cessation was observed in 63% of cases. In this particular study, 11 patients underwent multimodal monitoring in the ICU, and ketamine administration was linked to a stable mean arterial pressure, leading to reduced vasopressor requirements over time. Moreover, there were no discernible effects on intracranial pressure, cerebral blood flow, or cerebral perfusion pressure in cases involving both traumatic and nontraumatic brain injuries. These results indicate that ketamine treatment is advantageous for RSE, with high doses demonstrating improved hemodynamics without an increase in intracranial pressure. This study corroborates the results of previous case reports and series suggesting that ketamine is a favorable option for the management of patients with hemodynamically unstable RSE [34,35].
Considering the pathomechanism of SE, where GABAA receptors are internalized and glutamate receptors increase their concentration at the synapse, initiating c with a combination of a benzodiazepine and a second-line drug, such as LEV that modulates glutamate receptors, or other multi-action drugs, such as VPA, appears to be a practical approach. In third-line therapy, several studies have indicated favorable outcomes with early anesthetic combination therapies for RSE or super RSE, such as propofol-ketamine or midazolam-ketamine [35,36]. Early initiation of combined anesthetics may be beneficial, considering the underlying pathomechanism, and the need to mitigate side effects resulting from the high accumulation of propofol or barbiturates, by including propylene glycol.
Different anesthetics have similar efficacy for RSE, but are accompanied by a unique set of side effects. Side effects can impose limitations on the duration of infusion that can be administered. Consequently, the decision to continue should involve monitoring specific laboratory values (such as creatinine kinase, acid/base balance, serum lactate, and lipid profile) and conducting electrocardiograms and echocardiograms. Neurointensivists must assess whether or not each patient’s symptoms are related to the anesthetic drugs before proceeding further. Anesthetics management could be personalized, considering the individual characteristics of each patient.
The advantages and disadvantages, and initial and maintenance dose of each anesthetic which are summarized in Table 1, play a crucial role in their selection. When treating RSE with anesthesia, the primary goal is seizure suppression. However, the optimal extent of seizure suppression, including the need for burst suppression, remains unclear in the management of SE. In addition, while a continuous infusion is typically maintained for 24–48 hours before weaning [37], the duration of therapeutic coma is a subject of controversy. A recent observational study suggested that a deeper and shorter duration of therapeutic coma may be associated with a decreased risk of withdrawal seizures and complications related to prolonged hospitalization [38]. Therefore, considering the patient’s medical condition, we should aim to wean anesthetics as soon as possible after 24–48 hours with sufficient seizure suppression. Additionally, other nonanesthetic AEDs should be added to prevent the re-emergence of seizures during the anesthesia weaning process.
Volatile inhalational anesthetics have been explored as an alternative salvage therapy in cases of super RSE. These anesthetics are believed to suppress seizure activity by inhibiting NMDA excitotoxicity and activating GABA receptors [39]. Inhalational anesthetics offer advantages such as easy titration to EEG at the bedside and an ultrafast onset of action. Nevertheless, existing studies on inhalational anesthetics show disparity in outcomes, lack comparative groups, and exhibit variations in the treatment regimens. Furthermore, the utilization of these anesthetics is curtailed by the potential for serious adverse events, particularly following prolonged usage [39].
Table 2 summarizes the pharmacological characteristics of intravenous AEDs used in the management of SE. Traditionally, guidelines recommend the administration of intravenous fosphenytoin/PHT (20 mg/kg PHT equivalent), VPA (20–40 mg/kg), phenobarbital (PB; 15–20 mg/kg), and LEV (60 mg/kg or 3,000–4,500 mg) for the treatment of established SE resistant to benzodiazepines [7].
In the recent literature, the majority of studies pertaining to LEV have focused on its application in established SE. Three significant randomized trials published in 2019 examined larger loading doses of LEV specifically for established CSE. Notably, the Class I ESETT study involved 384 patients (ranging in age from 1 to 95 years old) with benzodiazepine-resistant established CSE. These patients were randomized using a Bayesian adaptive design to receive fosphenytoin at 20 mg PE/kg, LEV at 60 mg/kg, or VPA at 40 mg/kg [40]. Termination of SE was observed in comparable percentages with LEV (47%), fosphenytoin (45%), and VPA (46%). No significant disparities were noted in terms of enhanced consciousness levels or major safety incidents. However, there were numerically more instances of hypotension and intubation associated with fosphenytoin, while LEV was found to be linked to a higher number of fatalities. In the Class III EcLiPSE open-label randomized controlled trial conducted in the United Kingdom, 286 children experiencing CSE following initial treatment with a benzodiazepine were compared in terms of second-line therapy. This study evaluated the efficacy of intravenous LEV at a loading dose of 40 mg/kg and intravenous PHT at 20 mg/kg [41]. CSE was terminated more frequently with LEV compared to its frequency of termination with PHT (P=0.20). Furthermore, serious adverse events were reported with PHT, including life-threatening hypotension, exacerbated focal seizures, and a decreased level of consciousness. In the Class III open-label randomized controlled Convulsive Status Epilepticus Paediatric Trial (ConSEPT) conducted in Australia and New Zealand, 352 children experiencing CSE following initial benzodiazepine treatment were evaluated for second-line therapy. This study compared the effectiveness of intravenous LEV at a loading dose of 40 mg/kg, and intravenous PHT at 20 mg/kg [42]. Seizure activity ceased clinically within 5 minutes following the completion of the loading dose in 60% of children in the PHT group and 50% in the LEV group (P=0.16), and no significant adverse events were reported.
Lacosamide (LCS) is available as an IV solution. Between 2009 and 2019, a total of 32 clinical trials exploring the use of intravenous LCS for SE treatment were conducted. These trials employed varied definitions of RSE, with some encompassing a significant portion of focal SE or electrographic NCSE cases. The efficacy of LCS in managing recurrent electrographic nonconvulsive seizures was evaluated through a comparison with fosphenytoin (intravenous LCS 400 mg vs. fosphenytoin 20 mg; PHT equivalent per kilogram). In a prospective, multicenter, randomized controlled trial designed to assess noninferiority, LCS demonstrated superiority over fosphenytoin in preventing seizure recurrence, with rates of 63.3% vs. 50% (P=0.02) [43]. A recent comparative review involving 115 cases of LCS and 166 cases of PHT demonstrated comparable rates of seizure control and adverse events. However, patients with PHT exhibited a higher incidence of serious side effects compared to those associated with LCS (5.1 vs. 0.8%, P=0.049) [44]. In a recent review conducted by the American Epilepsy Society Treatment Committee, there is a suggestion that LCS might be effective at halting RSE for both children and adults [45].
PB, one of the first antiepileptic medications to be developed, can be employed as a second-line therapy for managing controlled SE. Nevertheless, the utilization of PB was restricted due to its increased incidence of sedation, respiratory depression, and hypotension, as well as a higher number of drug interactions than those associated with LEV or LCS. Recently, the use of PB is being reconsidered. During the eighth London-Innsbruck Colloquium on Status Epilepticus in 2022, Eugen Trinka emphasized the high efficacy of PB use due to its GABAergic and anti-glutamatergic properties. Consequently, considering its mechanisms of action, the application of PB in both early and established cases of SE, including RSE, appears to be justified [46]. In 2019, one study aimed to assess the relative effectiveness and safety of AEDs in adults experiencing CSE resistant to benzodiazepines. Five randomized controlled trials were analyzed, encompassing 349 patients. PB exhibited the highest likelihood of achieving optimal control of SE and seizure freedom, while VPA and LCS were found to have the best safety outcomes. No differences in the incidence of respiratory depression and hypotension were observed among the medications [47]. One prior study further investigated the effectiveness and safety of mega-dose PB (mega-dose [MDPB]; enteral or parenteral PB >10 mg/kg/day) for managing super refractory status epilepticus. Half of the patients achieved successful control of super RSE with a median duration of 45.5 days for MDPB treatment. The median maximum serum PB level reached 151.5 μg/mL [48]. Taking these findings into account, and considering the reduced risk of respiratory depression compared to those associated with other anesthetics, MDPB could be a viable and advantageous treatment choice for refractory NCSE.
NCSE, even if prolonged, may not require intensive care or anesthetic management, as the clinical variables are related to prognosis [39]. As yet, no extended prospective studies have yet been conducted to delineate the natural progression of NCSE or identify which patients would benefit from aggressive treatment. Therefore, when contemplating the use of anesthetic drugs for third-line therapy in refractory NCSE, it is crucial to assess whether this approach is more beneficial than it is detrimental to the patients involved. Considering the recent more favorable evidence data for intravenous AEDs, the initial consideration for third-line therapy in refractory NCSE could lean towards adding more second-line AEDs rather than opting for anesthetics.
In 2022, the International NORSE Consensus Group presented recommendations for managing NORSE, including FIRES [20]. In the acute phase of NORSE, an expert consensus supports the management of seizures with AEDs and anesthetics in the initial 48 hours should align with the acute treatment protocols followed for RSE in other conditions. Experts recommend initiating first-line immunotherapy, which may include corticosteroids, intravenous immunoglobulins, or therapeutic plasma exchange (TPE), within the initial 72 hours following the onset of SE in NORSE/FIRES cases. However, panel members exhibited significant disparities in their perspectives on the utilization of TPE. Consequently, this consensus document refrained from providing a specific recommendation regarding TPE, except for emphasizing that its use should not impede the initiation of subsequent treatments. Due to the probable engagement of immune mechanisms in perpetuating seizures, the consensus group also advocates for the initiation of a ketogenic diet and second-line immunotherapies within one week for noninfectious NORSE/FIRES patients exhibiting an insufficient response to first-line immune treatment. As existing evidence is insufficient to clearly endorse any particular second-line immunological treatment, the decision should be guided by the suspected underlying cause. For example, rituximab should be the first choice in the majority of cases where a pathogenic antibody is identified, or there is a strong suspicion of an autoimmune process. For cryptogenic NORSE/FIRES without evident clinical features of a specific autoimmune encephalitis syndrome, the administration of anakinra (interleukin 1 [IL]-1 receptor antagonists) or tocilizumab (IL-6 blockers) should be seriously contemplated.
In SE, early treatment and cessation of SE is crucial. However, when implementing aggressive treatment with general anesthesia and coma therapy, it is crucial to rely on evidence-based treatment modalities to prevent mortality and complications arising from RSE. Therefore, in the management of SE, status epilepticus could be classified into subgroups, such as CSE, NCSE and NORSE including FIRES. In refractory NCSE, the patient’s medical condition and weighing the benefits and risks of using anesthetics must be considered. Alternate options, such as add-on multiple non-sedative intravenous AEDs therapy, can be considered. Intravenous MDPB therapy could also be considered an effective treatment for super RSE when tapering out prolonged use of anesthetics. When choosing anesthetics, the choice should be individualized and it is important to consider the pros and cons of each drug before determining their use. Ketamine is favorable for children with RSE and adults with a hemodynamic unstable condition, and ketamine could be used as an add-on with other anesthetics with a GABAergic mechanism. In cases of NORSE, including FIRES, clinicians should consider administering immunotherapy and implementing a ketogenic diet. Management in each subgroup necessitates a multidisciplinary approach involving neurologists, intensivists, and other specialists. Individualized care based on each patient’s specific characteristics and underlying condition is paramount in improving outcomes in RSE.
REFERENCES
1. Legriel S, Oddo M, Brophy GM. What's new in refractory status epilepticus? Intensive Care Med. 2017; 43:543–6.
2. Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011; 134:2802–18.
3. Amengual-Gual M, Sánchez Fernández I, Wainwright MS. Novel drugs and early polypharmacotherapy in status epilepticus. Seizure. 2019; 68:79–88.
4. Joshi S, Rajasekaran K, Hawk KM, Chester SJ, Goodkin HP. Status epilepticus: role for etiology in determining response to benzodiazepines. Ann Neurol. 2018; 83:830–41.
5. Burman RJ, Rosch RE, Wilmshurst JM, Sen A, Ramantani G, Akerman CJ, et al. Why won't it stop? The dynamics of benzodiazepine resistance in status epilepticus. Nat Rev Neurol. 2022; 18:428–41.
6. Fujikawa DG. Starting ketamine for neuroprotection earlier than its current use as an anesthetic/antiepileptic drug late in refractory status epilepticus. Epilepsia. 2019; 60:373–80.
7. Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016; 16:48–61.
8. Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012; 17:3–23.
9. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus: report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015; 56:1515–23.
10. Trinka E, Leitinger M. Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy Behav. 2015; 49:203–22.
11. Hirsch LJ, Fong MW, Leitinger M, LaRoche SM, Beniczky S, Abend NS, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021; 38:1–29.
12. Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanisms of epileptic brain damage. Adv Neurol. 1986; 44:857–77.
13. Meldrum BS. Excitotoxicity and selective neuronal loss in epilepsy. Brain Pathol. 1993; 3:405–12.
14. Avdic U, Ahl M, Chugh D, Ali I, Chary K, Sierra A, et al. Nonconvulsive status epilepticus in rats leads to brain pathology. Epilepsia. 2018; 59:945–58.
15. Cheng JY. Latency to treatment of status epilepticus is associated with mortality and functional status. J Neurol Sci. 2016; 370:290–5.
16. Gutiérrez-Viedma Á, Parejo-Carbonell B, Cuadrado ML, Serrano-García I, Abarrategui B, García-Morales I. The relevance of timing in nonconvulsive status epilepticus: a series of 38 cases. Epilepsy Behav. 2018; 82:11–6.
17. Power KN, Gramstad A, Gilhus NE, Engelsen BA. Adult nonconvulsive status epilepticus in a clinical setting: semiology, aetiology, treatment and outcome. Seizure. 2015; 24:102–6.
18. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018; 59:739–44.
19. Gaspard N, Hirsch LJ, Sculier C, Loddenkemper T, van Baalen A, Lancrenon J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. 2018; 59:745–52.
20. Wickstrom R, Taraschenko O, Dilena R, Payne ET, Specchio N, Nabbout R, et al. International consensus recommendations for management of New Onset Refractory Status Epilepticus (NORSE) incl. Febrile Infection-Related Epilepsy Syndrome (FIRES): statements and supporting evidence. Epilepsia. 2022; 63:2840–64.
21. Hawkes MA, Hocker SE. Systemic complications following status epilepticus. Curr Neurol Neurosci Rep. 2018; 18:7.
22. Busl KM, Bleck TP. Neurogenic pulmonary edema. Crit Care Med. 2015; 43:1710–5.
23. Sutter R, Tschudin-Sutter S, Grize L, Fuhr P, Bonten MJ, Widmer AF, et al. Associations between infections and clinical outcome parameters in status epilepticus: a retrospective 5-year cohort study. Epilepsia. 2012; 53:1489–97.
24. Sutter R, Marsch S, Fuhr P, Rüegg S. Mortality and recovery from refractory status epilepticus in the intensive care unit: a 7-year observational study. Epilepsia. 2013; 54:502–11.
25. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis. An overview for clinicians. Crit Care. 2005; 9:158–69.
26. Minicucci F, Ferlisi M, Brigo F, Mecarelli O, Meletti S, Aguglia U, et al. Management of status epilepticus in adults: position paper of the Italian League against Epilepsy. Epilepsy Behav. 2020; 102:106675.
27. Chiu WT, Campozano V, Schiefecker A, Rodriguez DR, Ferreira D, Headlee A, et al. Management of refractory status epilepticus: an international cohort study (MORSE CODe) analysis of patients managed in the ICU. Neurology. 2022; 99:e1191–201.
28. Gaspard N, Foreman B, Judd LM, Brenton JN, Nathan BR, McCoy BM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013; 54:1498–503.
29. Espinosa L, Gomez M, Zamora A, Molano-Franco D. Refractory and super-refractory status epilepticus and evidence for the use of ketamine: a scope review. J Neurocrit Care. 2023; 16:1–9.
30. Groth CM, Droege CA, Connor KA, Kaukeinen K, Acquisto NM, Chui SH, et al. Multicenter retrospective review of ketamine use in the ICU. Crit Care Explor. 2022; 4:e0633.
31. Byun JI. Management of convulsive status epilepticus: recent updates. Encephalitis. 2023; 3:39–43.
32. Jacobwitz M, Mulvihill C, Kaufman MC, Gonzalez AK, Resendiz K, MacDonald JM, et al. Ketamine for management of neonatal and pediatric refractory status epilepticus. Neurology. 2022; 99:e1227–38.
33. Alkhachroum A, Der-Nigoghossian CA, Mathews E, Massad N, Letchinger R, Doyle K, et al. Ketamine to treat super-refractory status epilepticus. Neurology. 2020; 95:e2286–94.
34. Hurth KP, Jaworski A, Thomas KB, Kirsch WB, Rudoni MA, Wohlfarth KM. The reemergence of ketamine for treatment in critically ill adults. Crit Care Med. 2020; 48:899–911.
35. Choi JW, Shin JW. Early combination therapy of ketamine and midazolam in patients with refractory status epilepticus in hemodynamic unstable state. J Epilepsy Res. 2021; 11:150–3.
36. Sabharwal V, Ramsay E, Martinez R, Shumate R, Khan F, Dave H, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015; 52:264–6.
37. Trinka E, Leitinger M. Management of status epilepticus, refractory status epilepticus, and super-refractory status epilepticus. Continuum (Minneap Minn). 2022; 28:559–602.
38. Muhlhofer WG, Layfield S, Lowenstein D, Lin CP, Johnson RD, Saini S, et al. Duration of therapeutic coma and outcome of refractory status epilepticus. Epilepsia. 2019; 60:921–34.
39. Migdady I, Rosenthal ES, Cock HR. Management of status epilepticus: a narrative review. Anaesthesia. 2022; 77 Suppl 1:78–91.
40. Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019; 381:2103–13.
41. Lyttle MD, Rainford NE, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019; 393:2125–34.
42. Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019; 393:2135–45.
43. Husain AM, Lee JW, Kolls BJ, Hirsch LJ, Halford JJ, Gupta PK, et al. Randomized trial of lacosamide versus fosphenytoin for nonconvulsive seizures. Ann Neurol. 2018; 83:1174–85.
44. Panda PK, Panda P, Dawman L, Sharawat IK. Efficacy of lacosamide and phenytoin in status epilepticus: a systematic review. Acta Neurol Scand. 2021; 144:366–74.
45. Vossler DG, Bainbridge JL, Boggs JG, Novotny EJ, Loddenkemper T, Faught E, et al. Treatment of refractory convulsive status epilepticus: a comprehensive review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 2020; 20:245–64.
46. Trinka E. Phenobarbital in status epilepticus: rediscovery of an effective drug. Epilepsy Behav. 2023; 141:109104.
47. Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S. Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav. 2019; 101:106466.
48. Byun JI, Chu K, Sunwoo JS, Moon J, Kim TJ, Lim JA, et al. Mega-dose phenobarbital therapy for super-refractory status epilepticus. Epileptic Disord. 2015; 17:444–52.
Fig. 1.
Treatment approaches for refractory status epilepticus based on subgroups including convulsive status epilepticus, non-convulsive status epilepticus, and new-onset refractory status epilepticus (NORSE)/febrile infection-related epilepsy syndrome (FIRES). ABC, airway, breathing, circulation; IV, intravenous; EEG, electroencephalogram; AED, antiepileptic drug; IM, intramuscular; KD, ketogenic diet; VNS, vagus nerve stimulation; PB, phenobarbital; SE, status epilepticus; RSE, refractory status epilepticus; AE, autoimmune encephalitis; IL, interleukin.
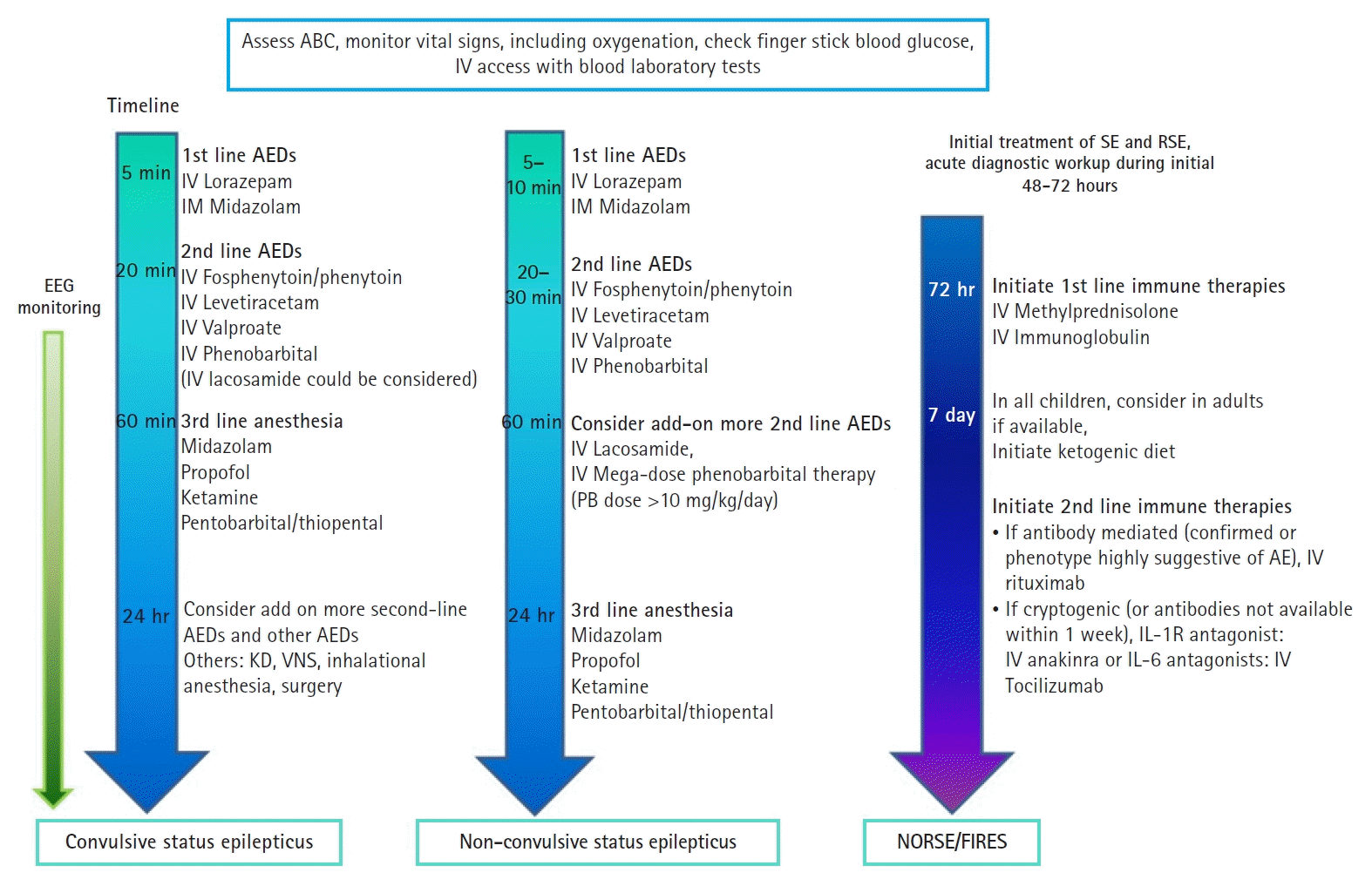
Table 1.
Anesthetics used for the treatment of refractory status epilepticus
Table 2.
Intravenous antiepileptic drugs used for the treatment of status epilepticus




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download