Abstract
Background
Cerebral malaria, caused by Plasmodium falciparum, can lead to severe neurological complications. It is more frequently observed in children than in adults. Because cerebral malaria is rare and has no specific symptoms or neurologic findings, it is not easy to diagnose.
Case report
We report a case of a 61-year-old male who returned from the Democratic Republic of the Congo with fever, fatigue, and confusion. With considering cerebral malaria, a peripheral blood smear confirmed P. falciparum infection (initial parasite load, 760,800/μL; ring form, 100%). Cerebrospinal fluid analyses showed high protein level (103.2 mg/dL). Electroencephalograms showed background slowing activity. Brain magnetic resonance imaging showed signal changes and cerebral swelling. The initial doxycycline and quinidine treatment for malaria was successful without sequalae.
Cerebral malaria is a rare symptom of Plasmodium falciparum [1]. This disease destroys the integrity of the blood-brain barrier through various mechanisms [2]. Cerebral malaria occurs mainly in children in endemic areas or in adult travelers who have not developed immunity. Therefore, clinicians should consider diagnosing cerebral malaria in patients with neurological symptoms and a history of travel to malaria-endemic areas [3]. Moreover, cerebral malaria is associated with severe neurological complications and a high mortality rate of approximately 15% [3]. The incidence of cerebral malaria is high in pediatric populations, particularly in those younger than 5 years, in sub-Saharan Africa, whereas cerebral malaria is relatively rare in adults [1]. Given the potential for sequelae, particularly in adults, early diagnosis and prompt treatment are critical for reducing the disease burden. In this report, we describe a case of decreased consciousness caused by P. falciparum infection in a patient who was transferred to the intensive care unit (ICU).
A 61-year-old man was admitted to the hospital with a fever of 38.9 ℃, progressive headache, and altered mental status. The patient had resided in the Democratic Republic of the Congo for business for 6 months before his admission and had recently returned to Korea. The patient received doxycycline for malarial prophylaxis along with vaccinations for yellow fever and typhoid disease.
Two days before the emergency room visit, he was confused, disoriented, and unable to recognize his house. His symptoms were intermittent and worsened upon awakening. The patient became increasingly delirious, and his violent behavior made it difficult for him to provide a comprehensive medical history. He had minimal oral intake for 4 days. His wife said that his symptoms started 2 weeks before presentation while he was in the Democratic Republic of the Congo. He experienced diarrhea but did not have any hematochezia or black stools. In addition, he had a cough without experiencing dyspnea or chest pain. The patient experienced nausea and vomiting, however, did not complain of visual disturbances, numbness, or focal weakness.
Upon arriving at the hospital, his vital signs revealed significant hypotension (blood pressure of 102/63 mm Hg), tachycardia (123 beats/min), a fever (38.9 °C), and an oxygen saturation level of 94% while breathing room air. The initial electrocardiogram (ECG) revealed sinus tachycardia with a QTc interval of 454 ms. He was admitted to the neuro-ICU for the management and continuous monitoring of his mental status, which included regular laboratory tests and ECGs. Several evaluations were performed for the diagnosis, and tropical infections were considered. Laboratory tests revealed pancytopenia, elevated C-reactive protein levels, and hyperlactatemia. Evaluation of cerebrospinal fluid (CSF) revealed no evidence of meningitis with elevated CSF protein level (103.2 mg/dL; normal range, 15.0–40.0 mg/dL) and normal adenosine deaminase (ADA) level (10 IU/L; normal range, 5.0–20.0 IU/L).
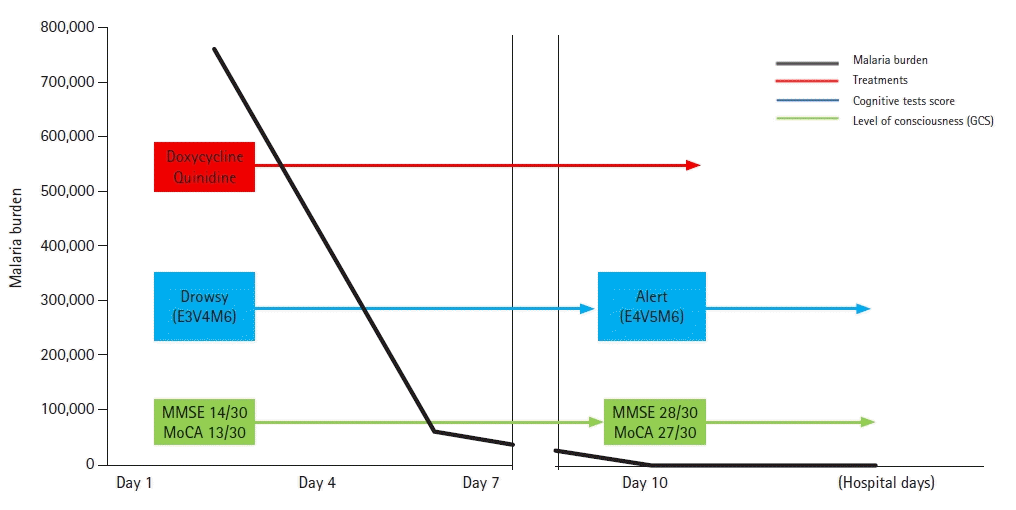
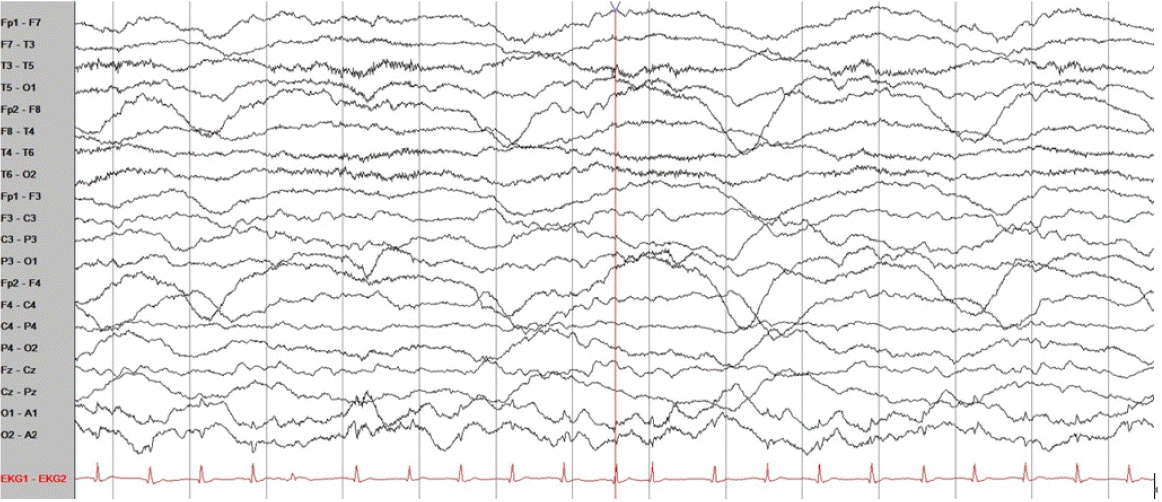
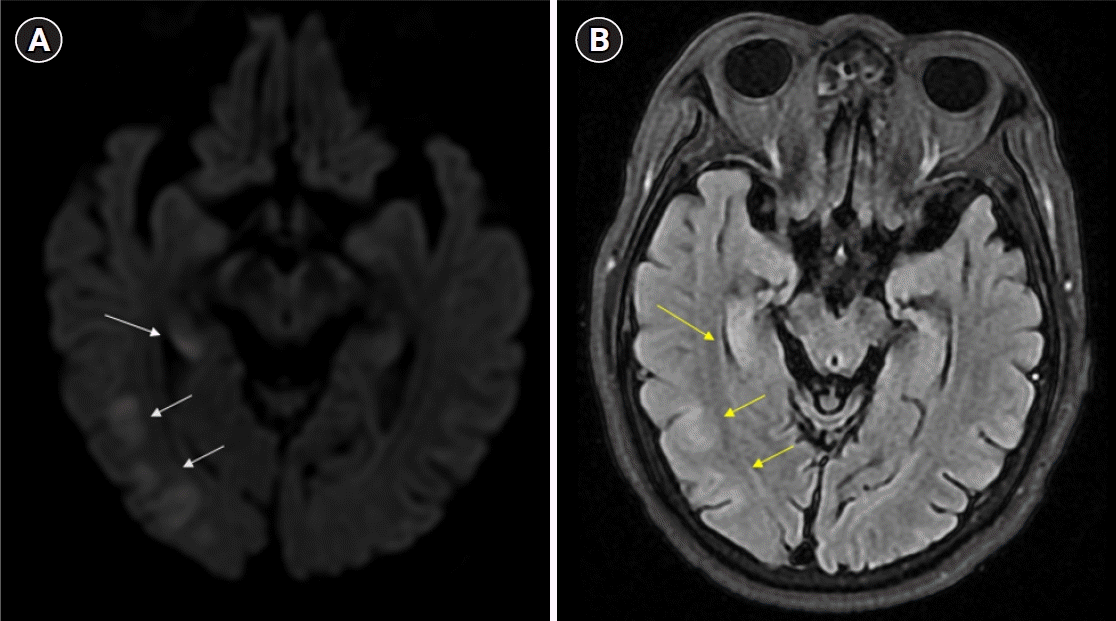
Further examination revealed no evidence of meningitis, enteric fever, Ebola virus disease, schistosomiasis, tick-borne Rickettsiae, filariasis, yellow fever, dengue, or chikungunya. Two days after admission, he developed continuous drowsiness (Glasgow Coma Scale [GCS] score, E3V4M6) and confusion (Fig. 1). Neurocognitive assessment yielded a score of 14/30 on the Mini-Mental State Examination (MMSE), 13/30 on the Montreal Cognitive Assessment (MoCA), and poor visuospatial and executive skills (Fig. 1). Phonemic and semantic paraphrasic fluency errors were present with mild ideomotor apraxia when following commands in motor tasks. No evidence of cerebellar dysfunction, frontal release signs, or depression was observed. Electroencephalography (EEG) was used to differentiate seizures, and EEG was performed. The EEG displayed background slowing activity (Fig. 2). Brain magnetic resonance imaging (MRI) revealed subtle changes with high signal intensity on the diffusion-weighted image (DWI) and swelling of the hippocampal, limbic, and parieto-occipital cortical lesions (Fig. 3). A peripheral blood smear isolated malarial parasites and confirmed P. falciparum infection (initial parasite load, 760,800/μL; ring form, 100%) under a microscope with multiple invasions of ring form in red blood cells (RBCs). Finally, cerebral malaria was diagnosed based on the patient’s clinical course and laboratory results.
The patient was treated for cerebral malaria with doxycycline and intravenous quinidine. Clinicians monitored drug toxicity. As intravenous quinidine can evoke hyperinsulinemia due to hypoglycemia, hourly blood glucose levels were assessed. Quinidine may also prolong the QT interval. Consequently, continuous ECG monitoring was required. In addition, clinicians consider discontinuing quinidine if QTc prolongs over 50% of the patient’s initial QTc and holding it until the QTc falls below 25% of the initial baseline value.
His consciousness continued to worsen and eventually declined. Following 6 days of intravenous quinidine treatment, a peripheral blood smear indicated a malarial load of 60,775/μL. His mental status improved (GCS, E4V4M6) as the malarial load decreased (250/μL) (Fig. 1). The patient's violent tendencies also disappeared.
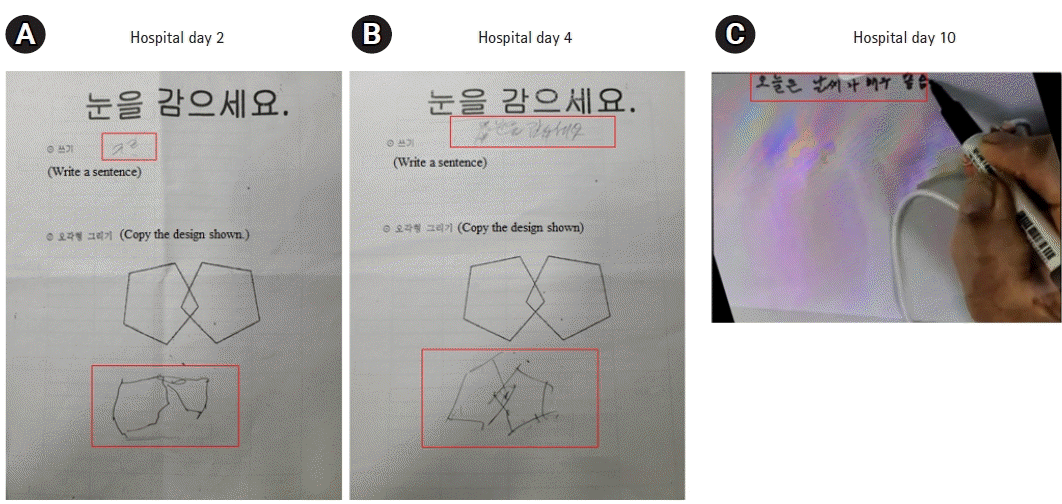
Ten days later, the patient was transferred to the general ward. His MMSE and MoCA scores improved to 28/30 and 27/30, respectively, with good visuospatial and executive skills (Fig. 4). His phonemic and semantic fluencies also improved. The patient was discharged on the 14th day of admission with no detectable parasite load (0/μL). His mental status and follow-up EEG findings were normal, and he could perform activities of daily living independently after discharge.
Here, we report an uncommon case of cerebral malaria in a patient with various neurological symptoms in Korea. In this case, the patient was properly diagnosed and treated, but when the medical staff initially met the patient, his symptoms were not specific. Therefore, we almost missed the first time and did not include the diagnostic criteria for cerebral malaria by P. falciparum.
Cerebral malaria poses a diagnostic challenge because of the nonspecific clinical features that may overlap with those of other conditions, leading to altered consciousness. Only P. falciparum, and no other malaria-causing pathogens, can cause cerebral malaria. The World Health Organization defines cerebral malaria as a clinical syndrome characterized by altered consciousness resulting from malarial infection that cannot be attributed to non-malarial causes [1]. As a result, cerebral malaria exhibits a heterogeneous array of symptoms rather than a homogenous syndrome [2].
Cerebral malaria caused by P. falciparum is a severe form that affects the brain and results in altered consciousness, neurological deficits, and death [3]. This condition is highly prevalent in endemic regions of Africa and has a significant impact on young children, with an estimated incidence of 1,120 per 100,000 per year and a peak incidence in preschool children [4]. Moreover, an estimated 575,000 children in Africa develop cerebral malaria annually [5].
Cerebral malaria presents with symptoms such as fever, headache, and body aches and can progress to altered consciousness (coma). Although neurological deficits, including blindness, ataxia, and hypotonia, have demonstrated improvement over time in some patients, approximately 25% of individuals have long-term impairments, including cognitive, motor, and behavioral dysfunctions, and a 10% incidence of epilepsy [3]. Contrary to its name, cerebral malaria does not involve P. falciparum invading the brain tissue. Other commonly observed systemic complications include anemia, metabolic acidosis, electrolyte imbalance, hypoglycemia, and shock. Evidence suggests that cerebral malaria is associated with multiple organ involvement [3].
The pathophysiology of cerebral malaria involves the sequestration of parasitized RBCs (pRBCs) in the cerebral microcirculation, leading to hypoxia and inadequate tissue perfusion. The sequestration of pRBCs in the cerebral microvasculature causes endothelial damage, cell apoptosis, and brain-blood-barrier dysfunction [6]. These events are associated with intracranial hypertension and brain swelling [7]. Cortical infarction and cerebral venous thrombosis can also occur due to disordered coagulation [8]. Severe metabolic factors, such as inflammatory cytokines and chemokines (tumor necrosis factor [TNF], interleukin [IL]-1b, IL-6, and IL-10) and mediators (quinolinic and kynurenic acid), may also exacerbate injury [9].
In addition, CSF examinations should exclude other causes of central nervous system infection. Furthermore, CSF results for cerebral malaria are generally within the normal range. Mild pleocytosis (10–50 cells/mm3), increased protein (up to 200 mg/dL), and/or ADA levels may sometimes be observed during CSF examination [10]. Our patient also demonstrated elevated CSF protein levels without central nervous system infection.
General conditions such as fever, nutritional status, diarrhea, mood, and medical conditions can affect a patient’s cognitive function [11]. Unlike in our case, the evaluation of cognitive function tests presents challenges in cases of cerebral malaria. Therefore, brain MRI can reveal additional information to determine the disease severity and brain injury in patients with cerebral malaria [12]. Brain MRI abnormalities are present in 78% of the patients with cerebral malaria and include signs of cerebral edema, infarcts, and subcortical white matter changes [13]. Brain swelling is related to venous congestion of sequestered pRBCs, causing increased cerebral blood volume [14]. Additionally, DWI abnormalities were few due to cytotoxic edema. In particular, fatal cases are associated with increased brain volume, leading to elevated cerebral pressure. In addition, predominant posterior cerebral swelling, such as in posterior reversible encephalopathy syndrome, has been reported [9]. Changes in MRI were related to the observed neurological symptoms. Compared to computed tomography, MRI is more sensitive for detecting brain abnormalities in patients with cerebral malaria. In addition, EEG findings have been associated with long-term neurocognitive morbidity in cerebral malaria [13]. Moreover, EEG findings suggest neurocognitive outcomes. A high average background voltage, fast dominant rhythm frequency, and vertex sharp waves are associated with better cognitive function [15].
Most patients regained consciousness within 48–72 hours of treatment initiation (median time, 32.3 hours) [1]. Therefore, cerebral malaria treatment aims to lower the malaria burden and reduce brain injury. Additionally, the signs of edema disappeared remarkably after antimalarial treatment within 72 hours in the brain MRI [9,14]. The clinical outcome of cerebral malaria may depend on the appropriate treatment of the disease mechanism to reduce complications. Recommended treatments for malaria include doxycycline and quinidine [2]. However, quinidine is requied caution when treating, because it can lead to torsades de pointes due to a prolonged QT interval and can also result in hypoglycemia due to hyperinsulinemia [2].
Several studies have investigated methods for preventing endothelial damage and improving blood flow. These include interventions targeting the pathogenesis and risk factors for brain injury [1]. Trials have been conducted on steroids, immunoglobulins, anti-TNF agents, micronutrients, and prophylactic anticonvulsants. Combining these adjuvant therapies may enhance neurocognitive outcomes [1].
This was a rare case of an adult with cerebral malaria who presented with altered mental status, behavioral changes, cognitive impairment, and decreased fluency. To diagnose this condition early, clinicians should be vigilant of neurocognitive deficits and neuropsychiatric symptoms. Moreover, individuals returning from malaria-endemic regions should undergo screening when they exhibit changes in their levels of consciousness. The patient’s symptoms improved with immediate and appropriate cerebral malaria treatment. For cerebral malaria identification, a travel history to endemic areas, including sub-Saharan Africa, and observation of the peripheral blood smear under a microscope is essential. We also attempted to identify the P. falciparum parasite, ring form (multiple invasions), or banana-shaped gametocytes in the blood smear.
Notes
Ethics statement
This study complied with the principles of the Declaration of Helsinki. The study’s protocol was reviewed and approved by the Institutional Review Board of Inha University Hospital (IRB No. 2022-08-007-006). The IRB waived the requirement to obtain written informed consent from patients.
REFERENCES
1. Roberson MT, Smith AT. Cerebral malaria in a patient with recent travel to the Congo presenting with delirium: a case report. Clin Pract Cases Emerg Med. 2020; 4:533–6.
2. Marsh K, English M, Crawley J, Peshu N. The pathogenesis of severe malaria in African children. Ann Trop Med Parasitol. 1996; 90:395–402.
3. Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010; 68:267–74.
4. Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001; 64(1-2 Suppl):1–11.
5. van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997; 131(1 Pt 1):125–9.
6. Storm J, Craig AG. Pathogenesis of cerebral malaria: inflammation and cytoadherence. Front Cell Infect Microbiol. 2014; 4:100.
7. Sein KK, Maeno Y, Thuc HV, Anh TK, Aikawa M. Differential sequestration of parasitized erythrocytes in the cerebrum and cerebellum in human cerebral malaria. Am J Trop Med Hyg. 1993; 48:504–11.
8. Schiess N, Villabona-Rueda A, Cottier KE, Huether K, Chipeta J, Stins MF. Pathophysiology and neurologic sequelae of cerebral malaria. Malar J. 2020; 19:266.
9. Mohanty S, Benjamin LA, Majhi M, Panda P, Kampondeni S, Sahu PK, et al. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. mSphere. 2017; 2:e00193–17.
10. Misra UK, Kalita J, Prabhakar S, Chakravarty A, Kochar D, Nair PP. Cerebral malaria and bacterial meningitis. Ann Indian Acad Neurol. 2011; 14(Suppl 1):S35–9.
11. Gure TR, Langa KM, Fisher GG, Piette JD, Plassman BL. Functional limitations in older adults who have cognitive impairment without dementia. J Geriatr Psychiatry Neurol. 2013; 26:78–85.
12. Kumral E, Bayam FE, Köken B, Erdoğan CE. Clinical and neuroimaging determinants of minimally conscious and persistent vegetative states after acute stroke. J Neurocrit Care. 2019; 12:37–45.
13. Kampondeni SD, Potchen MJ, Beare NA, Seydel KB, Glover SJ, Taylor TE, et al. MRI findings in a cohort of brain injured survivors of pediatric cerebral malaria. Am J Trop Med Hyg. 2013; 88:542–6.
14. Karimi M, Nazari S, Shirani F, Alivirdiloo V, Siahposht-Khachaki A, Edalatkhah S, et al. Neuroprotective effects of chloroquine on neurological scores, blood-brain barrier permeability, and brain edema after traumatic brain injury in male rats. J Neurocrit Care. 2023; 16:18–27.
15. Clark DJ, Bond C, Andrews A, Muller DJ, Sarkisian A, Opoka RO, et al. Admission clinical and EEG features associated with mortality and long-term neurologic and cognitive outcomes in pediatric cerebral malaria. Neurology. 2023; 101:e1307–18.
Fig. 1.
Timeline of malaria burden and symptoms progression. The measured malaria burden (black line), level of consciousness (blue line), and cognitive function test results (green line) are related to the treatment. GCS, Glasgow Coma Scale; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download