Abstract
Background/Aims
Solitary rectal ulcer syndrome (SRUS) can be overlooked, diagnosed late, or misdiagnosed, particularly in childhood. This study reviewed the 13-year experience of the authors’ institution to increase clinicians' awareness of SRUS in the presence of symptoms. This paper reports the endoscopic and histopathological findings in children presenting with hematochezia.
Methods
The clinical and laboratory findings of 22 patients diagnosed with biopsy-proven SRUS in the authors’ clinic between 2007 and 2020 were evaluated retrospectively.
Results
The mean age at diagnosis was 12.5±2.6 years, and 59.1% of the patients were male. The median time of diagnosis was 24 months. A single ulcer lesion was found by colonoscopy in 18 patients (81.8%), two ulcers in two patients (9%), and more than two ulcers in two patients (9%). The pathology reports of all biopsies taken from the lesions were consistent with a solitary rectal ulcer. In the first stage, the treatment was started with toilet training, a high-fiber diet, and laxatives. In 11 patients (50%) who did not respond to the initial treatment, a 5-ASA enema was added. A glucocorticoid enema was added to treatment in five patients (22%) whose complaints did not regress despite this treatment. Clinical remission was achieved in five of the patients (18.1%). The time to diagnosis was significantly shorter in those in remission than those not in remission (p=0.04).
Solitary rectal ulcer syndrome (SRUS) is rarely seen in the pediatric population and is encountered more frequently in adolescent children. The common complaints are prolonged straining, tenesmus, and perineal pain, accompanied by rectal bleeding and mucous in the stool.1 The prevalence of SRUS is one per 100,000 person-years in children.2 Approximately 75–80% of the patients are males.3 The low prevalence of SRUS in children has been suggested to be because it is often unrecognized or misdiagnosed.4,5 The diagnosis can be difficult because the endoscopic and histopathological findings of SRUS may mimic other disorders of the rectum.1,4
The etiology of SRUS is unclear. In healthy individuals, the puborectalis muscle relaxes during defecation, allowing the rectum to flatten and empty. On the other hand, this relaxation does not occur in patients with SRUS, who show tertiary contractions. Rectal hypersensitivity, which causes the feelings of a constant need to defecate and incomplete excretion, results in trauma, ischemia, and ulceration of the mucosa because of the inappropriate contraction of the puborectalis muscle and rectal mucosal prolapse. The diagnosis is based on the clinical findings, detection of an ulcer on the anterior wall of the rectum by colonoscopy, and characteristic histopathological changes.6 Although suggestive of a single ulcer, lesions may be multiple and of different shapes and sizes (ulcerative, polypoidal/nodular, or erythematous mucosa only). The histopathological findings distinguishing these lesions from other rectal diseases are fibromuscular obliteration of the lamina propria and the disorientation of muscle fibers.5
Currently, no specified treatment guideline is available for SRUS. The treatment includes correcting the pathogenic mechanisms by softening the stool through behavioral changes (to avoid staying on the toilet for a long time, not straining for a long time, and using a squat toilet or putting a step under the feet if the feet do not reach the floor when using a European toilet), bowel training, and the use of laxatives. Topical treatments for ulcers include sucralfate, sulfasalazine/mesalazine, and corticosteroids. In recent years, argon plasma coagulation therapy has been included in the treatment.7
Because patients are admitted with a variety of clinical presentations, both clinicians and pathologists should be highly aware of SRUS. This study aimed to increase clinicians’ awareness of SRUS in children admitted with hematochezia and to contribute to the literature by reporting the symptoms and the endoscopic and histopathological findings in this first large series conducted on Turkish children. This article was previously presented as an oral presentation at the Anatolian Gastroenterology Days Congress between September 30 and October 2, 2021.
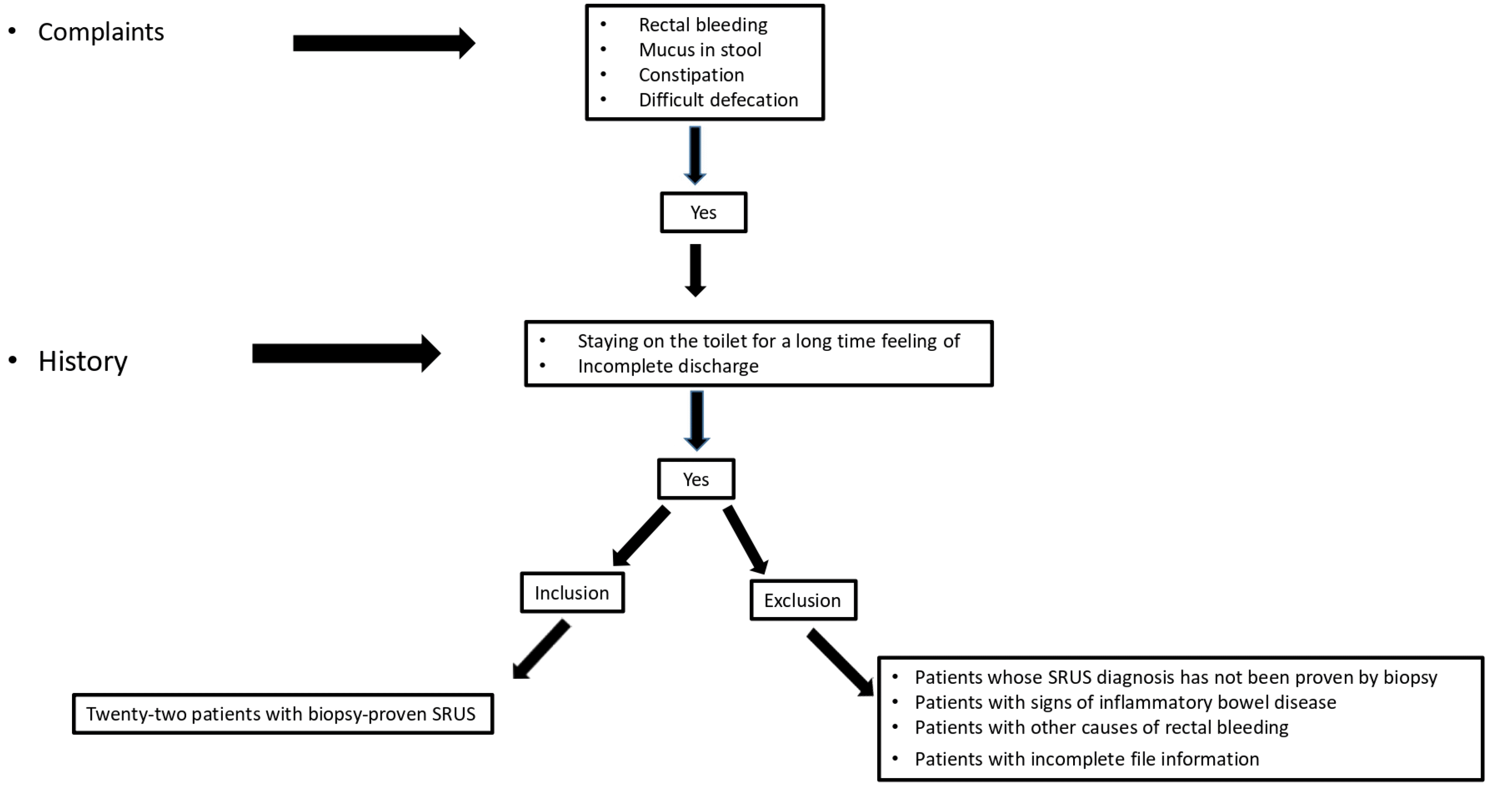
The clinical and laboratory findings of 22 patients diagnosed with biopsy-proven SRUS between 2007 and 2020 at the Pediatric Gastroenterology, Hepatology, and Nutrition Clinic of İnönü University Turgut Özal Medical Center were evaluated retrospectively. Fig. 1 presents a flow chart of patient selection. The patients' files were scanned retrospectively. The following were recorded: complaints (rectal bleeding, mucus in stool, constipation, and difficult defecation), history (staying on the toilet for a long time and feeling of incomplete discharge), weight, weight Z score, height, height Z score, rectal touch findings in physical examination, complete blood count, biochemical parameters, C-Reactive Protein (CRP), Erythrocyte sedimentation rate (ESR), Perinuclear-Anti-neutrophil Cytoplasmic Antibodies (P-ANCA), Saccharomyces Cerevisiae Antibody (ASCA), colonoscopy reports, and biopsy results. Rectal biopsy specimens were fixed in 10% formaldehyde after a routine tissue follow-up. They were then embedded in paraffin, cut to a four-micron thickness, stained with hematoxylin-eosin, and evaluated by optical microscopy. Masson Trichrome staining was used to reveal increased connective tissue in the lamina propria. Rectal biopsies of the study cases were reevaluated by a pathologist. Patients whose diagnosis of SRUS was not proven by biopsy, those with signs of inflammatory bowel disease, those with other causes of rectal bleeding, and patients with missing file information were excluded.
Ethical approval for the study was obtained from the Inonu University Scientific Research and Publication Ethics Committee Health Sciences Non-Interventional Clinical Research Ethics Committee (Decision no: 2020/1167).
Statistical analyses were performed using SPSS version 21.0 software (IBM Co., Armonk, NY, USA). The descriptive statistics are expressed as mean±standard deviation or numbers (percent). The normal distribution of the data was tested using Kolmogorov–Smirnov test. Normally distributed data were analyzed using one-way variance analysis or a Student's t-test. The data not fitting a normal distribution were analyzed using a Kruskal–Wallis test or Mann–Whitney U test. Statistical significance was evaluated using a Student's t-test, Mann–Whitney U test, or a chi-square test. A p-value <0.05 was considered significant.
The mean age at diagnosis was 12.5±2.6 years, and 59.1% (n=13) of the patients were male. The mean weight Z score of the patients was –0.25 (–1.6 to 1.69). The mean height Z score was −0.11 (−1.82 to 29). All the patients complained of rectal bleeding, mucous stool, constipation, and difficult defecation at admission. In addition, all the patients had a history of staying in the toilet too long and a feeling of incomplete evacuation. Table 1 lists the characteristics of the cases.
In a rectal examination, an anal fissure was present in 45.5% of the patients. At the time of diagnosis, the mean hemoglobin level of the patients was 12.4±1.17 g/dL. The mean platelet count was 278.7±57.3×103/mL, and the levels of acute phase markers, including CRP, ESR, and albumin, were normal. P-ANCA, a marker for ulcerative colitis and ASCA, a marker for Crohn's disease, were negative. Defecography was not applied in the authors’ center because it was unavailable. The median time to diagnosis was 24 (1–72) months.
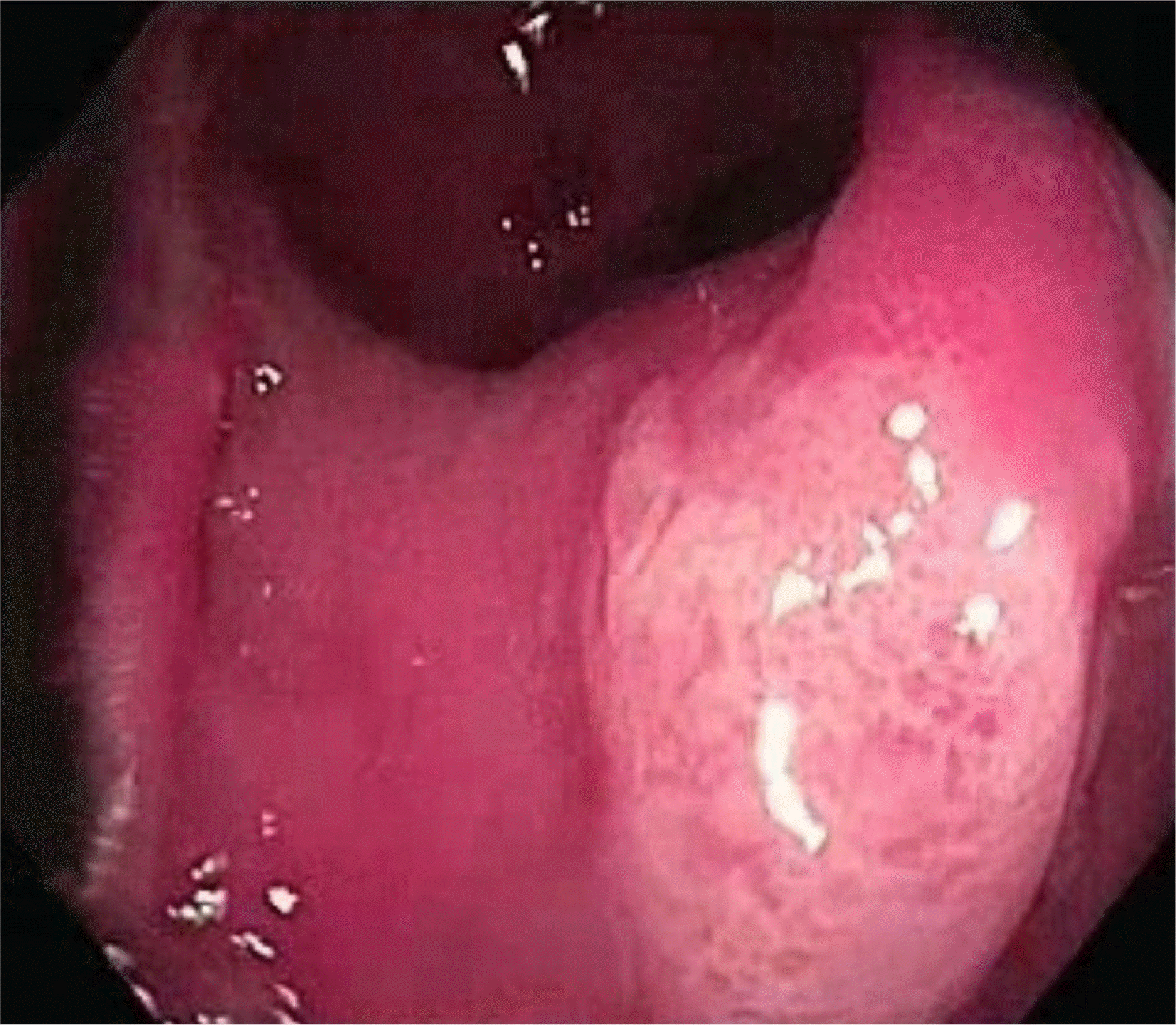
In the colonoscopy examination of the patients, ulcer(s) were observed approximately 5–15 cm away from the anal canal and located in front of the anorectal junction; the smallest and largest were 0.5 cm and 2 cm, respectively (Fig. 1). Colonoscopy detected a single ulcer was detected in 18 patients (81.8%) and more than two ulcers in two patients (9%). The pathology findings (superficial mucosal ulceration, fibromuscular hyperplasia in the lamina propria, dilatation of capillaries, and minimal inflammation) in all patients were consistent with a solitary rectal ulcer (Figs. 2, 3).
The treatment was started with toilet training, a high-fiber diet, and laxatives. The patients were followed up every three months, and 5-Aminosalicylic acid (5-ASA) enema was added to the treatment in 11 (50%) patients who had no clinical improvement during the follow-up. A glucocorticoid enema was added in five (18.1%) patients who did not show improvement despite the initial treatment. Biofeedback and argon plasma treatments could not be applied because they were unavailable in the author’ center. Sigmoidoscopy was performed to document mucosal healing in five patients who had been asymptomatic for at least three months after treatment was started or improvement in symptoms from the baseline and the ulcers had healed. In these five patients in remission, the minimum and maximum time between the symptoms and the diagnosis was three and nine months, respectively (mean: 5.8±2.3). In one patient in remission after behavioral changes, bowel education, and laxative use, the time from the beginning of the complaints to the diagnosis was three months. The time to diagnosis was significantly shorter in those in remission than in those who were not (p=0.04). The clinical treatment and follow-up of other patients have been continuing.
Cruveilhier first reported8 a case of rectal ulcers in four adult patients in 1829. Solitary rectal ulcer syndrome was widely recognized in 1969 when Madigan et al. reported 68 adult cases.6 SRUS is seen in ulcer form in only 40% of patients. The condition can be in different forms, varying from hyperemic mucosa to broad-based polypoidal lesions. In addition, the lesions are limited to the rectum and can be single or multiple, varying in size and shape in the sigmoid colon.9 Although solitary rectal ulcer syndrome is a common condition in adults, it is rarely diagnosed in pediatric practice because of a lack of awareness, or it is diagnosed late owing to inadequate diagnosis or misdiagnosis. Diagnosis is delayed in children because the symptoms are nonspecific and rare. In the literature, Godbole et al. reported the first SRUS cases in two children in 2000.10 Although it was widely recognized in adults since 1969, it could not be identified in pediatric patients until 2000. Later, 14 more case series of pediatric patients were reported.3-5,11,12 Poddar et al.13 published the largest case series. To the best of the authors’ knowledge, this study is the first large series investigating SRUS in Turkish children. Ertem et al.4 reported two cases, and Urgancı et al.14 reported six children in Türkiye. Increasing the awareness of this condition will result in the identification of more cases in Türkiye.
In the literature, in the series of pediatric SRUS cases, the mean or median age of the patients was above eight years.3-5,11,12 Consistent with the literature, in the present series, the mean age of the patients was 12.5±2.6 years. In this series, the median time to diagnosis was two years. Table 2 lists the mean or median age at diagnosis and time to diagnosis in pediatric case series reported in the literature.3-5,15-21
Thirumal et al.12 reported that the shortest median time between the onset of symptoms and diagnosis was 5.5 months. The researchers suggested that this short interval may be due to early clinical suspicion and early SRUS evaluation after excluding the common causes. SRUS patients are frequently diagnosed with constipation because they often present with constipation. Hence, SRUS may be overlooked because colonoscopy is not performed. For this reason, it is believed that a careful questioning of the history of dyssynergic defecation is important for diagnosing SRUS. Consistent with the literature, all of our patients had a history of dyssynergic defecation.13,19,20 Again, all of the present patients had complaints of rectal bleeding, prolonged stays on the toilet, excessive straining, and a feeling of incomplete evacuation. Poddar et al.13 reported that the rates of these complaints were 93.6%, 93.6%, 98.6%, and 92.8%, respectively. In the present study, ulcers were detected in all patients by a colonoscopy examination, and 77.3% had a single ulcer. In the series reported by Poddar et al.13, ulcers were detected in 72% of the patients, and 83% had a single ulcer. Kowalsk-Duplaga et al.20 detected ulcers in 97% of their cases, and 61% were solitary ulcers. Suresh et al.19 detected ulcers in 91% of the cases, and 68% were solitary ulcers. Poddar et al.13, found no ulcer in 26% of patients because of the low threshold for performing colonoscopy. In the present series, all the patients complained of bloody stool on admission, and ulcers were detected by colonoscopy. Therefore, the diagnosis can be made earlier before bloody stools if every patient with a history of dyssynergic defecation is referred to a pediatric gastroenterology specialist for evaluation in terms of SRUS. As the awareness for SRUS increases, a colonoscopy will be performed on every patient with dyssynergic complaints, and the rate of early diagnosis will increase.
The treatment of SRUS varies according to the severity of symptoms. Treatment planning is usually individualized. First, patient education and behavioral changes, such as not staying on the toilet for a long time, not straining for a long time, high fiber diet, and laxatives are recommended to prevent and treat constipation. If these conservative measures produce no improvement, topical treatments, including sucralfate, sulfasalazine/mesalazine, and corticosteroids, can be added.7 Similar to the literature, the treatment was started with toilet training, high fiber diet, and lactulose at the first stage, and a 5-ASA enema was added to the treatment in those who did not respond. Oral 5-ASA was added to the treatment in patients who did not improve clinically in the follow-up, and a glucocorticoid enema was added to the treatment in patients whose complaints did not regress despite this.
Blackburn et al.21 reported that behavioral therapy, high-fiber diet, and lactase therapy resulted in remission in seven out of eight children and in 18 of 24 children in a study of Thirumal et al.12, and in one patient in the present study. In this study, remission was observed in two patients given a mesalazine enema and two patients given a combination of a mesalazine enema and oral mesalazine treatment. On the other hand, similar to that reported by Thirumal et al.12, remission occurred earlier in the present patients who were diagnosed and treated early. In the study of Thirumal et al.12, the time between the onset of symptoms and diagnosis was less than six months in patients who showed remission. Similarly, in the present study, this time was a minimum and maximum of three and nine months, respectively (mean: 5.8±2.3). The time between the onset of symptoms and diagnosis was significantly shorter in patients who showed remission than in those who did not (p=0.04). No such comparison was found in the literature. This difference will increase as the number of patients increases.
This study is the first large series on Turkish children. Since it was not a randomized controlled study and it was a retrospective study, we could not compare the treatment methods. On the other hand, the rate of early diagnosis will increase if the awareness about SRUS in children increases, resulting in early treatment that will allow remission in more patients. Nevertheless, randomized controlled studies on the diagnosis and treatment of SRUS in children will be needed on a larger number of patients.
REFERENCES
1. Keshtgar AS. 2008; Solitary rectal ulcer syndrome in children. Eur J Gastroenterol Hepatol. 20:89–92. DOI: 10.1097/MEG.0b013e3282f402c1. PMID: 18188026.
2. Martin CJ, Parks TG, Biggart JD. 1981; Solitary rectal ulcer syndrome in Northern Ireland. 1971-1980. Br J Surg. 68:744–747. DOI: 10.1002/bjs.1800681021. PMID: 7284739.
3. Perito ER, Mileti E, Dalal DH, et al. 2012; Solitary rectal ulcer syndrome in children and adolescents. J Pediatr Gastroenterol Nutr. 54:266–270. DOI: 10.1097/MPG.0b013e318240bba5. PMID: 22094902. PMCID: PMC3719860.
4. Ertem D, Acar Y, Karaa EK, Pehlivanoglu E. 2002; A rare and often unrecognized cause of hematochezia and tenesmus in childhood: solitary rectal ulcer syndrome. Pediatrics. 110:e79. DOI: 10.1542/peds.110.6.e79. PMID: 12456946.
5. Martín de Carpi J, Vilar P, Varea V. 2007; Solitary rectal ulcer syndrome in childhood: a rare, benign, and probably misdiagnosed cause of rectal bleeding. Report of three cases. Dis Colon Rectum. 50:534–539. DOI: 10.1007/s10350-006-0720-1. PMID: 17080282.
6. Madigan MR, Morson BC. 1969; Solitary ulcer of the rectum. Gut. 10:871–881. DOI: 10.1136/gut.10.11.871. PMID: 5358578. PMCID: PMC1553062.
7. Zhu QC, Shen RR, Qin HL, Wang Y. 2014; Solitary rectal ulcer syndrome: clinical features, pathophysiology, diagnosis and treatment strategies. World J Gastroenterol. 20:738–744. DOI: 10.3748/wjg.v20.i3.738. PMID: 24574747. PMCID: PMC3921483.
8. Cruveilhier J. 1829. Ulcere chronique du rectum. Anatomie Pathologique du Corps Humain. JB Bailliere;Paris: p. 1829–1842.
9. Tjandra JJ, Fazio VW, Church JM, Lavery IC, Oakley JR, Milsom JW. 1992; Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum. 35:227–234. DOI: 10.1007/BF02051012. PMID: 1740066.
10. Godbole P, Botterill I, Newell SJ, Sagar PM, Stringer MD. 2000; Solitary rectal ulcer syndrome in children. J R Coll Surg Edinb. 45:411–414.
11. Kiriştioğlu I, Balkan E, Kiliç N, Doğruyol H. 2000; Solitary rectal ulcer syndrome in children. Turk J Pediatr. 42:56–60.
12. Thirumal P, Sumathi B, Nirmala D. 2020; A clinical entity often missed-solitary rectal ulcer syndrome in children. Front Pediatr. 8:396. DOI: 10.3389/fped.2020.00396. PMID: 32766189. PMCID: PMC7379901.
13. Poddar U, Yachha SK, Krishnani N, Kumari N, Srivastava A, Sen Sarma M. 2020; Solitary rectal ulcer syndrome in children: A report of 140 cases. J Pediatr Gastroenterol Nutr. 71:29–33. DOI: 10.1097/MPG.0000000000002680. PMID: 32097373.
14. Urgancı N, Kalyoncu D, Eken KG. 2013; Solitary rectal ulcer syndrome in children: a report of six cases. Gut Liver. 7:752–755. DOI: 10.5009/gnl.2013.7.6.752. PMID: 24312719. PMCID: PMC3848538.
15. Gabra HO, Roberts JP, Variend S, Shawis RN. 2005; Solitary rectal ulcer syndrome in children. A report of three cases. Eur J Pediatr Surg. 15:213–216. DOI: 10.1055/s-2004-821180. PMID: 15999319.
16. Somani SK, Ghosh A, Avasthi G, Goyal R, Gupta P. 2010; Healing of a bleeding solitary rectal ulcer with multiple sessions of argon plasma. Gastrointest Endosc. 71:578–582. DOI: 10.1016/j.gie.2009.10.038. PMID: 20189517.
17. Dehghani SM, Bahmanyar M, Geramizadeh B, Alizadeh A, Haghighat M. 2016; Solitary rectal ulcer syndrome: Is it really a rare condition in children? World J Clin Pediatr. 5:343–348. DOI: 10.5409/wjcp.v5.i3.343. PMID: 27610352. PMCID: PMC4978629.
18. Anjum MN, Cheema HA, Malik HS, Hashmi MA. 2017; Clinical spectrum of solitary rectal ulcer in children presenting with per-rectal bleed. J Ayub Med Coll Abbottabad. 29:74–77.
19. Suresh N, Ganesh R, Sathiyasekaran M. 2010; Solitary rectal ulcer syndrome: a case series. Indian Pediatr. 47:1059–1061. DOI: 10.1007/s13312-010-0177-0. PMID: 20453265.
20. Kowalska-Duplaga K, Lazowska-Przeorek I, Karolewska-Bochenek K, et al. 2017; Solitary rectal ulcer syndrome in children: A case series study. Adv Exp Med Biol. 1020:105–112. DOI: 10.1007/5584_2017_2. PMID: 28255911.
21. Blackburn C, McDermott M, Bourke B. 2012; Clinical presentation of and outcome for solitary rectal ulcer syndrome in children. J Pediatr Gastroenterol Nutr. 54:263–265. DOI: 10.1097/MPG.0b013e31823014c0. PMID: 22266488.
Fig. 3
Histopathologic findings. (A) Rectal mucosal fragment with ulcerated surface. H&E stain ×4. (B) Increased connective tissue, smooth muscle proliferation and crypt loss in the lamina propria. H&E stain ×10.
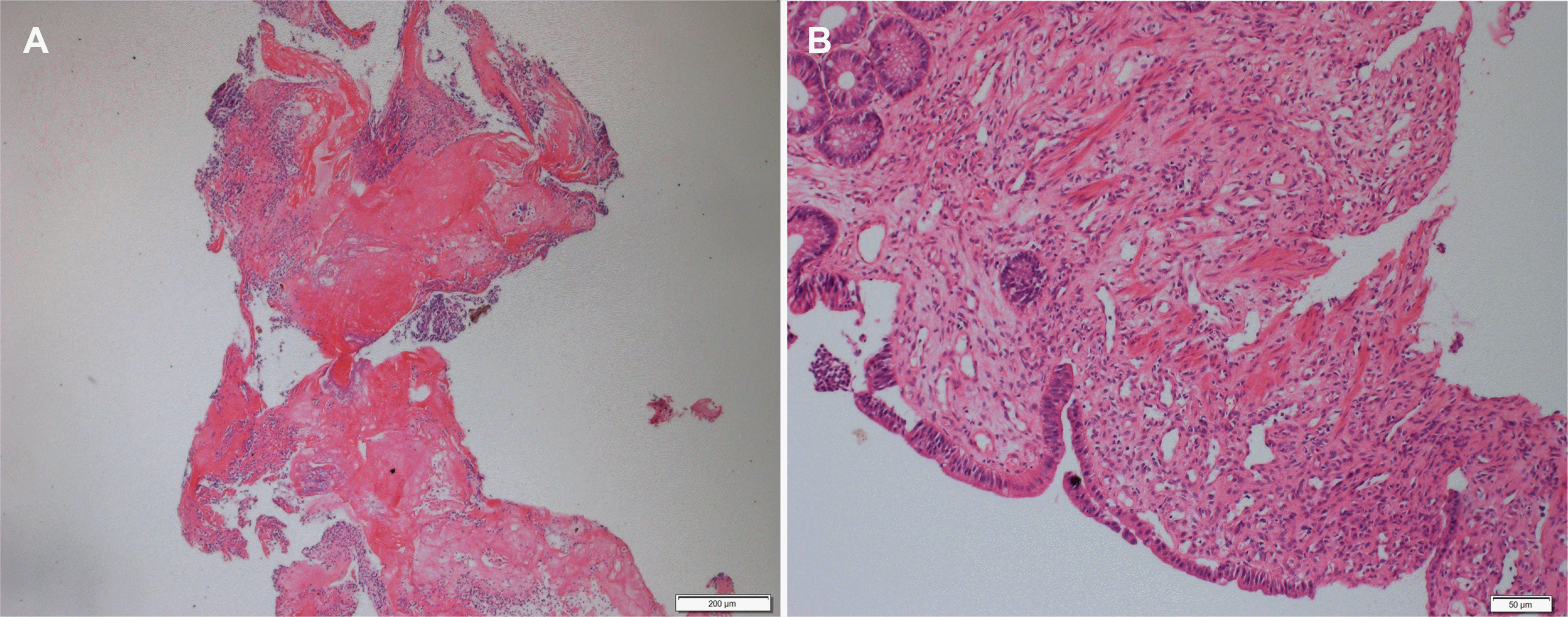
Table 1
Characteristics of the Patients
Table 2
Pediatric Case Series in the Literature
| Pediatric case series | Publication year | Number of cases | Age of diagnosis (year) | Duration from symptoms to diagnosis |
|---|---|---|---|---|
| Perito et al.3 | 2012 | 15 | Median: 13.9 (IQR 9.8–15.6) | Median: 3.2 years (IQR 1.2–5.5) |
| Ertem et al.4 | 2002 | 2 | Mean: 12.5±1.5 | 2–6 yeras |
| Martín de Carpi et al.5 | 2007 | 3 | Mean: 11±2.1 | Source not available |
| Godbole et al.10 | 2000 | 2 | Mean: 13±1 | 2–5 years |
| Kiriştioğlu et al.11 | 2000 | 4 | Source not available | Source not available |
| Gabra et al.15 | 2005 | 3 | Median: 2.5 (range 2–15) | 1–2 years |
| Somani et al.16 | 2010 | 24 | Source not available | Mean: 12.6±4.6 months |
| Suresh et al.19 | 2010 | 22 | Median: 10 (range 1.5–18) | Mean: 6 months |
| Blackburn et al.21 | 2012 | 8 | Mean:9.87 | Mean: 1.73 years (range 1 month–7 years) |
| Urgancı et al.14 | 2013 | 6 | Median: 13 (IQR 12–14) | Median: 1 year (IQR 0.25–4) |
| Dehghani et al.17 | 2016 | 55 | Mean: 10.4±3.7 | Mean: 15.5±11.2 months |
| Anjum et al.18 | 2017 | 21 | 8–12 | Source not available |
| Kowalska-Duplaga et al.20 | 2017 | 31 | 13 (range 5–18) | 1–48 months |
| Poddar et al.13 | 2020 | 140 | Median: 12 (IQR 10–14) | Median. 21 months (IQR 9–36 months) |
| Thirumal et al.12 | 2020 | 24 | Median: 8 (IQR 5.75–11) | Median: 5.5 months (IQR 3–6) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download