Abstract
Although WHO declared the end of the public health emergency for coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), XBB lineages continue to evolve and emerge globally. In particular, XBB.1.5 and XBB.1.16 are raising concerns because of their high immune evasion, leading to apprehensions regarding vaccine efficacy reduction and potential reinfection. We aimed to investigate the COVID-19 outbreak in Korea and predict the likelihood of reinfection by testing neutralizing activity against live viruses from the S clade and 19 Omicron sublineages. We found a significant risk of infection with the currently prevalent XBB lineage for individuals who were either vaccinated early or infected during the initial Omicron outbreak. Vaccinated individuals were better equipped than unvaccinated individuals to produce neutralizing antibodies for other SARS-CoV-2 variants upon infection. Therefore, unvaccinated individuals do not easily develop neutralizing activity against other variants and face the highest risk of reinfection by the XBB lineage. Our study provides important information to facilitate the development of strategies for monitoring populations that would be the most susceptible to new COVID-19 outbreaks.
Following the initial outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019, WHO declared the end of this public health emergency in May 2023 [1]. COVID-19 control measures have since been relaxed in Korea [2]. Nevertheless, since early 2023, the XBB lineage has been predominant in several countries, including Korea, and it continues to differentiate. According to the Korea Disease Control and Prevention Agency (KDCA), the variant detection rates for the third week of July 2023 were 5.9%, 22.7%, 27.1%, 20.0%, and 15.4% for XBB.1.5, XBB.1.9.1, XBB.1.9.2, XBB.1.16, and XBB.2.3, respectively, indicating that XBB lineage variants are predominant [3].
XBB.1.5 and XBB.1.16 have raised concerns regarding reinfection and reduced vaccine efficacy owing to their enhanced immune evasion [4, 5]. Conducting neutralization studies comparing sublineages in individuals who have been either vaccinated or previously infected with Omicron variants is important to provide evidence for public health control strategies and to support the development of suitable vaccines. Therefore, we used neutralization testing to investigate the state of the outbreak in Korea and to predict the likelihood of reinfection. Using recovery-phase serum from patients infected during the Omicron BA.1.1 outbreak and from third dose–vaccinated individuals, we performed neutralization testing for 19 major variants of Omicron sublineages that had been detected during a specific time (from January 2022 to May 2023) in Korea. This study was conducted at the KDCA with approval from KDCA’s Institutional Review Board (2021/12/2-PE-A).
We broadly divided participants into those who had been infected with Omicron variants and those who had not. Specimens were collected from January 2022 to March 2022. All participants in the infected group were from an infection cluster at a senior care facility, and Omicron sublineage BA.1.1 was detected using whole-genome sequencing of specimens from this group. We used convalescent specimens from the unvaccinated infected group (N=6) collected 15 days (on average) after symptom onset. The third dose–vaccinated breakthrough infection group individuals (N=16) were infected at an average of 64 days after their last vaccination, and specimens were collected 17 days (on average) post-infection. The uninfected group (N=7) consists entirely of third dose–vaccinated individuals, and specimens were collected at an average of 21 days after their last vaccination (Table 1).
We used live viruses from the S clade and 19 Omicron sublineages (BA.1, BA.1.1, BA.2, BA.2.12.1, BA.2.3, BA.4, BA.5.2.1, BA.2.75, BA.2.75.2, BA.4.6, XBC, BF.7, BQ.1, BQ.1.1, BN.1, XBB.1, XBB.1.5, XBB.1.9.1, and XBB.1.16) obtained from the KDCA (registered with Global Initiative on Sharing All Influenza Data). For immunogenicity analysis, we performed the plaque reduction neutralization test 50 (PRNT50) and measured the level of neutralizing antibodies for all 20 sublineages [6].
The unvaccinated infected group showed the highest neutralizing activity against BA.1.1 (geometric mean titer [GMT], 151), the same sublineage that infected the participants. This was followed by BA.1 (GMT, 83) and BA.2.12.1 (GMT, 37), whereas the neutralizing activity against BA.2 (GMT, 19), BA.2.3 (GMT, 13), BA.4 (GMT, 10), and XBC (GMT, 15) was low, close to the cutoff values. No neutralizing activity was observed against the S clade or XBB lineages. Overall, the neutralizing activity levels were either low or below the cutoff, rendering it difficult to compare sublineages (Fig. 1A).
The third dose–vaccinated uninfected group showed the highest neutralizing activity against the S clade (GMT, 1740), which includes the same variant as the vaccine. The neutralizing activity against XBB.1, XBB.1.5, XBB.1.9.1, and XBB.1.16 was 19.6-fold, 24.6-fold, 9.1-fold, and 19.6-fold lower than that against BA.1.1 (the dominant variant in the initial Omicron outbreak in Korea), respectively. Likewise, the neutralizing activity against XBB.1, XBB.1.5, XBB.1.9.1, and XBB.1.16 was 10.1-fold, 12.6-fold, 4.7-fold, and 10.1-fold lower than that against BA.5.2.1 (the dominant variant in the second half of 2022), respectively (Fig. 1B).
For the third dose–vaccinated BA.1.1 breakthrough infection group, neutralizing activity against the S clade variant used in the vaccine was the highest (GMT, 4,030). This group showed even higher neutralizing activity than the vaccinated uninfected group owing to the additional immunity acquired during infection. The neutralizing activity against XBB.1, XBB.1.5, XBB.1.9.1, and XBB.1.16 in this group was 36.5-fold, 67.7-fold, 25.7-fold, and 79.3-fold lower than that against BA.1.1, respectively. The neutralizing activity against XBB.1, XBB.1.5, XBB.1.9.1, and XBB.1.16 was 10.9-fold, 20.2-fold, 7.7-fold, and 23.6-fold lower than that against BA.5.2.1, respectively, demonstrating a marked decrease in neutralizing activity. The increased drop-off in neutralizing activity in this group may be attributed to the high neutralizing activity against the BA variants; however, the overall neutralizing activity was still higher than that in the other two groups (Fig. 1C).
Our findings demonstrated that the unvaccinated infected (BA.1.1), third dose–vaccinated uninfected, and third dose–vaccinated breakthrough infection (BA.1.1) groups all showed a marked decrease in neutralizing activity against all XBB lineages compared with that against BA.1.1 and BA.5.2.1. This finding indicates a significant risk of infection with the currently prevalent XBB lineage for individuals who exhibited waning vaccine efficacy after vaccination or who were infected during the initial Omicron outbreak [7, 8]. As our study involved older patients, we could not analyze whether the age of the participants was responsible for the reduced immune response. However, our results are consistent with those of other studies and show that the XBB lineages induce a higher reduction in neutralizing ability than other Omicron sublineages [9-13]. Moreover, the differences in neutralizing antibody formation against different Omicron sublineages between the unvaccinated infected and breakthrough infection groups demonstrate that the vaccine provides immunity against more recent Omicron sublineages. Unvaccinated infected individuals showed almost no neutralizing activity against Omicron sublineages other than the variant with which they had been infected. Conversely, despite differences in neutralizing activity levels, the vaccinated breakthrough infection group demonstrated neutralizing activity against all 19 tested Omicron sublineages, including those with which they had not been infected. This result is consistent with the findings of other studies, demonstrating that COVID-19 vaccines induce both antibody and T-cell immune responses [14-16]. This finding further implies that vaccinated individuals are better able to produce neutralizing antibodies against other SARS-CoV-2 variants when infected than unvaccinated individuals, owing to vaccine-acquired and hybrid immunity [17, 18]. In other words, as unvaccinated individuals do not easily acquire neutralizing activity against other variants, they face the highest risk of reinfection by the XBB lineage.
Although the small sample size limits the statistical significance of our results, these findings would help identify individuals at increased risk of reinfection and establish control and vaccination strategies for immunologically vulnerable groups. In particular, as of June 1, 2023, the mandatory isolation of infected patients at hospitals and palliative care institutes in Korea has been relaxed [19]. As unvaccinated infected individuals are unlikely to generate significant neutralizing activity against variants they have not been infected with, the risk of infection or reinfection in unvaccinated people is high. Therefore, vaccination or other protection strategies are required for unvaccinated high-risk groups.
Notes
AUTHOR CONTRIBUTIONS
Lee EJ, Lee H, O SW, Kim J-M, Kim DJ, No JS, Park AK, Kim J-A, and Lee CY contributed to specimen preparation and performed the experiments; Choi Y-K and Kim E-J conceived and planned the experiments; Lee EJ, Lee H, Rhee JE, Kim I-H, and Kim E-J interpreted the results; Lee EJ took the lead in writing the manuscript; and Kim E-J supervised the study. All authors provided critical feedback that contributed to the research and final manuscript. All authors have read and approved the final manuscript.
References
1. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Updated on May 2023.
2. KDCA press release. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=722664&cg_code=&act=view&nPage=2. Updated on May 2023.
3. KDCA press release. https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=723107&cg_code=C01&act=view&nPage=1. Updated on July 26, 2023.
4. Qu P, Faraone JN, Evans JP, Zheng YM, Carlin C, Anghelina M, et al. 2023; Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants. Cell Rep. 42:112443. DOI: 10.1016/j.celrep.2023.112443. PMID: 37104089. PMCID: PMC10279473.
5. Yamasoba D, Uriu K, Plianchaisuk A, Kosugi Y, Pan L, Zahradnik J, et al. 2023; Virological characteristics of the SARS-CoV-2 omicron XBB.1.16 variant. Lancet Infect Dis. 23:655–6. DOI: 10.1016/S1473-3099(23)00278-5. PMID: 37148902.
6. Kim HM, Lee EJ, O SW, Choi YJ, Lee H, Oh SJ, et al. 2023; A seroprevalence study on residents in a senior care facility with breakthrough SARS-CoV-2 Omicron infection. Viral Immunol. 36:203–8. DOI: 10.1089/vim.2022.0133. PMID: 36951666. PMCID: PMC10621659.
7. Menegale F, Manica M, Zardini A, Guzzetta G, Marziano V, d'Andrea V, et al. 2023; Evaluation of waning of SARS-CoV-2 vaccine-induced immunity: a systematic review and meta-analysis. JAMA Netw Open. 6:e2310650. DOI: 10.1001/jamanetworkopen.2023.10650. PMID: 37133863. PMCID: PMC10157431.
8. Harapan H, Ar Royan H, Tyas II, Nadira A, Abdi IF, Anwar S, et al. 2023; Waning anti-SARS-CoV-2 receptor-binding domain total antibody in CoronaVac-vaccinated individuals in Indonesia. F1000Res. 11:300. DOI: 10.12688/f1000research.109676.2. PMID: 37260419. PMCID: PMC10209622.
9. Zhang L, Jiang L, Tian T, Li W, Pan Y, Wang Y. 2022; Efficacy and safety of COVID-19 vaccination in older adults: a systematic review and meta-analysis. Vaccines (Basel). 11:33. DOI: 10.3390/vaccines11010033. PMID: 36679878. PMCID: PMC9862835.
10. Yang J, Hong W, Lei H, He C, Lei W, Zhou Y, et al. 2023; Low levels of neutralizing antibodies against XBB Omicron subvariants after BA.5 infection. Signal Transduct Target Ther. 8:252. DOI: 10.1038/s41392-023-01495-4. PMID: 37336889. PMCID: PMC10279763.
11. Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. 2023; Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N Engl J Med. 388:89–91. DOI: 10.1056/NEJMc2214302. PMID: 36476720. PMCID: PMC9749618.
12. Xia S, Jiao F, Wang L, Yu X, Lu T, Fu Y, et al. 2023; SARS-CoV-2 Omicron XBB subvariants exhibit enhanced fusogenicity and substantial immune evasion in elderly population, but high sensitivity to pan-coronavirus fusion inhibitors. J Med Virol. 95:e28641. DOI: 10.1002/jmv.28641. PMID: 36890632.
13. Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, et al. 2023; Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 186:279–86.e8. DOI: 10.1016/j.cell.2022.12.018. PMID: 36580913. PMCID: PMC9747694.
14. Wherry EJ, Barouch DH. 2022; T cell immunity to COVID-19 vaccines. Science. 377:821–2. DOI: 10.1126/science.add2897. PMID: 35981045.
15. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. 2022; mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 185:457–66.e4. DOI: 10.1016/j.cell.2021.12.033. PMID: 34995482. PMCID: PMC8733787.
16. Dulipsingh L, Schaefer EJ, Wakefield D, Williams K, Halilovic A, Crowell R. 2023; Comparing SARS-CoV-2 neutralizing antibody levels in convalescent unvaccinated, convalescent vaccinated, and naive vaccinated subjects. Heliyon. 9:e17410. DOI: 10.1016/j.heliyon.2023.e17410. PMID: 37366522. PMCID: PMC10276490.
17. Suarez Castillo MS, Khaoua H, Courtejoie N. 2022; Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro Surveill. 27:2200250. DOI: 10.2807/1560-7917.ES.2022.27.16.2200250. PMID: 35451363. PMCID: PMC9027152.
18. Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. 2023; Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 23:556–67. DOI: 10.1016/S1473-3099(22)00801-5. PMID: 36681084.
19. Guide to quarantine measures following the lowering of the COVID-19 alert level [in Korean]. https://www.kdca.go.kr/gallery.es?mid=a20503020000&bid=0003&b_list=9&act=view&list_no=146120&nPage=1&vlist_no_npage=1&keyField=&keyWord=&orderby=. Updated on May 2023.
Fig. 1
Comparison of neutralizing antibody titers against Omicron sublineages. (A) Neutralizing activity in the unvaccinated group infected with BA.1.1 (N=6). This group had neutralizing activity against BA.5.2.1 below the cutoff, and there was no observable neutralizing activity against the XBB lineages, making it impossible to compare sublineages. (B) Neutralizing activity in the third dose–vaccinated uninfected group (N=7). This group showed lower neutralizing activity against XBB lineages than against BA.1.1 and BA.5.2.1. (C) Neutralizing activity in the third dose–vaccinated breakthrough infection (BA.1.1) group (N=16). Similar to that in the B group, the neutralizing activity against XBB lineages is the lowest, whereas the overall neutralizing ability is the highest among the three groups, indicating a hybrid immunity caused by breakthrough infections. The individual circles represent the GMT obtained from two independent experiments, each consisting of two replicates. The lines and error bars represent the geometric means and 95% confidence intervals for each group, respectively. The corresponding cutoff for PRNT50 was indicated by a dotted line at 10. The numbers at the top of each figure represent the GMT of neutralizing antibodies for each respective variant. The reference for comparative analysis, BA.1.1 and BA.5.2.1, is represented by blue circles, the analyzed XBB lineages are represented by red circles, and other variants are represented by black circles. The parentheses next to P values indicate the fold reduction in the GMTs of XBB lineages with reference to BA.1.1 and BA.5.2.1. P values were calculated via unpaired Mann–Whitney U test using Prism version 9 (GraphPad Software, San Diego, CA, USA).
Abbreviations: GMT, geometric mean titer; PRNT50, 50% plaque reduction neutralization titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ns, not significant.
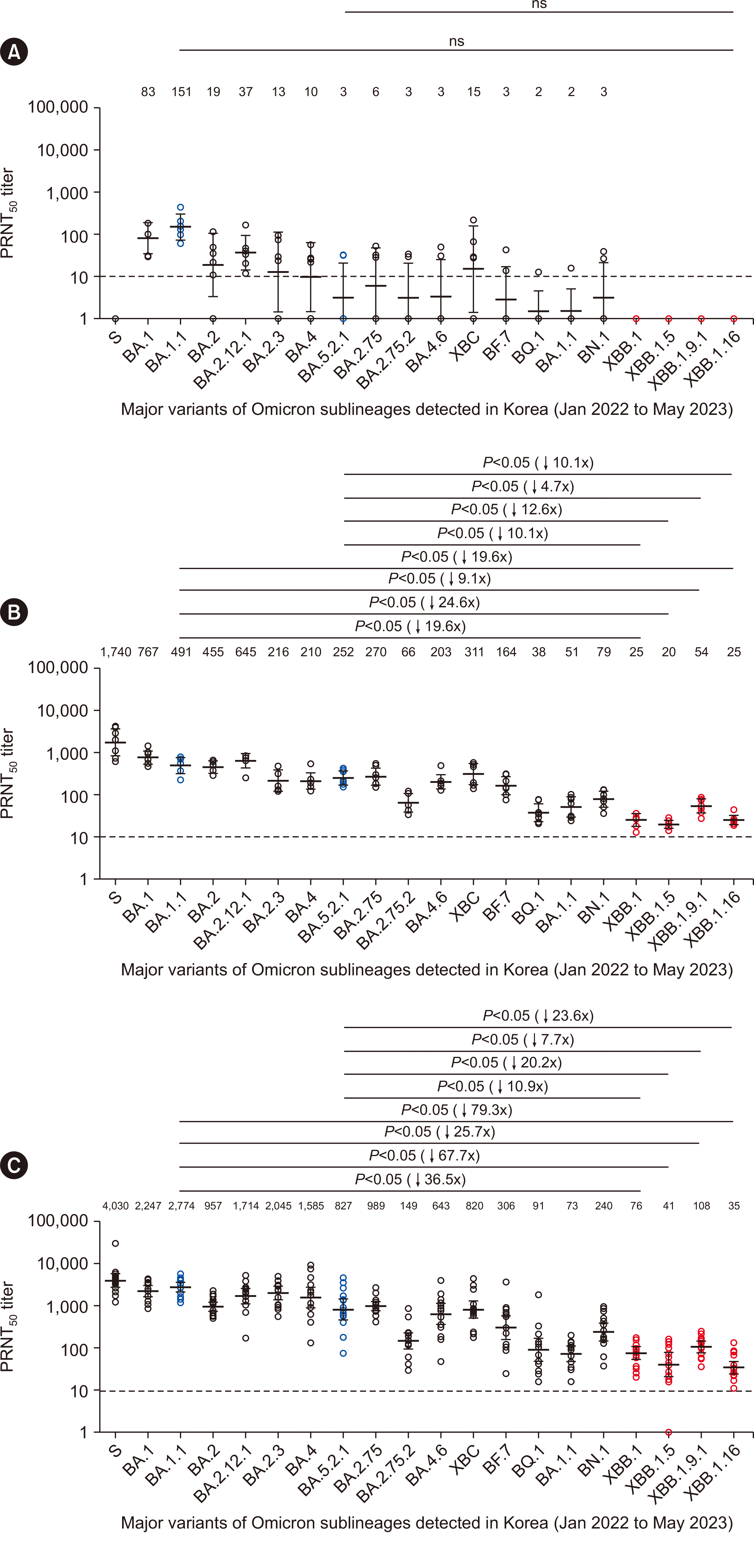
Table 1
Information on the study participants




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download