4. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. 2019; Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 380:45–56. DOI:
10.1056/NEJMoa1804980. PMID:
30501490.
5. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. 2018; Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 378:439–48. DOI:
10.1056/NEJMoa1709866. PMID:
29385370. PMCID:
PMC5996391.
6. Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. 2022; Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 28:325–32. DOI:
10.1038/s41591-021-01622-0. PMID:
34921238.
7. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. 2017; Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 377:2531–44. DOI:
10.1056/NEJMoa1707447. PMID:
29226797. PMCID:
PMC5882485.
8. Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. 2022; Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 23:91–103. DOI:
10.1016/S1470-2045(21)00591-X. PMID:
34895487.
9. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. 2021; KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 398:491–502. DOI:
10.1016/S0140-6736(21)01222-8. PMID:
34097852.
10. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. 2020; KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 382:1331–42. DOI:
10.1056/NEJMoa1914347. PMID:
32242358. PMCID:
PMC7731441.
11. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. 2020; Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 396:839–52. DOI:
10.1016/S0140-6736(20)31366-0. PMID:
32888407.
12. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. 2022; Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 386:640–54. DOI:
10.1056/NEJMoa2116133. PMID:
34891224.
13. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. 2022; Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 399:2294–308. DOI:
10.1016/S0140-6736(22)00662-6. PMID:
35717989.
14. Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. 2021; Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 384:705–16. DOI:
10.1056/NEJMoa2024850. PMID:
33626253.
15. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. 2021; Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 398:314–24. DOI:
10.1016/S0140-6736(21)00933-8. PMID:
34175021.
16. Bücklein V, Perez A, Rejeski K, Iacoboni G, Jurinovic V, Holtick U, et al. 2023; Inferior outcomes of EU versus US patients treated with CD19 CAR-T for relapsed/refractory large B-cell lymphoma: association with differences in tumor burden, systemic inflammation, bridging therapy utilization, and CAR-T product use. Hemasphere. 7:e907. DOI:
10.1097/HS9.0000000000000907. PMID:
37449196. PMCID:
PMC10337711.
17. Hoffmann MS, Hunter BD, Cobb PW, Varela JC, Munoz J. 2023; Overcoming barriers to referral for chimeric antigen receptor T cell therapy in patients with relapsed/refractory diffuse large B cell lymphoma. Transplant Cell Ther. 29:440–8. DOI:
10.1016/j.jtct.2023.04.003. PMID:
37031747.
18. Bethge WA, Martus P, Schmitt M, Holtick U, Subklewe M, von Tresckow B, et al. 2022; GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood. 140:349–58. DOI:
10.1182/blood.2021015209. PMID:
35316325.
19. Zafar A, Huang CY, Lo M, Arora S, Chung A, Wong SW, et al. 2023; Intensity of cyclophosphamide-based bridging therapy before chimeric antigen receptor T cell therapy in myeloma. Transplant Cell Ther. 29:504e1–7. DOI:
10.1016/j.jtct.2023.05.016. PMID:
37244643.
20. Ladbury C, Dandapani S, Hao C, Fabros M, Amini A, Sampath S, et al. 2023; Long-term follow-up of bridging therapies prior to CAR T-cell therapy for relapsed/refractory large B cell lymphoma. Cancers (Basel). 15:1747. DOI:
10.3390/cancers15061747. PMID:
36980632. PMCID:
PMC10046245.
21. Roddie C, Neill L, Osborne W, Iyengar S, Tholouli E, Irvine D, et al. 2023; Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. 7:2872–83. DOI:
10.1182/bloodadvances.2022009019. PMID:
36724512. PMCID:
PMC10300297.
22. Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. 2019; The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 133:1876–87. DOI:
10.1182/blood-2018-11-887067. PMID:
30782611. PMCID:
PMC6484391.
24. Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. 2019; Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 54:1643–50. DOI:
10.1038/s41409-019-0487-3. PMID:
30809033.
25. Yoo JW. 2023; Management of adverse events in young adults and children with acute B-cell lymphoblastic leukemia receiving anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. Blood Res. 58:S20–8. DOI:
10.5045/br.2023.2023026. PMID:
36891576. PMCID:
PMC10133856.
26. Li Y, Ming Y, Fu R, Li C, Wu Y, Jiang T, et al. 2022; The pathogenesis, diagnosis, prevention, and treatment of CAR-T cell therapy-related adverse reactions. Front Pharmacol. 13:950923. DOI:
10.3389/fphar.2022.950923. PMID:
36313336. PMCID:
PMC9616161.
27. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. 2018; Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 15:47–62. DOI:
10.1038/nrclinonc.2017.148. PMID:
28925994. PMCID:
PMC6733403.
28. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. 2018; Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 24:739–48. DOI:
10.1038/s41591-018-0036-4. PMID:
29808007.
29. Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. 2013; Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 368:1509–18. DOI:
10.1056/NEJMoa1215134. PMID:
23527958. PMCID:
PMC4058440.
30. Si S, Teachey DT. 2020; Spotlight on tocilizumab in the treatment of CAR-T-cell-induced cytokine release syndrome: clinical evidence to date. Ther Clin Risk Manag. 16:705–14.
31. Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. 2018; FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 23:943–7. DOI:
10.1634/theoncologist.2018-0028. PMID:
29622697. PMCID:
PMC6156173.
32. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. 2019; ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 25:625–38. DOI:
10.1016/j.bbmt.2018.12.758. PMID:
30592986.
33. Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. 2021; Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 39:3978–92. DOI:
10.1200/JCO.21.01992. PMID:
34724386.
34. Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. 2018; Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8:958–71. DOI:
10.1158/2159-8290.CD-17-1319. PMID:
29880584. PMCID:
PMC6385599.
35. Freyer CW, Porter DL. 2020; Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 146:940–8. DOI:
10.1016/j.jaci.2020.07.025. PMID:
32771558.
36. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. 2017; Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7:1404–19. DOI:
10.1158/2159-8290.CD-17-0698. PMID:
29025771. PMCID:
PMC5718945.
38. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. 2015; T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 385:517–28. DOI:
10.1016/S0140-6736(14)61403-3. PMID:
25319501.
39. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. 2020; Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T Consortium. J Clin Oncol. 38:3119–28. DOI:
10.1200/JCO.19.02104. PMID:
32401634. PMCID:
PMC7499611.
40. Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F, et al. 2021; Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro Oncol. 23:112–21. DOI:
10.1093/neuonc/noaa183. PMID:
32750704. PMCID:
PMC7850044.
41. Nellan A, McCully CML, Cruz Garcia R, Jayaprakash N, Widemann BC, Lee DW, et al. 2018; Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 132:662–6. DOI:
10.1182/blood-2018-05-846428. PMID:
29954750. PMCID:
PMC6086204.
42. Neelapu SS. 2019; Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 37 Suppl 1:48–52. DOI:
10.1002/hon.2595. PMID:
31187535.
43. Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. 2021; Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 32:34–48. DOI:
10.1016/j.annonc.2020.10.478. PMID:
33098993.
44. Topp MS, van Meerten T, Houot R, Minnema MC, Bouabdallah K, Lugtenburg PJ, et al. 2021; Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 195:388–98. DOI:
10.1111/bjh.17673. PMID:
34590303. PMCID:
PMC9293158.
45. Wehrli M, Gallagher K, Chen YB, Leick MB, McAfee SL, El-Jawahri AR, et al. 2022; Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. 10:e003847. DOI:
10.1136/jitc-2021-003847. PMID:
34996813. PMCID:
PMC8744112.
46. Riegler LL, Jones GP, Lee DW. 2019; Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag. 15:323–35. DOI:
10.2147/TCRM.S150524. PMID:
30880998. PMCID:
PMC6400118.
47. Hines MR, Keenan C, Maron Alfaro G, Cheng C, Zhou Y, Sharma A, et al. 2021; Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy. Br J Haematol. 194:701–7. DOI:
10.1111/bjh.17662. PMID:
34263927. PMCID:
PMC8756350.
48. Major A, Collins J, Craney C, Heitman AK, Bauer E, Zerante E, et al. 2021; Management of hemophagocytic lymphohistiocytosis (HLH) associated with chimeric antigen receptor T-cell (CAR-T) therapy using anti-cytokine therapy: an illustrative case and review of the literature. Leuk Lymphoma. 62:1765–9. DOI:
10.1080/10428194.2021.1881507. PMID:
33559517. PMCID:
PMC8282706.
49. Hines MR, Knight TE, McNerney KO, Leick MB, Jain T, Ahmed S, et al. 2023; Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transplant Cell Ther. 29:438.e1–16. DOI:
10.1016/j.jtct.2023.03.006. PMID:
36906275.
50. Henter JI, Samuelsson-Horne A, Aricò M, Egeler RM, Elinder G, Filipovich AH, et al. 2002; Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 100:2367–73. DOI:
10.1182/blood-2002-01-0172. PMID:
12239144.
51. Rosado FGN, Kim AS. 2013; Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clinical Pathol. 139:713–27. DOI:
10.1309/AJCP4ZDKJ4ICOUAT. PMID:
23690113.
52. Sandler RD, Tattersall RS, Schoemans H, Greco R, Badoglio M, Labopin M, et al. 2020; Diagnosis and management of secondary HLH/MAS following HSCT and CAR-T cell therapy in adults; a review of the literature and a survey of practice within EBMT centres on behalf of the autoimmune diseases working party (ADWP) and transplant complications working party (TCWP). Front Immunol. 11:524. DOI:
10.3389/fimmu.2020.00524. PMID:
32296434. PMCID:
PMC7137396.
53. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007; 48:124–31. DOI:
10.1002/pbc.21039. PMID:
16937360.
55. Yoon SE, Eun Y, Huh K, Chung CR, Yoo IY, Cho J, et al. 2020; A comprehensive analysis of adult patients with secondary hemophagocytic lymphohistiocytosis: a prospective cohort study. Ann Hematol. 99:2095–104. DOI:
10.1007/s00277-020-04083-6. PMID:
32440790.
56. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. 2022; Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 20:387–405. DOI:
10.6004/jnccn.2022.0020. PMID:
35390769.
57. Rainone M, Ngo D, Baird JH, Budde LE, Htut M, Aldoss I, et al. 2023; Interferon-gamma blockade in CAR T-cell therapy-associated macrophage activation syndrome/hemophagocytic lymphohistiocytosis. Blood Adv. 7:533–6. DOI:
10.1182/bloodadvances.2022008256. PMID:
35917457. PMCID:
PMC9979758.
58. La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, et al. 2019; Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 133:2465–77. DOI:
10.1182/blood.2018894618. PMID:
30992265.
59. Nahas GR, Komanduri KV, Pereira D, Goodman M, Jimenez AM, Beitinjaneh A, et al. 2020; Incidence and risk factors associated with a syndrome of persistent cytopenias after CAR-T cell therapy (PCTT). Leuk Lymphoma. 61:940–3. DOI:
10.1080/10428194.2019.1697814. PMID:
31793821.
61. Juluri KR, Wu QV, Voutsinas J, Hou J, Hirayama AV, Mullane E, et al. 2022; Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 6:2055–68. DOI:
10.1182/bloodadvances.2020004142. PMID:
34666344. PMCID:
PMC9006285.
62. Rejeski K, Wu Z, Blumenberg V, Kunz WG, Müller S, Kajigaya S, et al. 2022; Oligoclonal T-cell expansion in a patient with bone marrow failure after CD19 CAR-T therapy for Richter-transformed DLBCL. Blood. 140:2175–9. DOI:
10.1182/blood.2022017015. PMID:
35776908. PMCID:
PMC9837444.
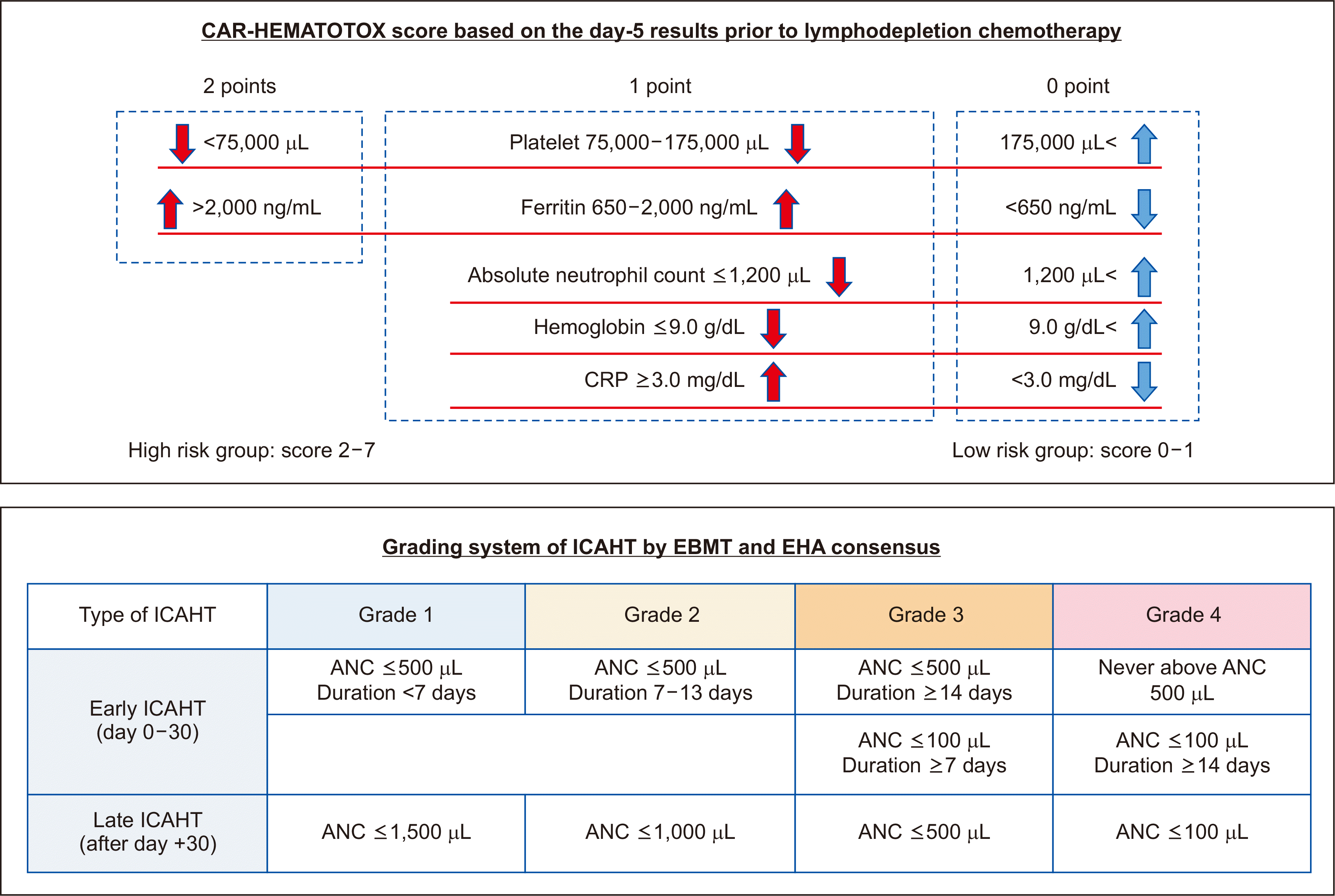
63. Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, et al. 2021; CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 138:2499–513. DOI:
10.1182/blood.2020010543. PMID:
34166502. PMCID:
PMC8893508.
64. Rejeski K, Perez A, Iacoboni G, Penack O, Bücklein V, Jentzsch L, et al. 2022; The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. 10:e004475. DOI:
10.1136/jitc-2021-004475. PMID:
35580927. PMCID:
PMC9114843.
65. Rejeski K, Subklewe M, Aljurf M, Bachy E, Balduzzi A, Barba P, et al. 2023; Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 142:865–77. DOI:
10.1182/blood.2023020578. PMID:
37300386.
66. Galli E, Allain V, Di Blasi R, Bernard S, Vercellino L, Morin F, et al. 2020; G-CSF does not worsen toxicities and efficacy of CAR-T cells in refractory/relapsed B-cell lymphoma. Bone Marrow Transplant. 55:2347–9. DOI:
10.1038/s41409-020-01006-x. PMID:
32719501.
67. Liévin R, Di Blasi R, Morin F, Galli E, Allain V, De Jorna R, et al. 2022; Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 57:431–9. DOI:
10.1038/s41409-021-01526-0. PMID:
35094012. PMCID:
PMC8907072.
68. Lemoine J, Bachy E, Cartron G, Beauvais D, Gastinne T, Di Blasi R, et al. 2023; Non-relapse mortality after CAR T-cell therapy for large B-cell lymphoma: a LYSA study from the DESCAR-T Registry. Blood Adv. 7:6589–98. DOI:
10.1182/bloodadvances.2023010624. PMID:
37672383. PMCID:
PMC10641092.
70. Harris CE, Vijenthira A, Ong SY, Baden LR, Hicks LK, Baird JH. 2023; COVID-19 and other viral infections in patients with hematologic malignancies. Am Soc Clin Oncol Educ Book. 43:e390778. DOI:
10.1200/EDBK_390778. PMID:
37163714.
71. Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, et al. 2018; Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 103:e215–8. DOI:
10.3324/haematol.2017.183459. PMID:
29419431. PMCID:
PMC5927974.
72. Plaks V, Rossi JM, Chou J, Wang L, Poddar S, Han G, et al. 2021; CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 138:1081–5. DOI:
10.1182/blood.2021010930. PMID:
34041526. PMCID:
PMC8462361.
73. Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, et al. 2021; Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol. 39:1650–9. DOI:
10.1200/JCO.20.02262. PMID:
33764809. PMCID:
PMC8274806.
74. Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. 2018; Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 378:449–59. DOI:
10.1056/NEJMoa1709919. PMID:
29385376. PMCID:
PMC6637939.
75. Myers RM, Taraseviciute A, Steinberg SM, Lamble AJ, Sheppard J, Yates B, et al. 2022; Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 40:932–44. DOI:
10.1200/JCO.21.01405. PMID:
34767461. PMCID:
PMC8937010.
76. Singh N, Lee YG, Shestova O, Ravikumar P, Hayer KE, Hong SJ, et al. 2020; Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T-cell dysfunction. Cancer Discov. 10:552–67. DOI:
10.1158/2159-8290.CD-19-0813. PMID:
32001516. PMCID:
PMC7416790.
77. Dekker L, Calkoen FG, Jiang Y, Blok H, Veldkamp SR, De Koning C, et al. 2022; Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia. Blood Adv. 6:1969–76. DOI:
10.1182/bloodadvances.2021006700. PMID:
35134115. PMCID:
PMC9006280.
78. Jain MD, Zhao H, Wang X, Atkins R, Menges M, Reid K, et al. 2021; Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 137:2621–33. DOI:
10.1182/blood.2020007445. PMID:
33512407. PMCID:
PMC8120145.
79. Mehta PH, Fiorenza S, Koldej RM, Jaworowski A, Ritchie DS, Quinn KM. 2021; T cell fitness and autologous CAR T cell therapy in haematologic malignancy. Front Immunol. 12:780442. DOI:
10.3389/fimmu.2021.780442. PMID:
34899742. PMCID:
PMC8658247.
80. Scholler N, Perbost R, Locke FL, Jain MD, Turcan S, Danan C, et al. 2022; Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat Med. 28:1872–82. DOI:
10.1038/s41591-022-01916-x. PMID:
36038629. PMCID:
PMC9499856.
81. Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. 2020; Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 26:1878–87. DOI:
10.1038/s41591-020-1061-7. PMID:
33020644. PMCID:
PMC8446909.
82. Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. 2016; Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 30:492–500. DOI:
10.1038/leu.2015.247. PMID:
26369987. PMCID:
PMC4746098.
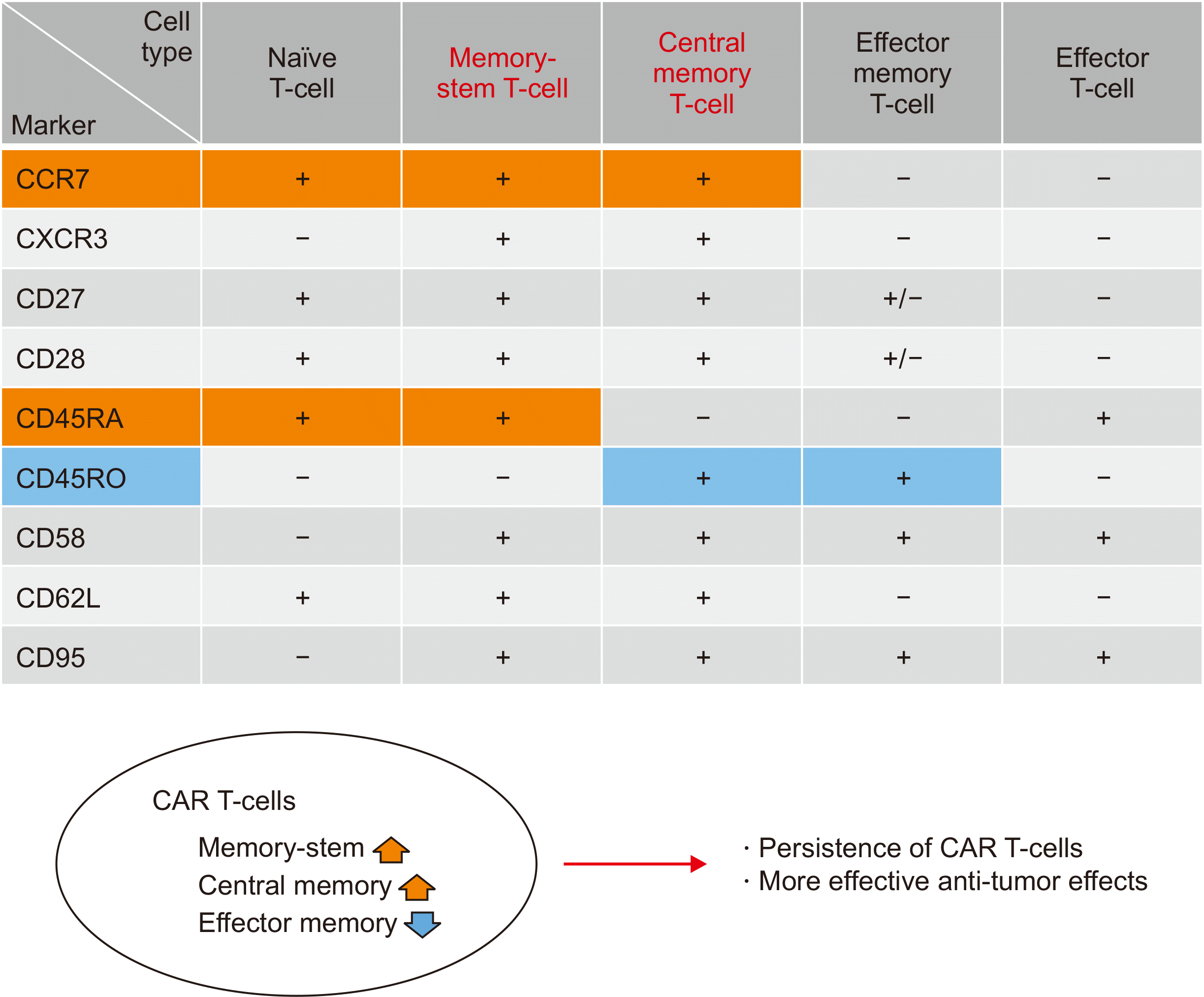
83. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. 2011; A human memory T cell subset with stem cell-like properties. Nat Med. 17:1290–7. DOI:
10.1038/nm.2446. PMID:
21926977. PMCID:
PMC3192229.
84. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. 2017; T memory stem cells in health and disease. Nat Med. 23:18–27. DOI:
10.1038/nm.4241. PMID:
28060797. PMCID:
PMC6354775.
85. Alvarez-Fernàndez C, Escribà-Garcia L, Caballero AC, Escudero-López E, Ujaldón-Miró C, Montserrat-Torres R, et al. 2021; Memory stem T cells modified with a redesigned CD30-chimeric antigen receptor show an enhanced antitumor effect in Hodgkin lymphoma. Clin Transl Immunology. 10:e1268. DOI:
10.1002/cti2.1268. PMID:
33968404. PMCID:
PMC8082716.
86. Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. 2020; Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 4:4898–911. DOI:
10.1182/bloodadvances.2020002394. PMID:
33035333. PMCID:
PMC7556133.
87. Aldoss I, Khaled SK, Wang X, Palmer J, Wang Y, Wagner JR, et al. 2023; Favorable activity and safety profile of memory-enriched CD19-targeted chimeric antigen receptor T-cell therapy in adults with high-risk relapsed/refractory ALL. Clin Cancer Res. 29:742–53. DOI:
10.1158/1078-0432.CCR-22-2038. PMID:
36255386. PMCID:
PMC10544259.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download