To the Editor:
Sirolimus, a mammalian target of rapamycin inhibitor, is commonly used as a maintenance immunosuppressive agent following kidney transplantation. Common mucocutaneous complications include oral ulcers, lymphedema, and poorly healing wounds. However, chronic nonhealing skin ulcers due to leukocytoclastic vasculitis (LCV) are rare. We present the case of a 40-year-old male kidney transplant recipient who developed nonhealing ulcers on his toes and abdominal wall while on sirolimus. A skin biopsy revealed LCV. After discontinuing sirolimus, the patient's skin lesions completely healed. Written informed consent was obtained for publication.
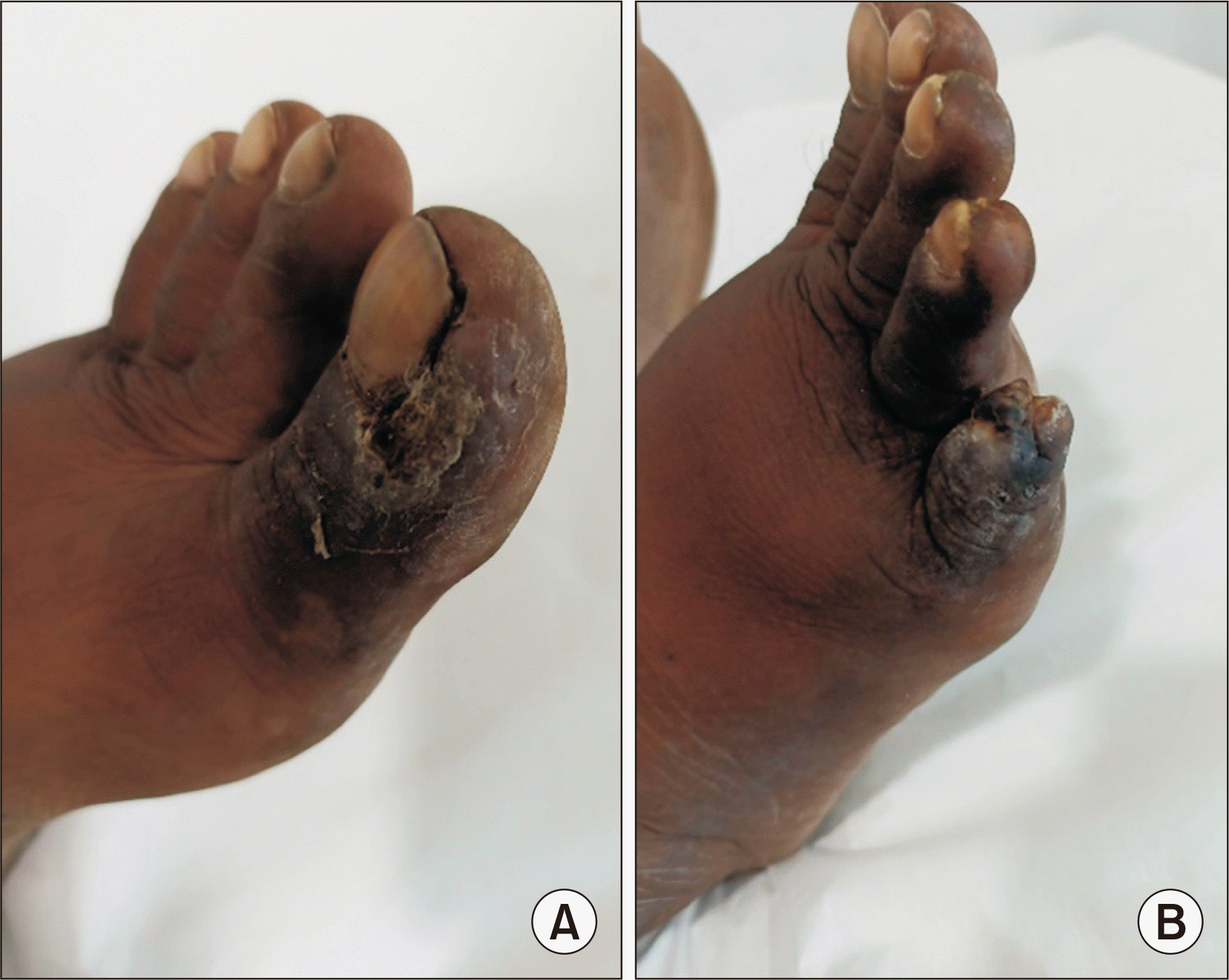
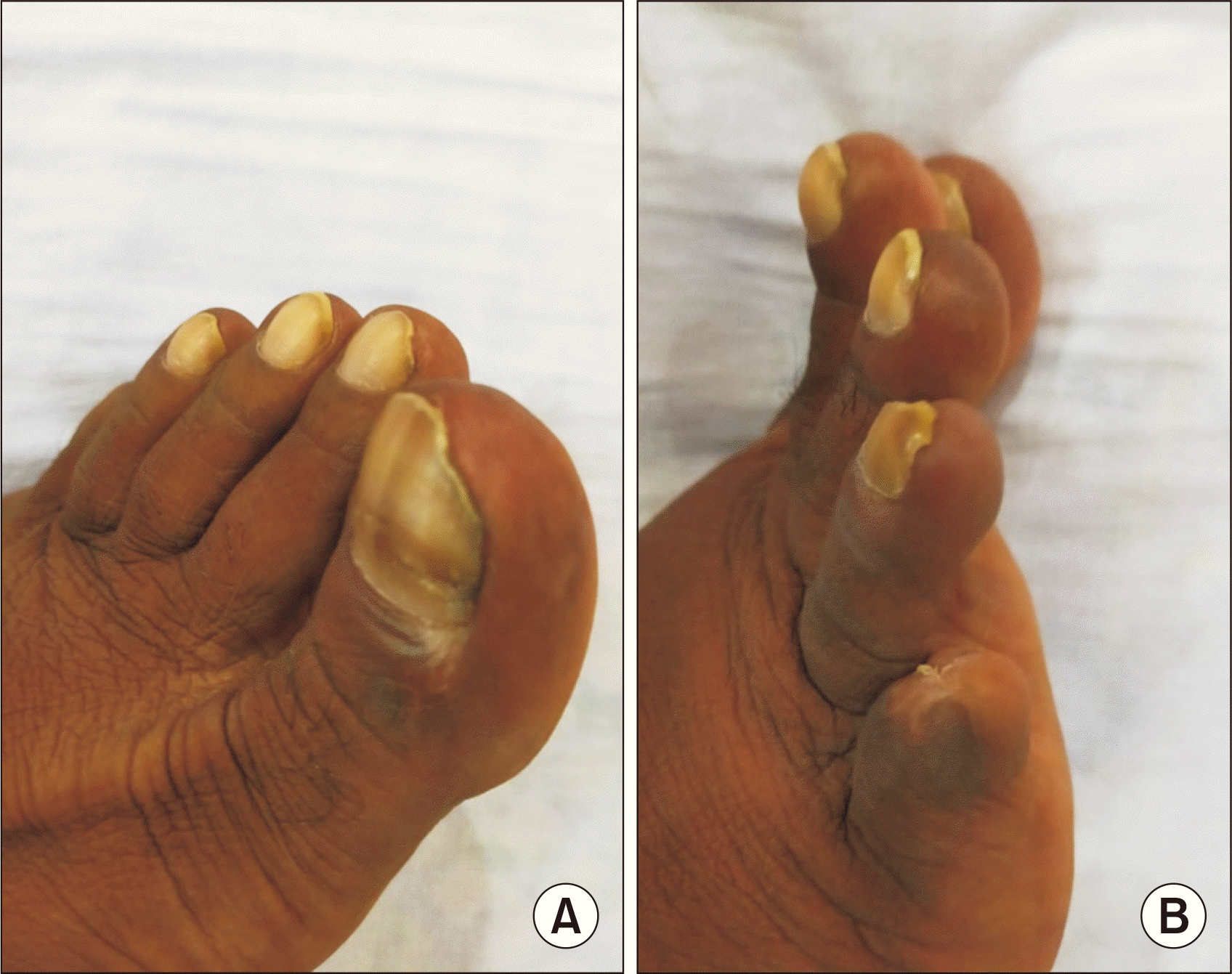
A 40-year-old male kidney transplant recipient presented with nonhealing, nonpruritic ulcerative lesions with scales on his great and little toes, as well as his abdominal wall, which had been present for the past 4 months (Fig. 1). He reported no history of contact with allergens or oral-genital lesions. Five years prior, he had undergone a living donor kidney transplant and received a 20-mg injection of basiliximab (administered on day 0 and day 4) for induction immunosuppression. This was followed by a regimen of tacrolimus, mycophenolate sodium, and prednisolone as maintenance immunosuppressants. His allograft function remained stable for 4 years posttransplant. However, during a regular follow-up evaluation, he was found to have allograft dysfunction, indicated by elevated creatinine levels, trace proteinuria on urinalysis, and normal transplant kidney ultrasound and Doppler study results. His tacrolimus trough level was 5.8 ng/dL. An ultrasound-guided kidney biopsy revealed signs of calcineurin nephrotoxicity. As a result, tacrolimus was discontinued, and the patient was started on a daily 1-mg dose of sirolimus. Eight weeks after starting sirolimus, the patient reported new-onset skin lesions on his great toe and sought consultation with a local dermatologist. The lesions were treated as a fungal infection (tinea pedis) with a topical 1% luliconazole cream. However, over the next 4–6 weeks, new lesions appeared on his little toe and abdominal wall. These lesions were painful and unresponsive to the topical antifungal treatment. The patient then reported to our center with the aforementioned complaints. Skin scraping for Gram staining, acid-fast bacilli staining, and potassium hydroxide mounting was performed to rule out an infectious etiology, but the results were negative. A skin punch biopsy from the toe lesion revealed LCV. A further workup for autoimmune causes, including antinuclear antibody immunoblot, myeloperoxidase and proteinase 3 antineutrophil cytoplasmic antibody, rheumatoid factor, and complement levels, was also negative. A hemogram, a liver function test, the erythrocyte sedimentation rate, and a syphilis test were all normal, and human immunodeficiency virus (HIV), hepatitis C RNA, and hepatitis B surface antigen tests were also normal. Sirolimus was considered the probable causative agent for the skin lesions, so it was discontinued and tacrolimus was resumed. Six weeks after discontinuing sirolimus, the skin lesions completely resolved, leaving only residual hyperpigmentation (Fig. 2).
LCV is a type of small-vessel vasculitis that affects dermal capillaries and venules. Approximately 50% of cases are idiopathic, while secondary causes can include infections (such as HIV, hepatitis B and C, syphilis, mycobacteria, and chlamydia), certain drugs (including beta-lactam antibiotics, sulfonamides, macrolides, loop and thiazide diuretics, phenytoin, allopurinol, and sirolimus), connective tissue disorders (e.g., systemic lupus erythematosus, Sjögren’s syndrome, Behçet’s disease, and rheumatoid arthritis), and malignancies (such as leukemia, lymphoma, and lung and intestinal carcinoma) [1]. LCV is characterized by necrotizing vasculitis, segmental transmural infiltration, and disruption of the vessels by neutrophils and fibrinoid necrosis. The association between LCV and sirolimus is not well understood, and it is a rare occurrence, with very few cases reported in the past that responded to discontinuation of the drug [2,3]. The time to recovery can range from a few days to 3–4 weeks after discontinuation. In our case, the diagnosis of sirolimus-induced LCV was based on the temporal profile of the occurrence of skin lesions after initiating sirolimus, biopsy-proven vascular inflammation, and the resolution of lesions upon discontinuation of the drug. With this report, we aim to make transplant physicians aware of this rare complication.
REFERENCES
1. Fiorentino DF. 2003; Cutaneous vasculitis. J Am Acad Dermatol. 48:311–40. DOI: 10.1067/mjd.2003.212. PMID: 12637912.
2. Hardinger KL, Cornelius LA, Trulock EP 3rd, Brennan DC. 2002; Sirolimus-induced leukocytoclastic vasculitis. Transplantation. 74:739–43. DOI: 10.1097/00007890-200209150-00025. PMID: 12352895.
3. Pasqualotto AC, Bianco PD, Sukiennik TC, Furian R, Garcia VD. 2004; Sirolimus-induced leukocytoclastic vasculitis: the second case reported. Am J Transplant. 4:1549–51. DOI: 10.1111/j.1600-6143.2004.00513.x. PMID: 15307846.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download