Abstract
Background
Liver transplantation has adverse effects from life-long immunosuppression that limit the improvement of long-term outcomes. Achieving clinical operational tolerance is a major goal in organ transplantation.
Methods
This study analyzed liver transplantation patients at a single institution from 1998 to 2020, excluding those who died within 1-year posttransplant. Operational tolerance was defined as normal liver function even after immunosuppressive drugs were discontinued. Propensity score matching was implemented at a 12 ratio for the tolerant group (TG) and the nontolerant group (NTG).
Results
Out of 2,300 recipients, 99 achieved operational tolerance without rejection. No significant differences in sex or body mass index (BMI) were found between the TG and NTG. There was a significantly higher percentage of children in the TG (24.0%) than in the NTG (10.1%). The NTG had more living donor liver transplants. Among 2,054 adult recipients, no significant differences in age, sex, or BMI were found between the TG and the NTG. However, the rate of living donor liver transplantation was 40.3% (29/75) in the TG and 84.8% in the NTG (P<0.001). The propensity score-matched analysis showed higher chronic renal failure rates and a higher graft recipient weight ratio in the TG, along with shorter warm ischemic times during surgery. After immunosuppressant withdrawal, a significant increase in the ratio of CD4+CD25+ T cells to total CD4+ T cells was observed in the TG.
In the 20th century, solid organ transplantation became the definitive treatment for patients with organ failure. In liver disease, after the first liver transplants were performed in the 1960s, the scope of transplantation was expanded from the original indication of end-stage liver disease (cirrhosis with severe complications), as well as irreversible acute liver failure and various metabolic liver diseases, to become firmly established since the late 20th century as a treatment for hepatocellular carcinoma (HCC) [1,2]. The development of immunosuppressants has undoubtedly played a major role in these advancements. The commercialization of calcineurin inhibitors (cyclosporin and tacrolimus) in the 1980s and 1990s played an important role in how liver transplantation has developed [3,4]. However, as the number of long-term survivors after transplantation increased, more attention was paid to the side effects of the long-term use of immunosuppressive drugs. It is well known that immunosuppression increases the risk of infections and de novo malignancies. In addition, long-term use of these drugs increases the risk of chronic diseases such as metabolic diseases, renal insufficiency, diabetes, dyslipidemia, osteoporosis, and vascular diseases, including cardiovascular disease. Since liver transplantation has become an established treatment for malignant tumors such as HCC, immunosuppressive drugs have also become a well-known risk factor for cancer recurrence [5-7]. Therefore, immunologic tolerance has received increasing attention in research on solid organ transplantation [6-8]. For instance, tolerance was induced in kidney transplantation by simultaneously transplanting the donor's bone marrow [9]. In liver transplantation, since the procedure’s inception by Thomas Starzl (1926–2017), it has been known that the liver achieves immune tolerance more easily than other solid organs [10]. Several factors explain the liver's immune tolerance, including the fact that the liver's unique function is to metabolize, store, and secrete absorbed nutrients from the large intestine; thus, tolerance is an innate mechanism of the liver, since it does not recognize absorbed material as foreign [10-13]. Immune tolerance after liver transplantation was discovered unexpectedly. A noncompliant adolescent patient did not take their medication against their doctor's orders [14]. However, the child's liver enzyme levels remained very stable, with no evidence of cirrhosis or rejection. Some studies have reported that immune tolerance was achieved in approximately 15% of patients who underwent whole liver transplantation [13,14]. Although many reports have been published regarding immune tolerance in whole liver transplantation, reports on partial liver transplantation, including living donor liver transplantation, are limited to pediatric transplantation. In this study, we analyzed the immunological, anatomical, and epidemiological characteristics of liver recipients who achieved immune tolerance at a single institution and studied specific T cell expression patterns to elucidate the mechanisms.
This research received approval from the Institutional Review Board of Samsung Medical Center (IRB No. 2023-11-171), which granted a waiver for the need for individual informed consent.
The study included all patients who underwent liver transplantation at a single institution from 1998 to 2020, excluding patients who died within 1 year of transplantation. Immune tolerance was defined as a state after transplantation in which immunosuppressive drugs were tapered off and liver function remained completely immunosuppressant-free. Normal liver function was defined as having stable serum liver enzyme levels, imaging liver findings, and an absence of clinical liver disease symptoms.
All patients received maintenance therapy based on triple therapy (steroid, calcineurin inhibitor, and mycophenolate mofetil) and, for adult patients, basiliximab induction therapy. A mammalian target of rapamycin inhibitor (sirolimus) was administered as needed. Steroids were usually tapered and discontinued within the first 6 months. If the liver enzyme levels were elevated at that time, immunosuppressive agents were increased or liver needle biopsy was performed if necessary, unless otherwise indicated after imaging studies. In patients with no history of rejection and stable liver enzyme levels, immunosuppressive agents were tapered from triple therapy to monotherapy, and then the drug dose was tapered and finally discontinued.
The baseline characteristics and demographics of patients who gained immune tolerance (tolerant group [TG]) and those who did not (nontolerant group [NTG]) were expressed as proportions, and the chi-square test and Student t-test were used for categorical and continuous variables, respectively. The analysis of variables that achieved tolerance was performed separately for pediatric and adult patients. For the adult living donor group, we conducted a 1:2 ratio propensity score matching analysis using the nearest neighbor method, comparing the TG with the NTG. The propensity score of each case was calculated using a multivariate logistic regression model which incorporated variables such as age, sex, and underlying disease, together with their Model for End-Stage Liver Disease values immediately before transplantation. In the multivariate analysis, logistic regression was performed. In addition, the numbers of CD4+ T cells and CD4+CD25+ T cells and their proportions in the TG after adult liver transplantation were ascertained.
Table 1 shows the clinical characteristics of patients according to operational tolerance. No differences were found in sex or body mass index (BMI) between the TG (n=99) and NTG (n=2,201). However, the percentage of children was significantly different, with 24.0% in the TG and 10.1% in the NTG. The proportion of living donor liver transplants was higher in the NTG. When comparing graft types, the percentage of left-sided grafts was higher in the TG (23.2% vs. 10.1% in the NTG), which may be due to the larger proportion of children.
The primary disease did not differ between the TG and NTG, but cases involving autoimmune liver disease, primary biliary cirrhosis, and primary sclerosing cholangitis were less common in the TG. Another notable exception was the achievement of immune tolerance in two liver transplant patients with incompatible blood types.
When the 246 pediatric patients under the age of 18 years who underwent transplantation and survived for more than 1 year were compared, no differences were found between the TG (n=24) and the NTG (n=222) in terms of age, sex, BMI, rate of living donor liver transplantation, or graft type.
A comparative analysis of the 2,054 adult patients aged 18 years and older who underwent transplantation during the period and survived for more than 1 year showed no differences in age, sex, BMI, etc. between the TG (n=75) and NTG (n=1,979), but the rate of living donor liver transplantation was lower in the TG (38.7%, 29/75) than in the NTG (84.8%, 1,678/1,979) (P<0.001). Graft type was also significantly higher in the TG than in the NTG (58.6% vs. 23.3%, P<0.001) (Table 2).
The pre- and postmatch results of 29 adult recipients showed that, before matching, the rate of posttransplant chronic renal failure was higher in the recipient group, the graft recipient weight ratio (GRWR) was higher in the recipient group, and the intraoperative warm ischemic time was shorter in the recipient group (Table 3). A comparative analysis of the results after 1:2 matching showed a higher rate of chronic renal failure in the TG, with statistically significant differences in GRWR and warm ischemic time (Table 4). Multivariate analysis of these results also showed significant differences in chronic kidney disease, GRWR, and warm ischemic time (Table 5).
When analyzing leukocytes from blood samples obtained from adult patients and comparing the percentage of CD4+CD25+ T cells among all CD4+ T cells, a significantly higher percentage of CD4+CD25+ T cells was found in the TG starting with the withdrawal of immunosuppressive drugs. Due to the small sample size, it was not possible to prove significance using statistical techniques. However, the overall number of CD4+ T cells was suppressed while taking immunosuppressive drugs, then increased after withdrawal, and the proportion of CD4+CD25+ T cells tended to be higher in the TG (Fig. 1).
The ongoing research on immune tolerance in liver transplantation is closely related to the expansion of indications for transplantation. Whereas patients with end-stage liver disease or acute liver failure accounted for more than 90% of all liver transplant recipients in the past, more than 50% of liver transplant recipients today are patients with HCC [1,2]. The use of immunosuppressive drugs in the treatment of solid tumors is the largest risk factor for cancer recurrence [8]. Therefore, many researchers have become interested in investigating this issue. In kidney transplant patients, the issue of immune tolerance has been focused on the deterioration of renal function and metabolic disease due to drug side effects. One such protocol is the cotransplantation of donor bone marrow cells [9]. However, these protocols are highly impractical for liver cancer patients. The patient's own bone marrow must be suppressed before transplantation, and this suppression can play a critical role in the spread of cancer. Therefore, liver transplantation research has focused on inducing spontaneous immune tolerance.
Studies on immune tolerance after liver transplantation have proposed various hypotheses. The most important suggestion relies on the anatomical characteristics of the liver, which has a specialized structure called the portal vein [10]. The explanation is that the body has a built-in immune tolerance: when the contents of the intestine enter the liver, an immune response is triggered against them on a daily basis, creating a situation that resembles chronic hepatitis. However, this is a poor explanation, since not all transplant patients achieve tolerance and only a small percentage of patients achieve immune tolerance [12].
Another hypothesis is T cell exhaustion, in which large numbers of cells enter the recipient's body and the recipient's T cells become too exhausted to attack them [15]. This hypothesis is supported by the fact that tolerance is frequently induced in children, frequently in adults in whole liver transplantation, and frequently in living donor liver transplantation with relatively large grafts.
A recent study’s findings confirm this hypothesis. The expression profile of CD4+CD25+ T cells (regulatory T [Treg] cells) in blood samples taken before and after induction of immune tolerance showed a significant increase in their proportion around the time when immunosuppressive drugs were withdrawn [16]. This demonstrates the role of Treg cells in immune tolerance. Because the finding validates the hypotheses of earlier studies, there is a need to conduct prospective studies with sufficient statistical power. As is well known, Treg cells play an immunomodulatory role through direct and indirect mechanisms, and direct suppression of progenitor cells in the graft has been considered an important mechanism for immune tolerance in liver transplantation [16-18].
The warm ischemic time in this study was a significant factor reflecting the graft’s qualitative state; that is, prolonged ischemic injury can cause cell damage in the graft. An especially long warm ischemic injury can cause severe cell damage after reperfusion, resulting in a sharp reduction in the number of cells. In other words, transplanting a well-preserved graft into a recipient with a short warm ischemic time has an advantage in inducing tolerance because more cells can be transplanted [16]. In this study, patients with renal failure were significantly more likely to achieve immune tolerance. However, there is no relationship between immune tolerance and renal function [19], and it is more likely that this is due to the late induction of tolerance after renal function has been compromised by prolonged immunosuppressive therapy.
Previous studies have reported that immune tolerance is frequent in pediatric or whole liver transplantation, but we have found several factors that may also induce tolerance in adults after living donor liver transplantation. This study found that transplantation of clinically large and healthy grafts was more favorable for inducing tolerance. It is possible also to speculate that the role of Treg cells is very important for achieving tolerance, which needs to be confirmed by further investigation.
REFERENCES
1. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. 2009; Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 10:35–43. DOI: 10.1016/S1470-2045(08)70284-5. PMID: 19058754.
2. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. 2007; Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 7:2587–96. DOI: 10.1111/j.1600-6143.2007.01965.x. PMID: 17868066.
3. Zhang W, Fung J. 2017; Limitations of current liver transplant immunosuppressive regimens: renal considerations. Hepatobiliary Pancreat Dis Int. 16:27–32. DOI: 10.1016/S1499-3872(16)60167-4. PMID: 28119255.
4. Rodríguez-Perálvarez M, Guerrero-Misas M, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. 2017; Maintenance immunosuppression for adults undergoing liver transplantation: a network meta-analysis. Cochrane Database Syst Rev. 3:CD011639. DOI: 10.1002/14651858.CD011639.pub2. PMID: 28362060.
5. Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, et al. 2001; A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 7:442–50. DOI: 10.1053/jlts.2001.23356. PMID: 11349266.
6. Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. 2002; Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: results from a double-blind randomized placebo-controlled trial. Liver Transpl. 8:132–42. DOI: 10.1053/jlts.2002.30302. PMID: 11862589.
7. Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. 2004; Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver Transpl. 10:1454–60. DOI: 10.1002/lt.20291. PMID: 15558584.
8. Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, et al. 2007; Tacrolimus monotherapy without steroids after liver transplantation: a prospective randomized double-blinded placebo-controlled trial. Am J Transplant. 7:1616–23. DOI: 10.1111/j.1600-6143.2007.01804.x. PMID: 17511685.
9. Lee KW, Park JB, Park H, Kwon Y, Lee JS, Kim KS, et al. 2020; Inducing transient mixed chimerism for allograft survival without maintenance immunosuppression with combined kidney and bone marrow transplantation: protocol optimization. Transplantation. 104:1472–82. DOI: 10.1097/TP.0000000000003006. PMID: 31634324.
10. Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. 1969; Induction of immunological tolerance by porcine liver allografts. Nature. 223:472–6. DOI: 10.1038/223472a0. PMID: 4894426.
11. Kamada N, Brons G, Davies HS. 1980; Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 29:429–31. DOI: 10.1097/00007890-198005000-00021. PMID: 6990572.
12. Kamada N, Davies HS, Roser B. 1981; Reversal of transplantation immunity by liver grafting. Nature. 292:840–2. DOI: 10.1038/292840a0. PMID: 7022223.
13. Subbotin V, Sun H, Aitouche A, Valdivia LA, Fung JJ, Starzl TE, et al. 1997; Abrogation of chronic rejection in a murine model of aortic allotransplantation by prior induction of donor-specific tolerance. Transplantation. 64:690–5. DOI: 10.1097/00007890-199709150-00005. PMID: 9311704. PMCID: PMC2957293.
14. Benítez C, Arancibia JP, Arrese M, Soza A, Domínguez P, Jarufe N, et al. 2011; Operational tolerance after liver transplantation, more common than we think: a case report. Ann Hepatol. 10:361–4. DOI: 10.1016/S1665-2681(19)31551-0. PMID: 21677341.
15. Choi GS. 2014; Clinical immune tolerance in liver transplantatiom: present and future. Hanyang Med Rev. 34:197–201. DOI: 10.7599/hmr.2014.34.4.197.
16. Ellias SD, Larson EL, Taner T, Nyberg SL. 2021; Cell-mediated therapies to facilitate operational tolerance in liver transplantation. Int J Mol Sci. 22:4016. DOI: 10.3390/ijms22084016. PMID: 33924646. PMCID: PMC8069094.
17. Cvetkovski F, Hexham JM, Berglund E. 2021; Strategies for liver transplantation tolerance. Int J Mol Sci. 22:2253. DOI: 10.3390/ijms22052253. PMID: 33668238. PMCID: PMC7956766.
18. Hann A, Oo YH, Perera MT. 2021; Regulatory T-cell therapy in liver transplantation and chronic liver disease. Front Immunol. 12:719954. DOI: 10.3389/fimmu.2021.719954. PMID: 34721383. PMCID: PMC8552037.
19. Syed-Ahmed M, Narayanan M. 2019; Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 26:8–15. DOI: 10.1053/j.ackd.2019.01.004. PMID: 30876622.
Fig. 1
(A) Serial T cell assays after liver transplantation. (B) Serial regulatory T (Treg) cell assays after liver transplantation. (C) Serial ratios of Treg cell and CD4+ T cell assay after liver transplantation. The blue arrow shows the time of operational tolerance.
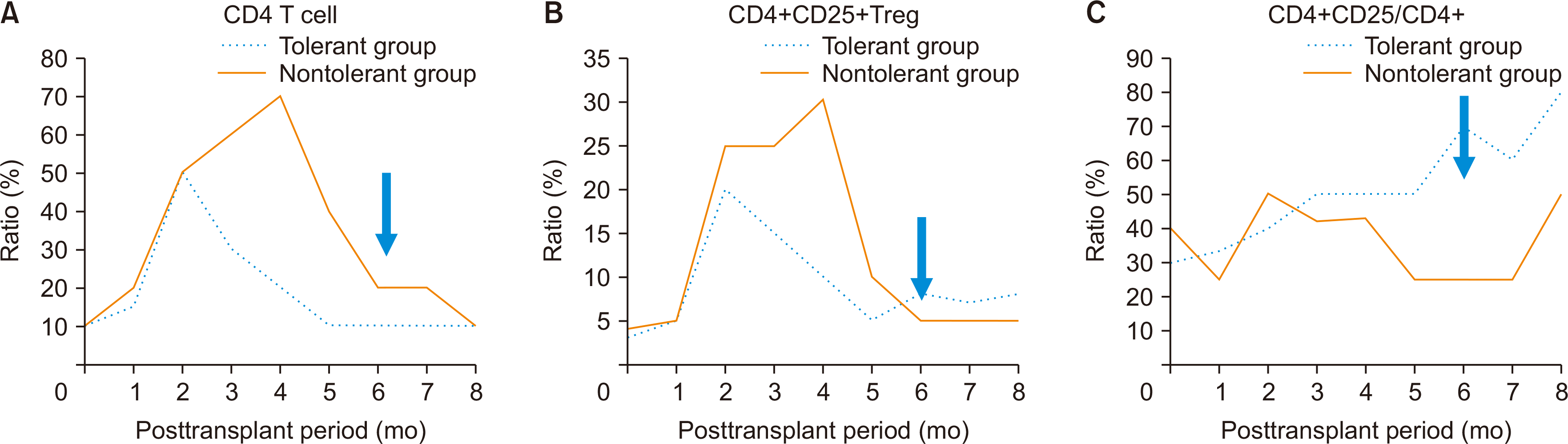
Table 1
Clinical characteristics of patients according to operational tolerance
Table 2
Clinical characteristics related to operational tolerance when dividing patient groups at 18 years of age
Table 3
Clinical characteristics of adult living donor recipients according to operational tolerance, before propensity score matching
Table 4
Clinical characteristics of patients according to operational tolerance in the propensity-matched analysis
Table 5
Univariate and multivariate analyses of factors associated with operational tolerance




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download