Abstract
Bronchobiliary fistula (BBF) is a very rare condition in children. Only a few pediatric BBF cases have been reported, in the context of a ruptured hydatid cyst or liver abscess. BBF after living donor liver transplantation (LDLT) has not been reported in the pediatric literature. We report a 7-year-old female child with Wilson disease, who developed BBF post-LDLT. She had a clinically uneventful course in the immediate post-transplant period. She was readmitted on postoperative day (POD) 75 with a productive cough and respiratory difficulty, which was diagnosed as bilioptysis secondary to BBF. Endoscopic retrograde cholangiopancreaticography was attempted but failed. Exploratory laparotomy showed a fistula from the strictured biliary anastomotic site to the right thoracic cavity; it was excised, and a Roux-en-Y hepaticojejunostomy was performed. She tolerated the procedure well and remained clinically well on follow-up through POD 185. BBF is extremely rare in children. This is the first case report of BBF in a child following LDLT. BBF requires a high index of suspicion for a timely intervention to prevent subsequent complications.
Biliary complication is regarded as the Achilles’ heel of living donor liver transplantation (LDLT). This diagnosis is usually straightforward, based on abnormalities of liver function tests and the appearance of bile in the drainage tubes. We report a bronchobiliary fistula (BBF) which became evident 3 months after LDLT. In 1850 Peacock et al. [1] reported the first BBF case in a young woman with a ruptured hydatid cyst. Both the acquired and the congenital varieties of BBF are very rare complications in children [2-5]. Acquired BBF usually occurs as a complication of a rupture of a hydatid cyst or subphrenic abscess. It could also occur following hepatic trauma, tumor, or hepatobiliary surgery [2,6-8]. The condition is universally characterized by the presence of yellow bile in the sputum (bilioptysis), which is pathognomonic of the condition [7,8]. A high index of suspicion and early diagnosis are crucial to prevent progressive corrosion of pulmonary and bronchial tissues. In the context of liver transplantation (LT), only a few cases have been reported in adults [4,5]. However, no such data exist in the pediatric LT setting to the best of our knowledge. Herein, we report a 7-year-old female child with Wilson disease, who developed BBF post-LT.
In our institution, a case report does not require Institutional Review Board approval. We have obtained informed consent from the patient’s guardian.
A 7-year-old female child presented to our center with decompensated chronic liver disease and portal hypertension. On evaluation, she was diagnosed with hepatic Wilson disease. She underwent ABO-compatible LDLT with the mother as the donor. The child received a 430-g left lobe graft with middle hepatic vein, with a graft-to-recipient weight ratio of 2.5. The grafted left hepatic duct was anastomosed to the recipient’s common hepatic duct. The immediate postoperative period was uneventful, and she was extubated after completion of the surgery on the operation table. She was started on triple immunosuppressive therapy (steroids, tacrolimus, and mycophenolate mofetil) and other supportive medicines as per our institution’s standard protocol [9,10]. The right and left abdominal drains were removed on postoperative day (POD) 3 and POD 11, respectively. On POD 14, she developed fever with a nonproductive cough, and on clinical examination, although there was no tachypnoea, the right subscapular region showed decreased air entry. Subsequent computed tomography (CT) imaging was suggestive of right pleural effusion with basal atelectasis, without any perihepatic fluid collection. The diagnostic pleural fluid aspiration suggested empyema (turbid fluid, total pleural fluid cells 2,760, neutrophils 90%, sterile culture), which was managed with pleural fluid drainage and antibiotics. Her graft function was excellent, and she was discharged on POD 24.
She was readmitted on POD 75 with a productive cough, respiratory difficulty, and intermittent fever spikes for 3 days. On examination, she had tachycardia, tachypnoea, and reduced air entry on the right infrascapular region; oxygen saturation on room air was 88%, which improved on supplemental oxygen and supportive measures. Chest imaging revealed heterogeneous opacity of the right lower lobe with mild pleural effusion (Fig. 1). Bronchoscopy for the unexplained pulmonary issues revealed enteric/bilious content in the right bronchus, which was interpreted as aspiration pneumonitis. She responded to antibiotics, chest physiotherapy, and nebulization. The liver graft function was persistently normal. She was discharged on POD 85 in a hemodynamically stable condition with mild respiratory symptoms.
During outpatient follow-up on POD 90, the patient’s sputum was noticed to be bilious (bilioptysis) (Fig. 2), which suggested a clinical diagnosis of BBF. She was admitted immediately and subjected to endoscopic retrograde cholangiopancreaticography (ERCP), which failed. She was then scheduled for exploratory laparotomy and surgical correction of the suspected communication. On laparotomy, a fistula was noticed from the strictured biliary anastomotic site to the right thoracic cavity, as shown in Fig. 3. The fistula was excised, and Roux-en-Y hepaticojejunostomy was performed. A drain was inserted in the right subphrenic space. The child’s symptoms improved, the drain was removed, and she was discharged with normal graft functions on POD 102. She was doing well on follow-up as per the last visit on POD 185.
BBFs can be divided broadly into congenital and acquired types. Congenital BBF usually presents early in infancy with poor feeding, bilioptysis, and respiratory difficulty. Acquired BBF can develop from pathology in the lung, diaphragm, or biliary tract [4]. Subphrenic abscesses, hydatid cysts, malignancies, and trauma are the leading causes of BBF. Table 1 shows a compilation of relevant case reports of acquired BBF in children. Patients with acquired BBF usually present with fever spikes, persistent productive cough, and respiratory difficulty. Bilioptysis remains the pathognomonic and most common clinical feature of BBF. Clinical features suggestive of biliary obstruction are often present. BBF is associated with a high risk of morbidity and mortality (12%) [7,16]. ERCP with or without surgical correction is the mainstay of management. A systematic review of 68 adult cases of BBF showed bilioptysis in all patients, 41% of which required surgical correction [7].
To the best of our knowledge there are only two case reports of BBF in post-LT patients and a few after hepatectomy, as shown in Table 2. Chaing et al. [4] reported a BBF in a 40-year-old post-LT woman from Taiwan. She developed biliary stricture and right subphrenic bile collection leading to BBF, for which she underwent percutaneous transhepatic biliary drainage (PTBD) [4]. A similar case was reported by Yeatman et al. [5]. The likely mechanism of the development of BBF in a post-LT setting is hypothesized to be because of the biliary stricture and bile leak in the subphrenic space, where bile acts as a tissue-corrosive agent, leading to the erosion of the diaphragm and subsequent communication with the right-hand bronchial tract. There was no rise in bilirubin level despite the biliary stricture. This can be explained by diversion of bile from the biliary system into the bronchi instead of the duodenum.
The diagnosis is based on demonstrating the abnormal communication between the biliary tree and the bronchial tract, which is usually done by cholangiography via magnetic resonance cholangiopancreaticography or ERCP. CT and hepatobiliary iminodiacetic acid scans are other modalities for diagnosis. Chest imaging usually shows right lower lung heterogenous opacities with reactionary pleural effusion, and bronchoscopy shows the bilious content in the bronchi [7]. In our case, bronchoscopy showed bilious content, which was initially misinterpreted as the aspiration of the content of the stomach and was treated as aspiration pneumonitis. Later, the child presented with frank bilioptysis, which raised the possibility of BBF; exploratory laparotomy confirmed the diagnosis.
Management focused on correcting the primary hepatobiliary insult. The first step was to ensure adequate drainage of bile from the biliary tract to the duodenum without any obstruction in the path. The commonly used modalities are ERCP with sphincterotomy and biliary stenting [17] and PTBD (especially in postsurgical cases) [18]. Surgical closure by excision of the BBF remains the treatment of choice for complicated cases. Interventional radiological procedures, such as endobronchial embolization with silicone spigots and transhepatic microcoil embolization, have also been mentioned in the literature [19]. From the largest series on hydatid cyst BBF by Tocchi et al. [20], the thoracoabdominal incision approach was found to be better for post-surgical outcomes than abdominal- or thoracic-only incision. Refractory cases need thoracotomy and lung resection, which is the last resort when all other conservative modalities fail.
The pediatric literature is limited. From the Indian subcontinent, one case of BBF was reported in a 3-year-old child with ruptured liver abscess, where it was managed with right lateral thoracotomy, right lower lobectomy, and excision of the communicating tract [2]. Yang et al. [3] published a case report of a 10-year-old child who experienced liver rupture following a road traffic accident, for which he underwent repeated operations. He subsequently developed obstruction of the iatrogenic hepatic venous outflow tract and BBF and had to undergo LT [3].
In our case, the child was suspected to have BBF based on bilioptysis. On failure of the ERCP, exploratory laparotomy and successful surgical closure were undertaken with a postoperative right subphrenic drain. The child did well after the procedure and was discharged in hemodynamically stable condition with normal graft functions. This is the first case report of BBF in a child following LDLT. BBF is extremely rare in children, and it requires a high index of suspicion for the timely intervention and prevention of subsequent complications.
REFERENCES
1. Peacock TB. 1850; Case in which hydatids were expectorated, and one of suppuration in a hydatid cyst of the liver communicating with the lungs. Edinb Med Surg J. 74:33–46.
2. Kumar P, Mehta P, Ismail J, Agarwala S, Jana M, Lodha R, et al. 2015; Brocho-biliary fistula: a rare complication after ruptured liver abscess in a 3½ year old child. Lung India. 32:489–91. DOI: 10.4103/0970-2113.164157. PMID: 26628766. PMCID: PMC4587006.
3. Yang L, Guo Z, Yang L, Ju W, Wang D, He X. Orthotopic liver transplantation in a pediatric patient with iatrogenic Budd-Chiari syndrome complicated by bronchobiliary fistula. Pediatr Transplant. 2017; 21:DOI: 10.1111/petr.13008. PMID: 28745424.
4. Chaing MH, Chen CW, Lu CH. 2020; Successful treatment of bronchobiliary fistula after living donor liver transplantation: a case report. Transplant Proc. 52:2778–80. DOI: 10.1016/j.transproceed.2020.01.171. PMID: 32434746.
5. Yeatman CF, Fisher RA, Carucci LR, Halvorsen RA. 2004; Bronchobiliary fistula after liver transplantation. J Comput Assist Tomogr. 28:717–20. DOI: 10.1097/01.rct.0000131583.52386.cf. PMID: 15480050.
6. Eryigit H, Oztas S, Urek S, Olgac G, Kurutepe M, Kutlu CA. 2007; Management of acquired bronchobiliary fistula: 3 case reports and a literature review. J Cardiothorac Surg. 2:52. DOI: 10.1186/1749-8090-2-52. PMID: 18053192. PMCID: PMC2217537.
7. Liao GQ, Wang H, Zhu GY, Zhu KB, Lv FX, Tai S. 2011; Management of acquired bronchobiliary fistula: a systematic literature review of 68 cases published in 30 years. World J Gastroenterol. 17:3842–9. DOI: 10.3748/wjg.v17.i33.3842. PMID: 21987628. PMCID: PMC3181447.
8. Pappas SC, Sasaki A, Minuk GY. 1982; Bronchobiliary fistula presenting as cough with yellow sputum. N Engl J Med. 307:1027. DOI: 10.1056/NEJM198210143071623. PMID: 7110295.
9. Pandey VK, Prabhudesai A, Goyal S, Nasa V, Yadav V, Singh SA, et al. 2022; Safety and feasibility of immediate tracheal extubation of small pediatric patients after living donor liver transplantation. Pediatr Transplant. 26:e14401. DOI: 10.1111/petr.14401. PMID: 36177941.
10. Pandey Y, Varma S, Chikkala BR, Acharya R, Verma S, Balradja I, et al. 2020; Outcome of pediatric liver transplants in patients with less than 10 kg of body weight is not worse. Exp Clin Transplant. 18:707–11. DOI: 10.6002/ect.2020.0308. PMID: 33187463.
11. Rahimi MT, Hares R, Rahman H, Hoshang MS, Hofiani SM. 2023; Management of acquired bronchobiliary fistula: a case report. J Pediatr Surg Case Rep. 95:102669. DOI: 10.1016/j.epsc.2023.102669.
12. Kuo YS, Lee SC, Chang H, Hsieh CB, Huang TW. 2014; Thoracoscopic surgery for bronchobiliary fistula: a case report. J Cardiothorac Surg. 9:139. DOI: 10.1186/s13019-014-0139-z. PMID: 25230847. PMCID: PMC4172870.
13. Hai S, Iimuro Y, Hirano T, Suzumura K, Yada A, Fujimoto J. 2016; Bronchobiliary fistula caused after hepatectomy for hepatocellular carcinoma: a case report. Surg Case Rep. 2:147. DOI: 10.1186/s40792-016-0273-z. PMID: 27921278. PMCID: PMC5138177.
14. Miranda García M, Martakoush María A, Cobos Briz M. 2018; Bronchobiliary fistula, a late complication of liver surgery. Arch Bronconeumol (Engl Ed). 54:285–6. DOI: 10.1016/j.arbr.2017.09.015. PMID: 29102340.
15. Lazarou V, Moris D, Papalampros A, Tsilimigras DI, Karachaliou GS, Petrou A. 2019; Bronchobiliary fistula after hepatectomy: a case report and review of the literature. Mol Clin Oncol. 11:602–6. DOI: 10.3892/mco.2019.1935. PMID: 31798877. PMCID: PMC6870047.
16. Moumen M, el Fares F. 1991; Bilio-bronchial fistula of hydatid origin. Apropos of 8 cases. J Chir (Paris). 128:188–92.
17. Thrumurthy SG, Anuruddha AH, De Zoysa MI, Samarasekera DN. ERCP for the treatment of traumatic biliobronchial and biliocutaneous fistulas. Endoscopy. 2011; 43 Suppl 2 UCTN:E42. DOI: 10.1055/s-0030-1255898. PMID: 21287444.
18. Deshmukh H, Prasad S, Patankar T, Patel V. 1999; Percutaneous management of a broncho-biliary fistula complicating ruptured amebic liver abscess. Am J Gastroenterol. 94:289–90.
19. Kostopanagiotou K, George RS, Kefaloyannis E, Papagiannopoulos K. 2015; Novel technique in managing bronchobiliary fistula in adults: endobronchial embolization using silicone spigots in 2 cases. Ann Thorac Med. 10:67–8.
20. Tocchi A, Mazzoni G, Miccini M, Drumo A, Cassini D, Colace L, et al. 2007; Treatment of hydatid bronchobiliary fistulas: 30 years of experience. Liver Int. 27:209–14. DOI: 10.1111/j.1478-3231.2007.01435.x. PMID: 17311615.
Fig. 1
(A) Chest X-ray, posteroanterior view, showing confluent nonhomogeneous opacities in the right lower zone obscuring the diaphragm and right cardiac border with mild right-hand pleural effusion. (B) Computed tomography showing consolidation of the right lung.
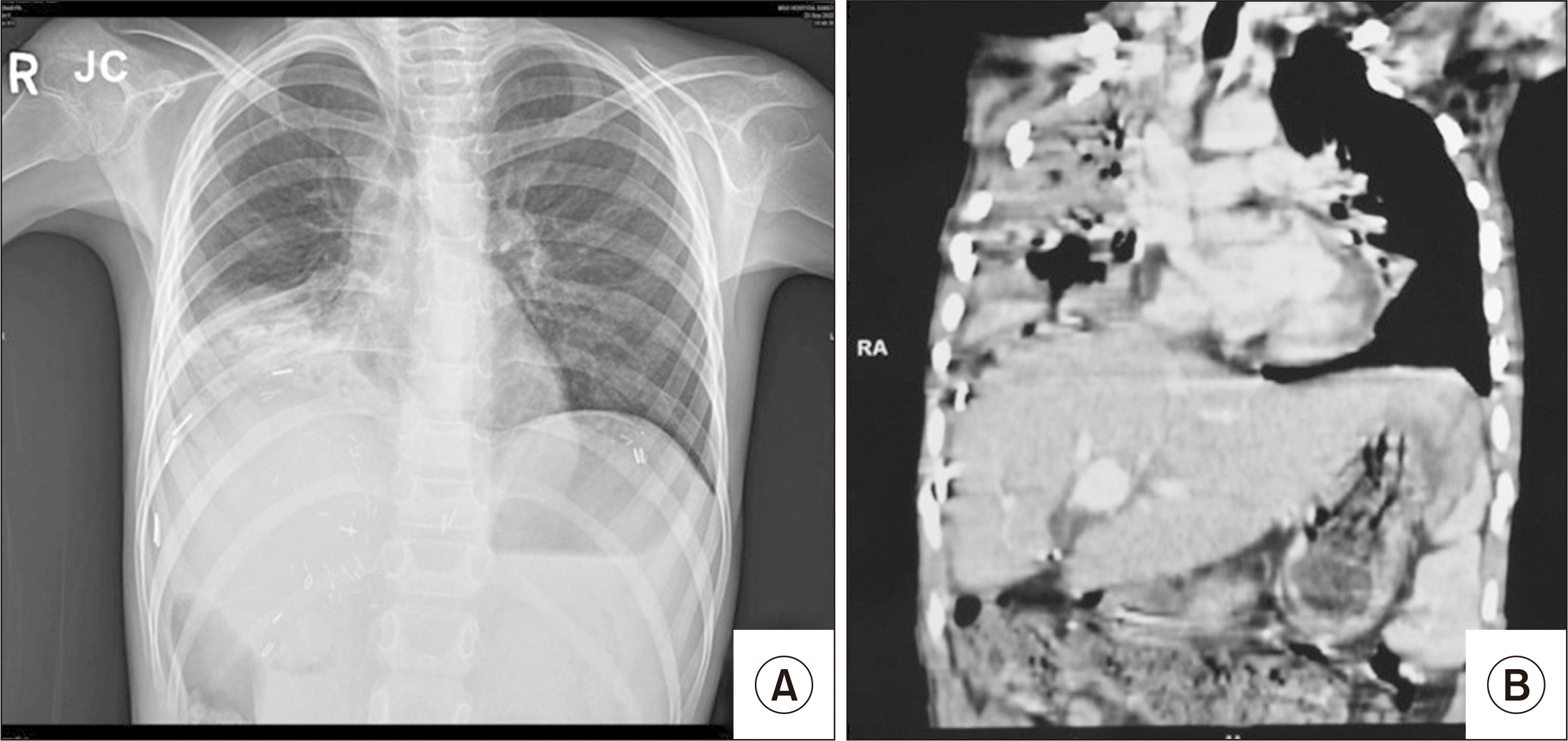
Fig. 2
Bilioptysis: yellow-colored bilious sputum. (A) Cup containing yellow colored sputum (bilioptysis), shown by parents in the outpatient clinic. (B) Tray showing yellow sputum after the admission to the intensive care unit
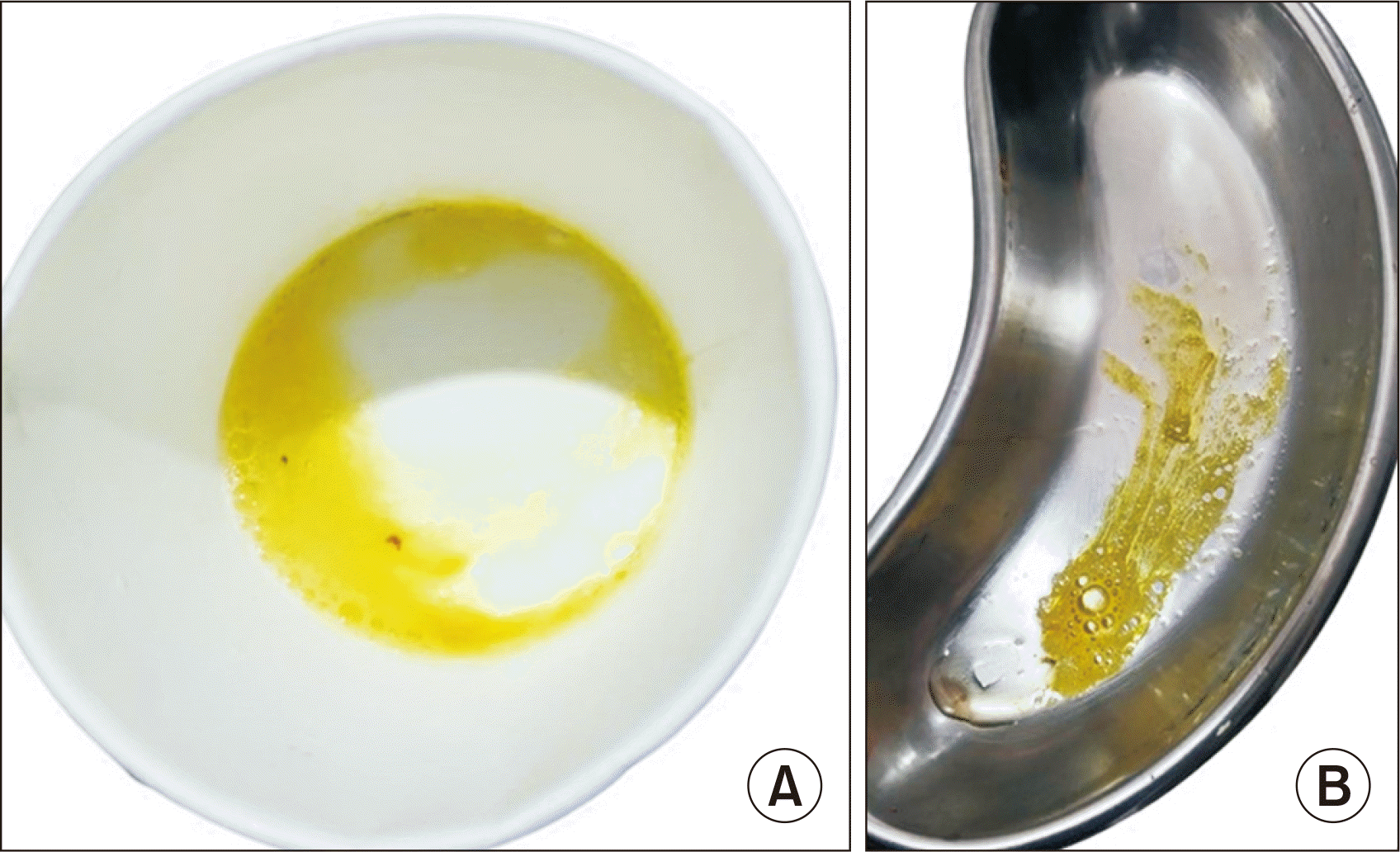
Fig. 3
Bronchobiliary fistula: communication from the strictured duct-duct anastomotic site to the right-hand thoracic cavity. (A) Before and (B) after examination of fistula tract by metallic probe (indicated by arrows).
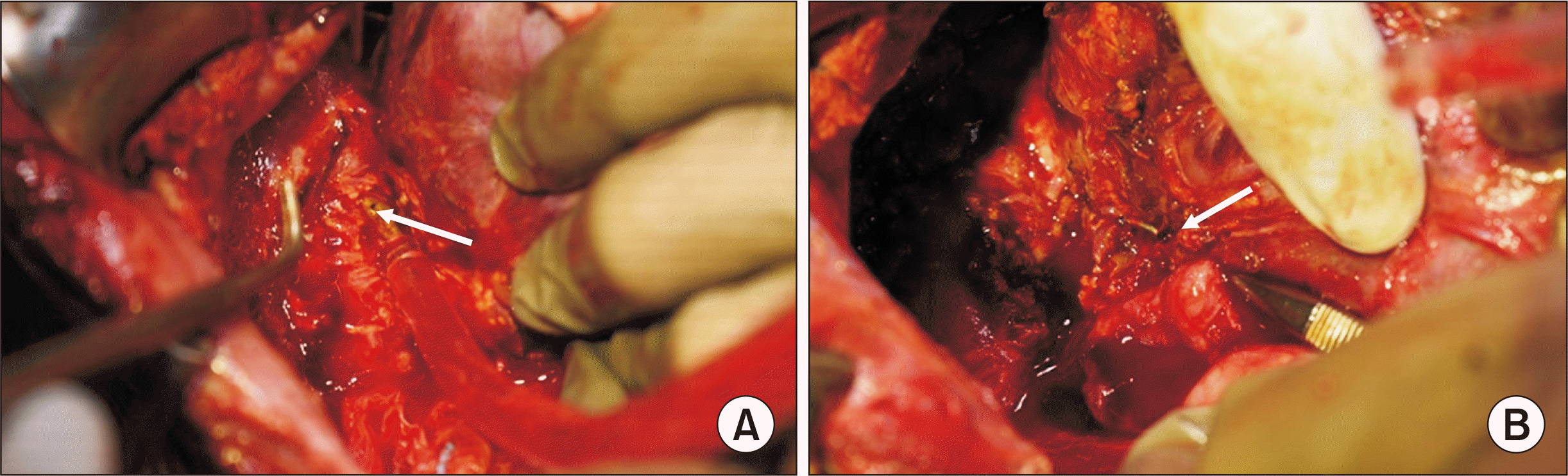
Table 1
Case reports of acquired BBF in children
| Study | Subject | Primary diagnosis | Fistulous communication | Interval between primary diagnosis and BBF (mo) | Clinical feature | Imaging/diagnostic modality | Treatment | Outcome on last follow-up | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Age (yr) | Sex | ||||||||
| Kumar et al. (2015) [2] | 3.5 | Female | Ruptured liver abscess | Right hepatic duct to a branch of right main bronchus | 7 | Bilioptysis | MRCP showed fistulous communication | Right lateral thoracotomy and right lower lobectomy with surgical excision of sinus tract | Alive and healthy |
| Yang et al. (2017) [3] | 10 | Male | Traumatic liver rupture | Unknown | 36 | Bilioptysis | CT could not identify the fistula | Orthotopic liver transplantation (persistent BBF and chronic HVOTO) | Alive and healthy |
| Rahimi et al. (2023) [11] | 17 | Female | Liver hydatid cyst | Unknown | 10 | Bilioptysis | CT chest and abdomen | Right thoracotomy, adhesiolysis, and fistula tract excision | Alive and healthy |
Table 2
Case reports of acquired BBF in post-liver surgery patients
| Study | Subject | Primary diagnosis | Type of fistula | Interval between primary diagnosis and BBF | Clinical feature | Imaging/diagnostic modality | Treatment | Outcome on last follow-up | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Age (yr) | Sex | ||||||||
| Post-liver transplantation | |||||||||
| Yeatman et al. (2004) [5] | 40 | Female | Ruptured hepatic adenoma | Right subphrenic biloma to right lower bronchus | 4 mo | Bilioptysis | Bronchoscopy showed bile in the bronchi | External biliary stent | Alive and healthy |
| Chaing et al. (2020) [4] | 40 | Female | Autoimmune hepatitis | Right subphrenic biloma to right lower bronchus | 1 yr | Bilioptysis | MRCP showed fistulous communication | Subphrenic drainage tube and PTBD with internalization | Alive and healthy |
| Current study (2023) | 7 | Female | Wilson disease | Strictured biliary anastomotic site to the right thoracic cavity | 3 mo | Bilioptysis | Bronchoscopy showed bilious content in the bronchi | Laparotomy, fistula tract excision, Roux-en-Y hepaticojejunostomy, and subphrenic drain | Alive and healthy |
| Post-hepatectomy | |||||||||
| Eryigit et al. (2007) [6] | 35 | Male | Liver hydatid cyst | Unknown | 2 mo | Bilioptysis | MRCP showed mild IHBRD; right thoracotomy showed fistula | Right thoracotomy, adhesiolysis, and fistula tract excision | Alive and healthy |
| 20 | Male | Gunshot injury to liver and diaphragm | Unknown | 15 day | Bilioptysis | None | Right inferior thoracotomy and lower lobectomy with postoperative chest and abdominal drains | Alive and healthy | |
| 38 | Male | Liver hydatid cyst | Unknown | 4 mo | Bilioptysis, jaundice | Percutaneous cholangiography | Right inferior thoracotomy and lower lobectomy with postoperative subcostal drain | Alive and healthy | |
| Kuo et al. (2014) [12] | 68 | Male | Hepatocellular carcinoma | Unknown | 2 yr | Reduced right-hand air entry | Flexible bronchoscopy | Video-assisted thoracoscopic surgery, adhesiolysis | Alive and healthy |
| Hai et al. (2016) [13] | 70 | Male | Recurrent hepatocellular carcinoma | Common bile duct to right lower lung bronchus | 2 wk | Bilioptysis | CT abdomen followed by fistulogram | Residual right bisectionectomy without resection of the fistulous tract and involved lung | Alive and healthy |
| Miranda García et al. (2018) [14] | 57 | Male | Sigmoid adenocarcinoma with metastatic liver disease | Subphrenic space to right lower lung | 8 mo | Bilioptysis, fever | CT chest and abdomen; MRCP | Percutaneous transhepatic biliary drainage | Alive and healthy |
| Lazarou et al. (2019) [15] | 64 | Male | Metastatic colon cancer | Intrahepatic collection to inferior lobular bronchus | 3 mo | Bilioptysis, fever, jaundice | CT chest and abdomen | ERCP with stenting and transhiatal fistula tract excision | Alive and healthy |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download