Abstract
Due to a critical organ shortage, pig organs are being explored for use in transplantation. Differences between species, particularly in cell surface glycans, can trigger elevated immune responses in xenotransplantation. To mitigate the risk of hyperacute rejection, genetically modified pigs have been developed that lack certain glycans and express human complement inhibitors. Nevertheless, organs from these pigs may still provoke stronger inflammatory and innate immune reactions than allotransplants. Dysregulation of coagulation and persistent inflammation remain obstacles in the transplantation of pig organs into primates. Regulatory macrophages (Mregs), known for their anti-inflammatory properties, could offer a potential solution. Mregs secrete interleukin 10 and transforming growth factor beta, thereby suppressing immune responses and promoting the development of regulatory T cells. These Mregs are typically induced via the stimulation of monocytes or macrophages with macrophage colony-stimulating factor and interferon gamma, and they conspicuously express the stable marker dehydrogenase/reductase 9. Consequently, understanding the precise mechanisms governing Mreg generation, stability, and immunomodulation could pave the way for the therapeutic use of Mregs generated in vitro. This approach has the potential to reduce the required dosages and durations of anti-inflammatory and immunosuppressive medications in preclinical and clinical settings.
Xenotransplantation refers to the transplantation of organs, tissues, cells, and the like across different species. The critical shortage of donor organs has led to the consideration of pig organs for this purpose. Pigs are deemed the most suitable candidates for xenotransplantation because they physiologically resemble humans, give birth to multiple offspring at once, and are easy to raise. However, substantial differences exist in molecular expression patterns between species, particularly in cell surface glycans, which are the carbohydrate components of glycoconjugates such as glycoproteins, glycolipids, or proteoglycans. These molecular discrepancies can elicit much stronger immune responses during xenotransplantation compared to allotransplantation. Galactose α1,3-galactose (α-Gal)- and N-glycolylneuraminic acid (Neu5Gc)-containing glycoconjugates exemplify glycans present in porcine vascular endothelial tissues but not in human tissues [1]. In early studies, transplanted organs such as hearts and kidneys were subjected to hyperacute rejection within minutes or hours. This was due to the presence of α-Gal, a sugar structure found on the surfaces of pig but not human cells. Natural antibodies in human blood that target this antigen bind to α-Gal on the endothelial cells of pig blood vessels. This binding activates the endothelial cells and triggers the complement system, leading to vascular damage and thrombus formation and constituting a hyperacute rejection response [2]. Consequently, the disparity in sugar structures between species is a primary factor inducing attacks by natural antibodies or innate immune cells. At present, research has pinpointed α-Gal and Neu5Gc as the key sugar structures on pig cell surfaces that bind to natural antibodies. Genetically engineered pigs have been created to eliminate these structures and to incorporate human molecules (CD46, CD55, or CD59) that inhibit complement activation. This genetic engineering has made it possible to overcome hyperacute rejection responses [3]. However, numerous sugar structures in these engineered pigs still differ from those in humans and can stimulate innate immune cells [4-6]. Additionally, pre-existing sugar structures may become more highly expressed, or new glycans may emerge, in genetically engineered pigs [4,6,7]. As a result, even with the use of current genetically engineered pigs, xenotransplantation may still face more intense inflammatory and innate immune responses than those observed in allotransplantation. The sugar structures can cause sustained systemic inflammation by continuously stimulating innate immune cells, including macrophages. In allotransplantation, inflammatory responses due to ischemia-reperfusion injury (IRI) during transplant preparation or surgical damage to the organ can be managed with anti-inflammatory agents, such as steroids. Due to the adverse effects of long-term steroid use, the drug dosage should be gradually decreased while monitoring the posttransplant prognosis. However, the sustained systemic inflammatory responses observed in xenotransplantation may preclude the gradual reduction of steroids [8]. Persistent systemic inflammation can further impair the function of the transplanted organ by interacting with the coagulation system. Therefore, it is uncertain whether immunosuppression protocols effective in allogeneic organ transplantation will also yield success in xenotransplantation.
Macrophages, as integral components of the innate immune system, play an important role in host defense. They achieve this by recognizing and responding to invading pathogens or their products during the initial stages of infection. This response includes activation, phagocytosis, induction of inflammatory responses, and antigen presentation to T cells. Although macrophages are crucial in the early inflammatory response to organ transplantation, they also contribute to the induction of transplantation tolerance [9]. Long-term transplant recipients who exhibit unresponsiveness to the transplanted organ often display graft-infiltrating macrophages with immunosuppressive properties [10]. A notable feature of macrophages is their plasticity, which enables them to easily switch between phenotypes in response to various physical and chemical stimuli from their environment [11]. Two well-established subpopulations of macrophages, each with distinct characteristics and functions, are the classically activated or inflammatory (M1) macrophages and the alternatively activated or anti-inflammatory (M2) macrophages. Recently, interest has been growing in regulatory macrophages (Mregs), a specialized subset that shares features with M2 cells and exhibits immunosuppressive capabilities. Human Mregs represent a unique state of macrophages capable of suppressing both inflammatory and T cell-mediated immune responses. The Hutchinson group in Germany has investigated the phenotype and immunological properties of Mregs derived from human peripheral blood mononuclear cells. Their findings highlight the clinical potential of these cells, demonstrating that pretransplant administration in allogeneic kidney transplantation can attenuate inflammatory and T cell-mediated immune responses, thereby reducing the need for prolonged and high-dose anti-inflammatory treatments [12]. Consequently, this therapeutic approach is anticipated to be even more effective in xenotransplantation, which involves substantially stronger immune responses. In this research, our objective was to explore this key obstacle currently facing xenotransplantation and to evaluate the utility of Mregs as a novel cell therapy to address the issue. We aimed to elucidate the principles and methods of the induction of human Mregs, define their typical phenotype, and examine their immunomodulatory functions. Furthermore, we discuss the mechanisms by which they exert their effects and the potential limitations on the practical application of this technology.
Recent evidence suggests that dysregulation of coagulation and persistent systemic inflammation are critical obstacles to successful pig-to-primate solid organ xenotransplantation. In the context of xenotransplantation, the primate coagulation system may be triggered by antibodies that target glycan antigens present on pig vascular endothelial cells, but not on primate cells [13-15]. Additionally, tissue factors (TFs) from both pig and primate sources contribute to the activation of coagulation [16,17], leading to the development of thrombotic microangiopathy [18,19]. Furthermore, incompatibilities between pig and primate molecules involved in the coagulation-anticoagulation systems exacerbate this dysregulation. Although pig thrombomodulin can bind to human thrombin, it does not effectively activate human protein C, an essential anticoagulant [20,21]. The pig TF pathway inhibitor is also insufficient in suppressing primate factor Xa, resulting in ineffective coagulation inhibition [22]. Pig von Willebrand factor spontaneously binds to primate platelet glycoprotein 1b and aggregates primate platelets, even without shear stress [23]. These activated platelets develop thrombosis after recruitment to damaged endothelial sites, leading to widespread activation of the coagulation system [24]. CD39 is an ectoenzyme present on the vascular endothelium that plays a vital role in preventing platelet aggregation triggered by extracellular adenosine triphosphate (ATP) and adenosine diphosphate. It accomplishes this by hydrolyzing these molecules into adenosine monophosphate and adenosine [25]. After transplantation, the expression of pig CD39 on vascular endothelial cells is reduced [26]. CD73, another enzyme involved in the metabolic process that converts ATP to adenosine, is expressed at much lower levels in pig endothelial cells compared to human cells, leading to decreased adenosine production and potentially causing intravascular coagulation [27]. Consequently, the introduction of human CD39 and CD73 genes into the pig genome has been suggested as a means to increase adenosine production. The expression of human CD39 in pigs has been demonstrated to significantly protect against myocardial damage in an ischemia-reperfusion model [28].
Inflammation is an unavoidable complication in solid organ transplantation, stemming from surgical trauma and IRI that occurs during the surgical procedure. Damage-associated molecular patterns (DAMPs) are released from injured tissues during IRI and are recognized by receptors on innate immune cells, which initiate inflammatory responses that can lead to graft rejection [29,30]. Therefore, early-phase suppression of inflammation in organ transplantation is critical for success. Despite this, recent research has shown that systemic inflammation can persist for several months following pig-to-primate solid organ xenotransplantation, even with the use of immunosuppressive therapy [8,31,32]. In recipients of heart and kidney transplants, serum levels of inflammatory cytokines and chemokines, such as tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin (IL)-12, and IL-18, are effectively managed by immunosuppressive therapy post-transplantation. However, serum levels of IL-6, monocyte chemoattractant protein 1 (MCP-1) [31], and C-reactive protein (CRP) [31-34] remain significantly elevated for an extended period. Notably, levels of these inflammatory mediators in nonhuman primate organ allograft recipients are significantly lower than those observed in organ xenograft recipients, suggesting that inflammatory responses are much more pronounced in xenograft recipients [31]. In the context of small artery patch xenotransplantation, recipient serum levels of inflammatory mediators also increased, apart from IL-6 and CRP, when no immunosuppressants were administered. When immunosuppressive medications were used, serum levels of inflammatory cytokines and chemokines were reduced, but levels of IL-6 and CRP remained high [31]. It has been hypothesized that IL-6, MCP-1, and CRP are involved in a positive feedback loop [35]. Given that IL-6 plays a pivotal role in chronic inflammatory diseases, autoimmune disorders, and cytokine storms [36-38], it likely occupies a central position in the sustained systemic inflammation seen in solid organ xenotransplantation. This inflammatory milieu contributes to the activation of monocytes, which then express TF [39], leading to the activation of coagulation [40,41]. The resulting amplification circuit between inflammation and coagulation promotes the release of additional inflammatory mediators and procoagulant factors [42], along with protease-activated receptor-mediated signaling and the further release of inflammatory cytokines. Therefore, preventing monocyte/macrophage-mediated inflammation therapeutically is critical for managing coagulation dysregulation following solid organ xenotransplantation.
Macrophages are phagocytic cells of the innate immune system that are essential for host defense, employing a variety of immunological and physiological mechanisms such as phagocytosis, inflammatory responses, antigen presentation, and wound healing. A notable feature of macrophages is their plasticity, which allows them to transition between subtypes [11]. In response to various stimuli, resting macrophages can undergo classical or alternative polarization to adopt distinct phenotypes. Two primary, functionally distinct macrophage subtypes are the classically activated macrophages (inflammatory macrophages, M1) and the alternatively activated macrophages (anti-inflammatory macrophages, M2) [43-46]. M1 macrophages arise in response to pathogen-associated molecular patterns or DAMPs at sites of infection or injury [47,48]. M2 macrophages, in turn, are polarized by macrophage colony-stimulating factor (M-CSF), IL-4, IL-10, and IL-13 [49]. Typically, M2 cells possess anti-inflammatory and regulatory properties, producing cytokines such as IL-4, IL-10, IL-33, and transforming growth factor beta (TGF-β) [50,51]. They demonstrate heightened phagocytic activity, express increased levels of scavenging, mannose, and galactose receptors, and produce greater concentrations of ornithine and polyamines due to an active arginase pathway. Additionally, they secrete substantial amounts of IL-10 and exhibit elevated levels of the IL-1 decoy receptor and IL-1Ra [52]. Overall, M2 macrophages exert anti-inflammatory effects and play a vital role in the antiparasite immune response necessary for parasite clearance, and they are involved in tissue remodeling, vasculogenesis, and tumor progression [11,44,53]. M2 cells can be identified by their distinct expression of surface proteins and secretion of various effector molecules [51]. M2 macrophages can be further categorized into subgroups M2a, M2b, M2c, and M2d [54-56]. M2a macrophages, also known as wound-healing macrophages, are stimulated by IL-4 and IL-13. They express high levels of the mannose receptor (also known as CD206), the decoy IL-1 receptor, and CCL17. They also secrete profibrotic factors such as TGF-β, insulin-like growth factor, and fibronectin, which aid in tissue repair [44,57-69]. M2b macrophages, or Mregs, are induced by toll-like receptor ligands [57,60-66] or IL-1R ligands [57,62,67-69] in conjunction with immune complexes or by various stimuli, including TGF, IL-10, or glucocorticoids [70]. They secret large quantities of IL-10 and small amounts of IL-12 [52,71], regulating both immune and inflammatory reactions. M2c macrophages are induced by IL-10 [45] or TGF-β [62] along with glucocorticoids. They are characterized by a strong anti-inflammatory profile, secreting large amounts of IL-10 and TGF-β, and express high levels of Mer receptor tyrosine kinase (MerTK), resulting in their efficient phagocytosis of apoptotic cells [62,72]. They are important in resolving inflammation, remodeling the extracellular matrix, and repairing tissue [73-75]. M2d macrophages, also referred to as tumor-associated macrophages, are induced by A2 adenosine receptor agonists or IL-6 [62,76-78]. These cells secrete high levels of IL-10, TGF-β, and vascular endothelial growth factor, but low levels of IL-12, TNF-α, and IL-1β [76,77,79-81], contributing to tumor angiogenesis, growth, and metastasis [62]. However, some overlap exists in the functional phenotypes of M2 macrophages. The subtypes of macrophages and their respective functions have been extensively covered in other studies [62,71,82].
Mregs are a type of macrophage known for their anti-inflammatory and immunosuppressive capabilities, sharing some of these properties with various M2 macrophage subsets [83-87]. The anti-inflammatory functions of Mregs are largely due to their capacity to inhibit M1 macrophages [84-86,88,89]. Mregs secrete high levels of the immunosuppressive cytokine IL-10, which curtails T cell proliferation [44,90], and promotes a T helper 2 response [12,91]. Conversely, Mregs produce minimal amounts of IL-12, a cytokine crucial for driving the differentiation of naïve T cells into T helper 1 cells, which are characterized by their robust production of IFN-γ. In human studies, Mregs have been shown to suppress mitogen-induced T cell proliferation in vitro [12] and to reduce T cell secretion of IL-2 and IFN-γ [91]. Additionally, they facilitate the induction of regulatory T cells (Tregs) through the release of TGF-β. Based on these distinctive immunosuppressive qualities, in vitro-generated Mregs have garnered interest as a potential cellular therapy for various inflammatory conditions, including sepsis [92], inflammatory bowel disease [93], autoimmune disorders [94,95] and organ transplantation [12,91].
Mregs can be differentiated from monocytes and macrophages within a particular microenvironment [96]. Two signals from this microenvironment are required for Mreg induction. The first signal is elicited by exposure to M-CSF, granulocyte M-CSF, prostaglandin E2, glucocorticoids, or apoptotic cells [81,89]. The second can be achieved through stimulation with IFN-γ [97-99], IL-10 [100], or immunosuppressive reagents such as glucocorticoids [101], vitamin D [102,103], or rapamycin [104]. The initial signal facilitates the transformation of monocytes into macrophages, while the subsequent signal imparts immunosuppressive characteristics to these cells [62] (Fig. 1). Although various methods of Mreg induction have been examined, including our own approach (in which human monocytes were cultured on a three-dimensional micropatterned polydimethylsiloxane surface coated with polydopamine and the common cell adhesion peptide motif arginylglycylaspartic acid [RGD]) [105], as well as activation with a combination of phorbol 12-myristate 13-acetate, RGD, and vitamin D3 [103], the most extensively studied method involves combined treatment with M-CSF and IFN-γ. Human Mregs originate from CD14+ peripheral blood monocytes, which are first stimulated with M-CSF for 6 days and then with IFN-γ [99]. Typically, Mregs exhibit a fibrous morphology and express high levels of tissue-resident macrophage markers, such as CD163, CD169, CD204, CD206, CD209, and MerTK [106]. While identifying stable, specific markers for Mregs induced by various stimuli remains challenging, dehydrogenase/reductase 9 has been identified as a stable marker for human Mregs [106].
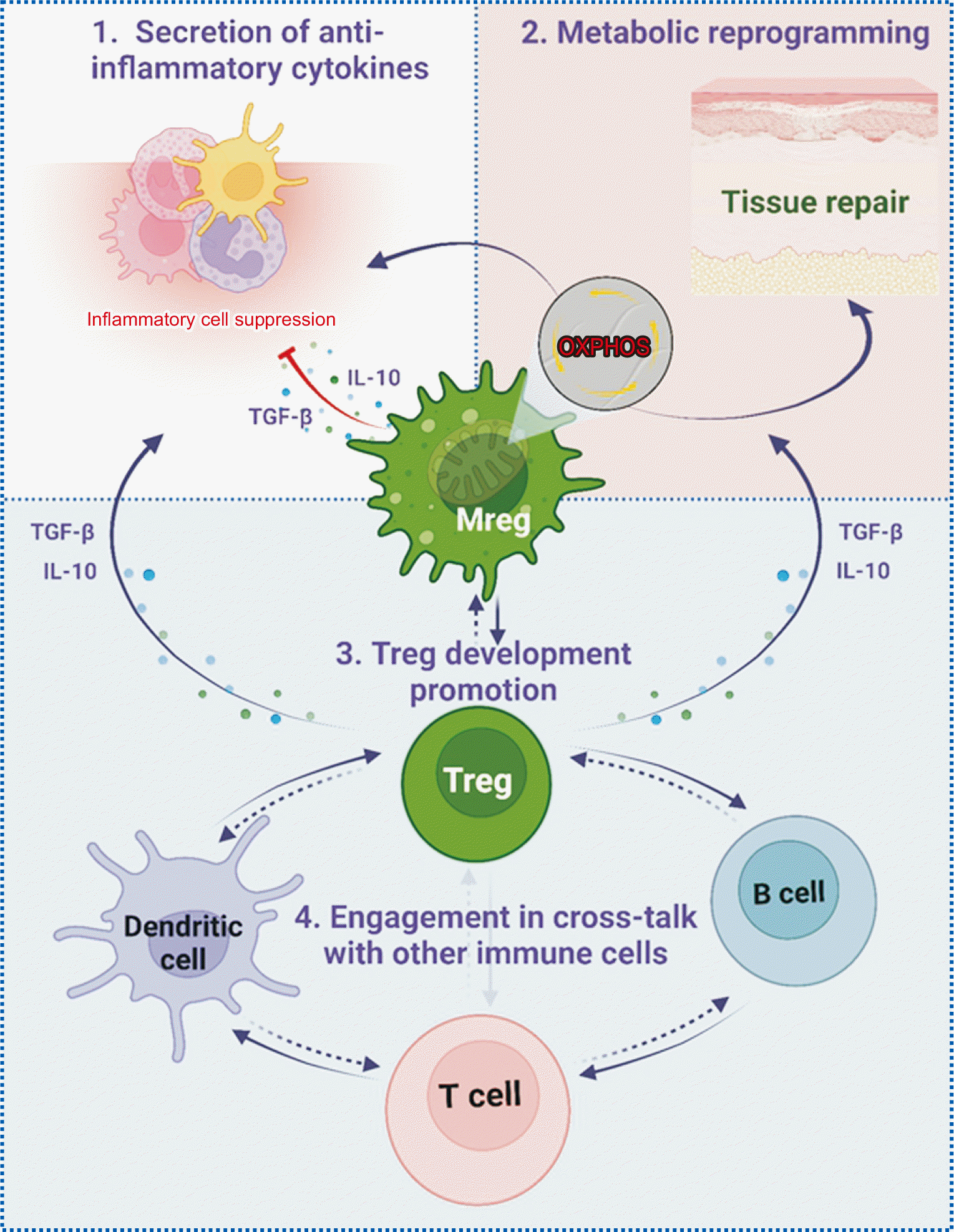
Although the mechanisms underlying the immunomodulatory functions of Mregs have not been fully elucidated, several have been identified: (1) Mregs secrete anti-inflammatory cytokines, such as IL-10 and TGF-β, which attenuate the proinflammatory response of other immune cells [91]; (2) Mregs can interact with and promote the development of Tregs, which possess potent suppressive capabilities to regulate immune responses and prevent immune-mediated tissue damage [52,91,103]; (3) Mregs undergo specific metabolic reprogramming, shifting their metabolism toward oxidative phosphorylation, a process associated with their anti-inflammatory and tissue-repair capabilities [107,108]; and (4) Mregs engage in cross-talk with other immune cells, such as dendritic cells, T cells, and B cells, thereby influencing their activation and function [52]. As such, Mregs contribute to a balanced and controlled immune response (Fig. 2).
Among these mechanisms, the interplay between Mregs and Tregs holds considerable importance in the modulation of the immune response at the site of graft transplantation. This modulation occurs through both direct and indirect interactions between these two cell types. Direct interactions involve cell-to-cell contact, in which T cells physically engage with Mregs, leading to the activation and proliferation of Tregs [12,91]. Indirectly, Mregs can influence Tregs by secreting anti-inflammatory cytokines, such as IL-10 and TGF-β. These cytokines are instrumental in enhancing the suppressive capabilities of Tregs, thereby promoting immune tolerance [91,109]. By interacting with one another and with other immune cells, Mregs and Tregs work in concert to preserve immune homeostasis.
One challenge in employing induced Mregs as a therapeutic approach for inflammatory diseases is their lack of stability. Numerous studies have shown that extended exposure to an inflammatory milieu can cause these cells to revert to a resting state or even transition to the proinflammatory M1 phenotype [110-114]. Furthermore, if the initial stimulants are not consistently provided, differentiated macrophages rapidly revert to their original phenotype [115]. Moreover, when a macrophage with one phenotype is exposed to a polarizing factor of the opposite type, it may tend to switch to a phenotype that aligns with the new activating environment [116]. Notably, the stability of these engineered cells is crucial to the success of any clinical therapy. Therefore, a deeper understanding of the mechanisms underlying macrophage plasticity is essential for developing therapeutic strategies that involve Mregs. The source of the precursor cells is another key factor in determining the fate of the activated cells. Selecting the appropriate cell source is vital for achieving a successful outcome in cell therapy. Research by various groups has indicated that macrophages with different origins exhibit unique responses to the same stimulant, leading to a range of phenotypes, each with specific functions [117]. Recent findings suggest that the propensity for phenotypic instability is largely due to the proliferation process of these macrophages [118,119]. The discovery of continuously maintained Mregs revealed that while newly formed cells lose their regulatory phenotype, the original parental macrophages retain their regulatory characteristics.
As a form of cellular therapy, the use of Tregs in organ allotransplantation offers considerable potential to improve transplant outcomes by promoting immune tolerance and reducing the likelihood of graft rejection. Nevertheless, xenotransplantation is hindered by the persistence of systemic inflammation, a critical contributor to immune rejection. Such inflammation may hinder the proliferation of Tregs and lead to increased cell death, thereby reducing the availability of these cells and undermining their capacity to suppress immune responses. Moreover, this inflammatory milieu may induce the conversion of Tregs into proinflammatory effector T cells, exacerbating the overall immune response and inflammation.
To address these challenges, it is imperative to explore alternative or complementary approaches. Mregs have emerged as a promising solution for mitigating systemic inflammation. Xenotransplantation presents certain advantages over allotransplantation, such as the potential for genetic modification and the opportunity for pre-conditioning prior to the transplant. Therefore, by gaining a thorough understanding of the precise mechanisms governing the generation, stability, and immunomodulatory functions of Mregs, the application of Mregs generated in vitro stands poised to reduce the dosage and duration of the anti-inflammatory and immunosuppressive drugs required in future preclinical and clinical settings.
ARTICLE INFORMATION
REFERENCES
1. Galili U. 2005; The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 83:674–86. DOI: 10.1111/j.1440-1711.2005.01366.x. PMID: 16266320.
2. Cooper DK, Ekser B, Tector AJ. 2015; Immunobiological barriers to xenotransplantation. Int J Surg. 23(Pt B):211–6. DOI: 10.1016/j.ijsu.2015.06.068. PMID: 26159291. PMCID: PMC4684773.
3. Denner J. 2014; Xenotransplantation-progress and problems: a review. J Transplant Technol Res. 4:1000133. DOI: 10.4172/2161-0991.1000133.
4. Burlak C, Bern M, Brito AE, Isailovic D, Wang ZY, Estrada JL, et al. 2013; N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation. 20:277–91. DOI: 10.1111/xen.12047. PMID: 24033743. PMCID: PMC4593510.
5. Nanno Y, Shajahan A, Sonon RN, Azadi P, Hering BJ, Burlak C. 2020; High-mannose type N-glycans with core fucosylation and complex-type N-glycans with terminal neuraminic acid residues are unique to porcine islets. PLoS One. 15:e0241249. DOI: 10.1371/journal.pone.0241249. PMID: 33170858. PMCID: PMC7654812.
6. Choe HM, Luo ZB, Xuan MF, Quan BH, Kang JD, Oh MJ, et al. Sialylation and fucosylation changes of cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) and glycoprotein, alpha1, 3-galactosyltransferase (GGTA1) knockout pig erythrocyte membranes. BioRxiv [Preprint]. 2020. Available from: https://doi.org/10.1101/2020.08.07.240846. cited 2023 Aug 23. DOI: 10.1101/2020.08.07.240846.
7. Yeh P, Ezzelarab M, Bovin N, Hara H, Long C, Tomiyama K, et al. 2010; Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 17:197–206. DOI: 10.1111/j.1399-3089.2010.00579.x. PMID: 20636540.
8. Ezzelarab MB, Cooper DK. 2015; Systemic inflammation in xenograft recipients (SIXR): a new paradigm in pig-to-primate xenotransplantation? Int J Surg. 23(Pt B):301–5. DOI: 10.1016/j.ijsu.2015.07.643. PMID: 26209584. PMCID: PMC4684785.
9. Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, et al. 2010; Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 120:2486–96. DOI: 10.1172/JCI41628. PMID: 20551515. PMCID: PMC2898596.
10. Ochando J, Conde P, Bronte V. 2015; Monocyte-derived suppressor cells in transplantation. Curr Transplant Rep. 2:176–83. DOI: 10.1007/s40472-015-0054-9. PMID: 26301174. PMCID: PMC4541698.
11. Biswas SK, Mantovani A. 2010; Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 11:889–96. DOI: 10.1038/ni.1937. PMID: 20856220.
12. Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. 2011; Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 187:2072–8. DOI: 10.4049/jimmunol.1100762. PMID: 21804023.
13. Ezzelarab M, Hara H, Busch J, Rood PP, Zhu X, Ibrahim Z, et al. 2006; Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation. 13:400–7. DOI: 10.1111/j.1399-3089.2006.00320.x. PMID: 16925663.
14. Baumann BC, Stussi G, Huggel K, Rieben R, Seebach JD. 2007; Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation. 83:193–201. DOI: 10.1097/01.tp.0000250478.00567.e5. PMID: 17264816.
15. Pierson RN 3rd, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, et al. 2009; Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 16:263–80. DOI: 10.1111/j.1399-3089.2009.00534.x. PMID: 19796067. PMCID: PMC2866107.
16. Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. 2008; Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 86:702–9. DOI: 10.1097/TP.0b013e31818410a3. PMID: 18791452. PMCID: PMC2637773.
17. Zelaya H, Rothmeier AS, Ruf W. 2018; Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 16:1941–52. DOI: 10.1111/jth.14246. PMID: 30030891.
18. Bühler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. 2000; Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 70:1323–31. DOI: 10.1097/00007890-200011150-00010. PMID: 11087147.
19. Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. 2005; Heart transplantation in baboons using alpha1,3-galactosyltransferase gene- knockout pigs as donors: initial experience. Nat Med. 11:29–31. DOI: 10.1038/nm1171. PMID: 15619628.
20. Siegel JB, Grey ST, Lesnikoski BA, Kopp CW, Soares M, Esch JS, et al. 1997; Xenogeneic endothelial cells activate human prothrombin. Transplantation. 64:888–96. DOI: 10.1097/00007890-199709270-00017. PMID: 9326416.
21. Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d'Apice AJ, Cowan PJ. 2008; Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 8:1101–12. DOI: 10.1111/j.1600-6143.2008.02210.x. PMID: 18444940.
22. Cowan PJ, d'Apice AJ. 2009; Complement activation and coagulation in xenotransplantation. Immunol Cell Biol. 87:203–8. DOI: 10.1038/icb.2008.107. PMID: 19153592.
23. Pareti FI, Mazzucato M, Bottini E, Mannucci PM. 1992; Interaction of porcine von Willebrand factor with the platelet glycoproteins Ib and IIb/IIIa complex. Br J Haematol. 82:81–6. DOI: 10.1111/j.1365-2141.1992.tb04597.x. PMID: 1419806.
24. Gawaz M. 2004; Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 61:498–511. DOI: 10.1016/j.cardiores.2003.11.036. PMID: 14962480.
25. Antonioli L, Pacher P, Vizi ES, Haskó G. 2013; CD39 and CD73 in immunity and inflammation. Trends Mol Med. 19:355–67. DOI: 10.1016/j.molmed.2013.03.005. PMID: 23601906. PMCID: PMC3674206.
26. Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. 2008; Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 172:1471–81. DOI: 10.2353/ajpath.2008.070672. PMID: 18467706. PMCID: PMC2408408.
27. Khalpey Z, Yuen AH, Kalsi KK, Kochan Z, Karbowska J, Slominska EM, et al. 2005; Loss of ecto-5'nucleotidase from porcine endothelial cells after exposure to human blood: Implications for xenotransplantation. Biochim Biophys Acta. 1741:191–8. DOI: 10.1016/j.bbadis.2005.03.008. PMID: 15955461.
28. Wheeler DG, Joseph ME, Mahamud SD, Aurand WL, Mohler PJ, Pompili VJ, et al. 2012; Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol. 52:958–61. DOI: 10.1016/j.yjmcc.2012.01.002. PMID: 22269791. PMCID: PMC3327755.
29. Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG. 2009; An innate response to allogeneic nonself mediated by monocytes. J Immunol. 183:7810–6. DOI: 10.4049/jimmunol.0902194. PMID: 19923456.
30. Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, et al. 2014; Non-self recognition by monocytes initiates allograft rejection. J Clin Invest. 124:3579–89. DOI: 10.1172/JCI74370. PMID: 24983319. PMCID: PMC4109551.
31. Ezzelarab MB, Ekser B, Azimzadeh A, Lin CC, Zhao Y, Rodriguez R, et al. 2015; Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 22:32–47. DOI: 10.1111/xen.12133. PMID: 25209710. PMCID: PMC4329078.
32. Iwase H, Ekser B, Zhou H, Liu H, Satyananda V, Humar R, et al. 2015; Further evidence for sustained systemic inflammation in xenograft recipients (SIXR). Xenotransplantation. 22:399–405. DOI: 10.1111/xen.12182. PMID: 26292982. PMCID: PMC4575631.
33. Li T, Lee W, Hara H, Long C, Ezzelarab M, Ayares D, et al. 2017; An investigation of extracellular histones in pig-to-baboon organ xenotransplantation. Transplantation. 101:2330–9. DOI: 10.1097/TP.0000000000001676. PMID: 28157735. PMCID: PMC5856196.
34. Iwase H, Liu H, Li T, Zhang Z, Gao B, Hara H, et al. 2017; Therapeutic regulation of systemic inflammation in xenograft recipients. Xenotransplantation. 24:e12296. DOI: 10.1111/xen.12296. PMID: 28294424. PMCID: PMC5397335.
35. Kim JY. 2019; Macrophages in xenotransplantation. Korean J Transplant. 33:74–82. DOI: 10.4285/jkstn.2019.33.4.74. PMID: 35769982. PMCID: PMC9188951.
36. Ishihara K, Hirano T. 2002; IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 13:357–68. DOI: 10.1016/S1359-6101(02)00027-8. PMID: 12220549.
37. Hunter CA, Jones SA. 2015; IL-6 as a keystone cytokine in health and disease. Nat Immunol. 16:448–57. DOI: 10.1038/ni.3153. PMID: 25898198.
38. Jones SA, Jenkins BJ. 2018; Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 18:773–89. DOI: 10.1038/s41577-018-0066-7. PMID: 30254251.
39. Lin CC, Ezzelarab M, Shapiro R, Ekser B, Long C, Hara H, et al. 2010; Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 10:1556–68. DOI: 10.1111/j.1600-6143.2010.03147.x. PMID: 20642682. PMCID: PMC2914318.
40. Chu AJ. 2011; Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011:367284. DOI: 10.4061/2011/367284. PMID: 21941675. PMCID: PMC3176495.
41. Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. 2008; C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 28:698–704. DOI: 10.1161/ATVBAHA.107.160903. PMID: 18276908.
42. Strukova S. 2006; Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 11:59–80. DOI: 10.2741/1780. PMID: 16146714.
43. Stein M, Keshav S, Harris N, Gordon S. 1992; Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 176:287–92. DOI: 10.1084/jem.176.1.287. PMID: 1613462. PMCID: PMC2119288.
44. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004; The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677–86. DOI: 10.1016/j.it.2004.09.015. PMID: 15530839.
45. Curi R, Mendes R, Crispin LA, Norata GD, Sampaio SC, Newsholme P. 2017; A past and present overview of macrophage metabolism and functional outcomes. Clin Sci (Lond). 131:1329–42. DOI: 10.1042/CS20170220. PMID: 28592702.
46. Ip WK, Hoshi N, Shouval DS, Snapper S, Medzhitov R. 2017; Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 356:513–9. DOI: 10.1126/science.aal3535. PMID: 28473584. PMCID: PMC6260791.
47. Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. 2002; Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci U S A. 99:1503–8. DOI: 10.1073/pnas.022649799. PMID: 11805289. PMCID: PMC122220.
48. Martinez FO, Gordon S, Locati M, Mantovani A. 2006; Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 177:7303–11. DOI: 10.4049/jimmunol.177.10.7303. PMID: 17082649.
49. Yao Y, Xu XH, Jin L. 2019; Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 10:792. DOI: 10.3389/fimmu.2019.00792. PMID: 31037072. PMCID: PMC6476302.
50. Giacomelli R, Ruscitti P, Alvaro S, Ciccia F, Liakouli V, Di Benedetto P, et al. 2016; IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol. 12:849–55. DOI: 10.1586/1744666X.2016.1168293. PMID: 26999417.
51. Di Benedetto P, Ruscitti P, Vadasz Z, Toubi E, Giacomelli R. 2019; Macrophages with regulatory functions, a possible new therapeutic perspective in autoimmune diseases. Autoimmun Rev. 18:102369. DOI: 10.1016/j.autrev.2019.102369. PMID: 31404701.
52. Zhang F, Zhang J, Cao P, Sun Z, Wang W. 2021; The characteristics of regulatory macrophages and their roles in transplantation. Int Immunopharmacol. 91:107322. DOI: 10.1016/j.intimp.2020.107322. PMID: 33418238.
53. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. 2013; Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 229:176–85. DOI: 10.1002/path.4133. PMID: 23096265.
54. Martinez FO, Gordon S. 2014; The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6:13. DOI: 10.12703/P6-13. PMID: 24669294. PMCID: PMC3944738.
55. Sudan B, Wacker MA, Wilson ME, Graff JW. 2015; A systematic approach to identify markers of distinctly activated human macrophages. Front Immunol. 6:253. DOI: 10.3389/fimmu.2015.00253. PMID: 26074920. PMCID: PMC4445387.
56. Hesketh M, Sahin KB, West ZE, Murray RZ. 2017; Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 18:1545. DOI: 10.3390/ijms18071545. PMID: 28714933. PMCID: PMC5536033.
57. Colin S, Chinetti-Gbaguidi G, Staels B. 2014; Macrophage phenotypes in atherosclerosis. Immunol Rev. 262:153–66. DOI: 10.1111/imr.12218. PMID: 25319333.
58. De Paoli F, Staels B, Chinetti-Gbaguidi G. 2014; Macrophage phenotypes and their modulation in atherosclerosis. Circ J. 78:1775–81. DOI: 10.1253/circj.CJ-14-0621. PMID: 24998279.
59. Wynn TA, Vannella KM. 2016; Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 44:450–62. DOI: 10.1016/j.immuni.2016.02.015. PMID: 26982353. PMCID: PMC4794754.
60. Gensel JC, Zhang B. 2015; Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619:1–11. DOI: 10.1016/j.brainres.2014.12.045. PMID: 25578260.
61. Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. 2012; Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 287:21816–25. DOI: 10.1074/jbc.M111.327031. PMID: 22549785. PMCID: PMC3381144.
62. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. 2018; Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 233:6425–40. DOI: 10.1002/jcp.26429. PMID: 29319160.
63. Ito I, Asai A, Suzuki S, Kobayashi M, Suzuki F. 2017; M2b macrophage polarization accompanied with reduction of long noncoding RNA GAS5. Biochem Biophys Res Commun. 493:170–5. DOI: 10.1016/j.bbrc.2017.09.053. PMID: 28917839.
64. Wilcock DM. 2012; A changing perspective on the role of neuroinflammation in Alzheimer's disease. Int J Alzheimers Dis. 2012:495243. DOI: 10.1155/2012/495243. PMID: 22844636. PMCID: PMC3403314.
65. Ohlsson SM, Linge CP, Gullstrand B, Lood C, Johansson A, Ohlsson S, et al. 2014; Serum from patients with systemic vasculitis induces alternatively activated macrophage M2c polarization. Clin Immunol. 152:10–9. DOI: 10.1016/j.clim.2014.02.016. PMID: 24631966.
66. Schulert GS, Fall N, Harley JB, Shen N, Lovell DJ, Thornton S, et al. 2016; Monocyte microRNA expression in active systemic juvenile idiopathic arthritis implicates microRNA-125a-5p in polarized monocyte phenotypes. Arthritis Rheumatol. 68:2300–13. DOI: 10.1002/art.39694. PMID: 27014994. PMCID: PMC5001902.
67. Orme J, Mohan C. 2012; Macrophage subpopulations in systemic lupus erythematosus. Discov Med. 13:151–8.
68. MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, et al. 2017; Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano. 11:2428–43. DOI: 10.1021/acsnano.6b06245. PMID: 28040885.
69. Fujiwara Y, Hizukuri Y, Yamashiro K, Makita N, Ohnishi K, Takeya M, et al. 2016; Guanylate-binding protein 5 is a marker of interferon-γ-induced classically activated macrophages. Clin Transl Immunology. 5:e111. DOI: 10.1038/cti.2016.59. PMID: 27990286. PMCID: PMC5133363.
70. Rőszer T. 2015; Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015:816460. DOI: 10.1155/2015/816460. PMID: 26089604. PMCID: PMC4452191.
71. Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. 2019; M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 106:345–58. DOI: 10.1002/JLB.3RU1018-378RR. PMID: 30576000. PMCID: PMC7379745.
72. Zizzo G, Hilliard BA, Monestier M, Cohen PL. 2012; Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 189:3508–20. DOI: 10.4049/jimmunol.1200662. PMID: 22942426. PMCID: PMC3465703.
73. Lee C, Jeong H, Lee H, Hong M, Park SY, Bae H. 2020; Magnolol attenuates cisplatin-induced muscle wasting by M2c macrophage activation. Front Immunol. 11:77. DOI: 10.3389/fimmu.2020.00077. PMID: 32117241. PMCID: PMC7018987.
74. Miki S, Suzuki JI, Takashima M, Ishida M, Kokubo H, Yoshizumi M. 2021; S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci Rep. 11:22469. DOI: 10.1038/s41598-021-01866-3. PMID: 34789834. PMCID: PMC8599840.
75. Tian L, Yu Q, Liu D, Chen Z, Zhang Y, Lu J, et al. 2022; Epithelial-mesenchymal transition of peritoneal mesothelial cells is enhanced by M2c macrophage polarization. Immunol Invest. 51:301–15. DOI: 10.1080/08820139.2020.1828911. PMID: 34490837.
76. Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, et al. 2007; Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 110:4319–30. DOI: 10.1182/blood-2007-02-072587. PMID: 17848619.
77. Wang Q, Ni H, Lan L, Wei X, Xiang R, Wang Y. 2010; Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 20:701–12. DOI: 10.1038/cr.2010.52. PMID: 20386569.
78. Novak ML, Koh TJ. 2013; Macrophage phenotypes during tissue repair. J Leukoc Biol. 93:875–81. DOI: 10.1189/jlb.1012512. PMID: 23505314. PMCID: PMC3656331.
79. Martinez FO, Sica A, Mantovani A, Locati M. 2008; Macrophage activation and polarization. Front Biosci. 13:453–61. DOI: 10.2741/2692. PMID: 17981560.
80. Ferrante CJ, Leibovich SJ. 2012; Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle). 1:10–6. DOI: 10.1089/wound.2011.0307. PMID: 24527272. PMCID: PMC3623587.
81. Atri C, Guerfali FZ, Laouini D. 2018; Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 19:1801. DOI: 10.3390/ijms19061801. PMID: 29921749. PMCID: PMC6032107.
82. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. 2019; The metabolic signature of macrophage responses. Front Immunol. 10:1462. DOI: 10.3389/fimmu.2019.01462. PMID: 31333642. PMCID: PMC6618143.
83. Mosser DM, Edwards JP. 2008; Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 8:958–69. DOI: 10.1038/nri2448. PMID: 19029990. PMCID: PMC2724991.
84. Sutterwala FS, Noel GJ, Salgame P, Mosser DM. 1998; Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 188:217–22. DOI: 10.1084/jem.188.1.217. PMID: 9653099. PMCID: PMC2525554.
85. Sutterwala FS, Noel GJ, Clynes R, Mosser DM. 1997; Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 185:1977–85. DOI: 10.1084/jem.185.11.1977. PMID: 9166427. PMCID: PMC2196339.
86. Lucas M, Zhang X, Prasanna V, Mosser DM. 2005; ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 175:469–77. DOI: 10.4049/jimmunol.175.1.469. PMID: 15972681.
87. Chandrasekaran P, Izadjoo S, Stimely J, Palaniyandi S, Zhu X, Tafuri W, et al. 2019; Regulatory macrophages inhibit alternative macrophage activation and attenuate pathology associated with fibrosis. J Immunol. 203:2130–40. DOI: 10.4049/jimmunol.1900270. PMID: 31541024.
88. Edwards JP, Zhang X, Frauwirth KA, Mosser DM. 2006; Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 80:1298–307. DOI: 10.1189/jlb.0406249. PMID: 16905575. PMCID: PMC2642590.
89. Fleming BD, Chandrasekaran P, Dillon LA, Dalby E, Suresh R, Sarkar A, et al. 2015; The generation of macrophages with anti-inflammatory activity in the absence of STAT6 signaling. J Leukoc Biol. 98:395–407. DOI: 10.1189/jlb.2A1114-560R. PMID: 26048978. PMCID: PMC4541501.
90. Gordon S. 2003; Alternative activation of macrophages. Nat Rev Immunol. 3:23–35. DOI: 10.1038/nri978. PMID: 12511873.
91. Riquelme P, Haarer J, Kammler A, Walter L, Tomiuk S, Ahrens N, et al. 2018; TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity. Nat Commun. 9:2858. DOI: 10.1038/s41467-018-05167-8. PMID: 30030423. PMCID: PMC6054648.
92. Yeung ST, Ovando LJ, Russo AJ, Rathinam VA, Khanna KM. 2023; CD169+ macrophage intrinsic IL-10 production regulates immune homeostasis during sepsis. Cell Rep. 42:112171. DOI: 10.1016/j.celrep.2023.112171. PMID: 36867536. PMCID: PMC10123955.
93. Vos AC, Wildenberg ME, Arijs I, Duijvestein M, Verhaar AP, de Hertogh G, et al. 2012; Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis. 18:401–8. DOI: 10.1002/ibd.21818. PMID: 21936028.
94. Ziegler T, Rausch S, Steinfelder S, Klotz C, Hepworth MR, Kühl AA, et al. 2015; A novel regulatory macrophage induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J Immunol. 194:1555–64. DOI: 10.4049/jimmunol.1401217. PMID: 25589067.
95. Nie H, Wang A, He Q, Yang Q, Liu L, Zhang G, et al. 2017; Phenotypic switch in lung interstitial macrophage polarization in an ovalbumin-induced mouse model of asthma. Exp Ther Med. 14:1284–92. DOI: 10.3892/etm.2017.4699. PMID: 28810589. PMCID: PMC5526127.
96. Zhang G, Hara H, Yamamoto T, Li Q, Jagdale A, Li Y, et al. 2018; Serum amyloid a as an indicator of impending xenograft failure: experimental studies. Int J Surg. 60:283–90. DOI: 10.1016/j.ijsu.2018.11.027. PMID: 30521954. PMCID: PMC6310230.
97. Hamilton TA, Zhao C, Pavicic PG Jr, Datta S. 2014; Myeloid colony-stimulating factors as regulators of macrophage polarization. Front Immunol. 5:554. DOI: 10.3389/fimmu.2014.00554. PMID: 25484881. PMCID: PMC4240161.
98. Munn DH, Armstrong E. 1993; Cytokine regulation of human monocyte differentiation in vitro: the tumor-cytotoxic phenotype induced by macrophage colony-stimulating factor is developmentally regulated by gamma-interferon. Cancer Res. 53:2603–13.
99. Hutchinson JA, Riquelme P, Geissler EK, Fändrich F. 2011; Human regulatory macrophages. Methods Mol Biol. 677:181–92. DOI: 10.1007/978-1-60761-869-0_13. PMID: 20941611.
100. Rückerl D, Hessmann M, Yoshimoto T, Ehlers S, Hölscher C. 2006; Alternatively activated macrophages express the IL-27 receptor alpha chain WSX-1. Immunobiology. 211:427–36. DOI: 10.1016/j.imbio.2006.05.008. PMID: 16920482.
101. Desgeorges T, Caratti G, Mounier R, Tuckermann J, Chazaud B. 2019; Glucocorticoids shape macrophage phenotype for tissue repair. Front Immunol. 10:1591. DOI: 10.3389/fimmu.2019.01591. PMID: 31354730. PMCID: PMC6632423.
102. Chen L, Eapen MS, Zosky GR. 2017; Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci Rep. 7:45172. DOI: 10.1038/srep45172. PMID: 28345644. PMCID: PMC5366921.
103. Pham HL, Hoang TX, Kim JY. 2023; Human regulatory macrophages derived from THP-1 cells using arginylglycylaspartic acid and vitamin D3. Biomedicines. 11:1740. DOI: 10.3390/biomedicines11061740. PMID: 37371835. PMCID: PMC10296385.
104. Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. 2013; The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 4:2834. DOI: 10.1038/ncomms3834. PMID: 24280772. PMCID: PMC3876736.
105. Pham HL, Yang DH, Chae WR, Jung JH, Hoang TX, Lee NY, et al. 2023; PDMS micropatterns coated with PDA and RGD induce a regulatory macrophage-like phenotype. Micromachines (Basel). 14:673. DOI: 10.3390/mi14030673. PMID: 36985080. PMCID: PMC10052727.
106. Riquelme P, Amodio G, Macedo C, Moreau A, Obermajer N, Brochhausen C, et al. 2017; DHRS9 is a stable marker of human regulatory macrophages. Transplantation. 101:2731–8. DOI: 10.1097/TP.0000000000001814. PMID: 28594751. PMCID: PMC6319563.
107. Du L, Lin L, Li Q, Liu K, Huang Y, Wang X, et al. 2019; IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab. 29:1363–75. DOI: 10.1016/j.cmet.2019.01.006. PMID: 30745181.
108. Suzuki H, Hisamatsu T, Chiba S, Mori K, Kitazume MT, Shimamura K, et al. 2016; Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol Lett. 176:18–27. DOI: 10.1016/j.imlet.2016.05.009. PMID: 27208804.
109. Schmidt A, Zhang XM, Joshi RN, Iqbal S, Wahlund C, Gabrielsson S, et al. 2016; Human macrophages induce CD4(+)Foxp3(+) regulatory T cells via binding and re-release of TGF-β. Immunol Cell Biol. 94:747–62. DOI: 10.1038/icb.2016.34. PMID: 27075967.
110. Hörhold F, Eisel D, Oswald M, Kolte A, Röll D, Osen W, et al. 2020; Reprogramming of macrophages employing gene regulatory and metabolic network models. PLoS Comput Biol. 16:e1007657. DOI: 10.1371/journal.pcbi.1007657. PMID: 32097424. PMCID: PMC7059956.
111. Raggi F, Pelassa S, Pierobon D, Penco F, Gattorno M, Novelli F, et al. 2017; Regulation of human macrophage M1-M2 polarization balance by hypoxia and the triggering receptor expressed on myeloid cells-1. Front Immunol. 8:1097. DOI: 10.3389/fimmu.2017.01097. PMID: 28936211. PMCID: PMC5594076.
112. Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, et al. 1999; Alternative versus classical activation of macrophages. Pathobiology. 67:222–6. DOI: 10.1159/000028096. PMID: 10725788.
113. Mantovani A, Sica A, Locati M. 2005; Macrophage polarization comes of age. Immunity. 23:344–6. DOI: 10.1016/j.immuni.2005.10.001. PMID: 16226499.
114. Lawrence T, Natoli G. 2011; Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 11:750–61. DOI: 10.1038/nri3088. PMID: 22025054.
115. Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, et al. 2009; Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 114:3244–54. DOI: 10.1182/blood-2009-04-217620. PMID: 19567879. PMCID: PMC2759649.
116. Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. 2005; Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 175:342–9. DOI: 10.4049/jimmunol.175.1.342. PMID: 15972667.
117. Davies LC, Jenkins SJ, Allen JE, Taylor PR. 2013; Tissue-resident macrophages. Nat Immunol. 14:986–95. DOI: 10.1038/ni.2705. PMID: 24048120. PMCID: PMC4045180.
118. Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. 2011; A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 41:2155–64. DOI: 10.1002/eji.201141817. PMID: 21710478.
119. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. 2011; Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 332:1284–8. DOI: 10.1126/science.1204351. PMID: 21566158. PMCID: PMC3128495.
Fig. 1
Schematic diagram illustrating the two signals essential for regulatory macrophage (Mreg) induction. The first signal, triggered by macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage CSF (GM-CSF), prostaglandin E2, and glucocorticoids, is necessary for inducing the differentiation of monocytes into macrophages. The second signal, induced by interferon gamma (IFN-γ), interleukin 10 (IL-10), or other immunosuppressive agents, is required to confer anti-inflammatory characteristics upon Mregs. MER-TK, Mer receptor tyrosine kinase.
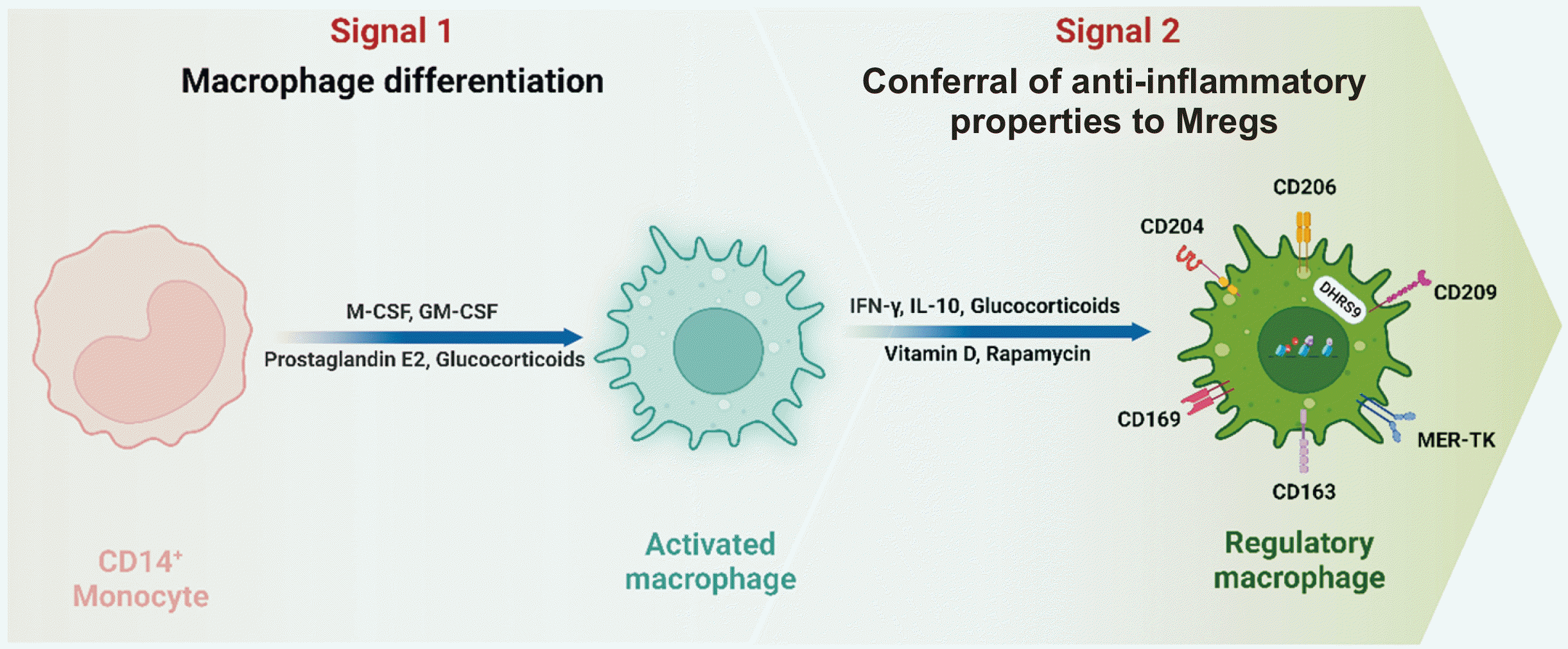
Fig. 2
Proposed immunomodulatory mechanisms of regulatory macrophages (Mregs). Mregs exert their immunomodulatory functions through four potential mechanisms: (1) the secretion of anti-inflammatory cytokines; (2) metabolic reprogramming toward oxidative phosphorylation; (3) the promotion of regulatory T cell development; and (4) engagement in cross-talk with other immune cells. Together, these processes contribute to the pivotal role of Mregs in immune regulation and the maintenance of immune balance. TGF-β, transforming growth factor beta; IL-10, interleukin 10; OXPHOS, oxidative phosphorylation; Treg, regulatory T cell.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download