Abstract
Olfaction is one of the five basic human senses, and it is known to be one of the most primitive senses. The sense of olfaction may have been critical for human survival in prehistoric society, and although many believe its importance has diminished over time, it continues to have an impact on human interaction, bonding, and propagation of the species. Even if we are unaware of it, the sense of smell greatly affects our lives and is closely related to overall quality of life and health. Nonetheless, olfaction has been neglected from a scientific perspective compared to other senses. However, olfaction has recently received substantial attention since the loss of smell and taste has been noted as a key symptom of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Studies investigating olfaction loss in association with coronavirus disease 2019 (COVID-19) have revealed that olfactory dysfunction can be both conductive and sensorineural, possibly causing structural changes in the brain. Olfactory training is an effective treatment for olfactory dysfunction, suggesting the reorganization of neural associations. A reduced ability to smell may also alert suspicion for neurodegenerative or psychiatric disorders. Here, we summarize the basic knowledge that we, as otorhinolaryngologists, should have about the sense of smell and the peripheral and central olfactory pathways for managing and helping patients with olfactory dysfunction.
Olfaction is one of the most primitive senses when considered in the context of evolution. Approximately 400 genes code for olfactory receptors in the human genome, accounting for 2.4% of the entire genome and constituting the largest gene family [1]. Until recently, it was a common belief that the human ability to smell is inferior to that of other mammals, primarily due to the small size of the olfactory bulbs compared to the entire brain. However, studies have suggested that although the relative olfactory bulb size and the number of olfactory receptor coding genes fall behind those of rodents, humans have an olfactory system with complex networks and pathways that allow us to perform as well as or even better than some other mammals [2]. A study that examined the resolution of human olfaction calculated that humans can discriminate at least 1 trillion olfactory stimuli [3]. Although we may not realize it, our behavior is strongly influenced by the sense of smell, and the decisions we make may often be driven by the nose telling us what to do [4]. Olfaction starts in the womb, as the developing fetus reacts to olfactory stimuli at around 30 weeks. The fetus becomes familiar with the components of the maternal diet, which are transferred via the amniotic fluid [5]. The development of olfaction is vital in bonding between infants and mothers [6]. Olfactory dysfunction can also lead to malnutrition, from a distorted sense of smell and taste resulting in diminished enjoyment of food [7]. The sense of smell can impact one’s survival, as some odors, such as gas leaks, spoiled food, or smoke, can provide warning signs. A study showed that olfactory dysfunction in the elderly is one of the strongest predictors of 5-year mortality, although this certainly reflects correlation and not causation [8]. Olfactory neurons have the ability to regenerate, and recent studies on olfactory training have shown that olfactory function can be improved through practice [9]. After Buck and Axel were awarded the Nobel Prize in Physiology or Medicine in 2004 for their work on odorant receptors and the olfactory system [10], olfaction has attracted considerable interest from researchers in various fields. Olfactory dysfunction in neurodegenerative diseases, including Parkinson disease (PD) and Alzheimer disease (AD) is one of the earliest signs of the impending cognitive impairment. Recently, during the coronavirus disease 2019 (COVID-19) pandemic, smell and taste loss have been well recognized as one of the key symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [11,12]. This has raised interest in olfaction and taste among both physicians and the general public. In this review, we outline the essentials that otorhinolaryngologists should know when they manage patients with olfactory dysfunction and help patients, including the basic anatomy and physiology of the olfactory system and related diseases.
Depending on the method of evaluation, studies report varying prevalence of olfactory dysfunction, from as low as 1.4% to as high as 62.4% [13]. In the US and Korean Health and Nutrition Examination Surveys, the prevalence of olfactory dysfunction has been estimated to be about 10% to 14% and 4.5%, respectively [14-16]. A systematic review and meta-analysis investigating the prevalence of olfactory dysfunction in the general population reported the overall prevalence to be about 22.2% [13]. While olfactory function can change suddenly from some etiologies, other etiologies can cause it to decline slowly over time. In these cases, one may not perceive the severity until there is no function (anosmia). Thus, the actual prevalence of olfactory dysfunction is expected to be higher than those estimated by self-reports in surveys. In the analysis by Desiato et al. [13], the prevalence was 28.8% when determined by objective testing, which was significantly higher than the rate of 9.5% determined by subjective means.
Olfactory dysfunction can be described as qualitative or quantitative in nature. Hyposmia (a diminished sense of smell) and anosmia (the complete inability to sense odors) belong to the quantitative category. Qualitative dysfunction, which is usually accompanied by quantitative dysfunction, consists of parosmia (a distorted sense of smell) and phantosmia (sensing an odor that is not present). Infectious diseases, such as upper respiratory viral infections, and inflammatory diseases such as chronic rhinosinusitis, especially with polyposis, can cause olfactory dysfunction [17]. In these cases, olfactory dysfunction can be both conductive and sensorineural [18]. Conductive-type olfactory dysfunction results from airflow blockage that prevents odorants from binding with the olfactory sensory neurons while damage or disorder of the olfactory sensory neurons or the olfactory pathways results in sensorineural-type olfactory loss. Rhinologic diseases, such as chronic rhinosinusitis with nasal polyps, can cause both conductive and sensorineural-type olfactory dysfunction. Patients with neurodegenerative diseases such as AD or PD can also present with gradual-onset olfactory loss [19]. Trauma, neoplasms, congenital anosmia, medications and toxins, metabolic and endocrine disorders, normal aging, and many other factors can also be etiologies of olfactory dysfunction. According to previous studies, post-infectious olfactory dysfunction (PIOD) is one of the most common causes of olfactory dysfunction [20]. As smell and taste loss have been identified as symptoms of SARS-CoV-2 infection, many are now realizing the importance of olfaction in health and quality of life [11].
Of the five basic human senses, smell and taste are chemical senses. Olfactory receptor neurons act as distant chemoreceptors that sense odorant molecules. Odorant molecules in the air come in contact with the olfactory mucosa in two ways. The first is through the orthonasal pathway, in which odorant molecules pass through the nasal vestibule into the nasal cavity via airflow. The second pathway involves odorant molecules being transported to the nasal cavity in a retrograde fashion through the nasopharynx from the oral cavity and oropharynx [21]. After the odorant molecules dissolve into the mucus lining the olfactory mucosa, the molecules bind to olfactory receptor neurons, resulting in depolarization and the relay of action potentials [22]. These action potentials are delivered to the olfactory bulb and are relayed to the olfactory cortex through the olfactory tract (Fig. 1) [23].
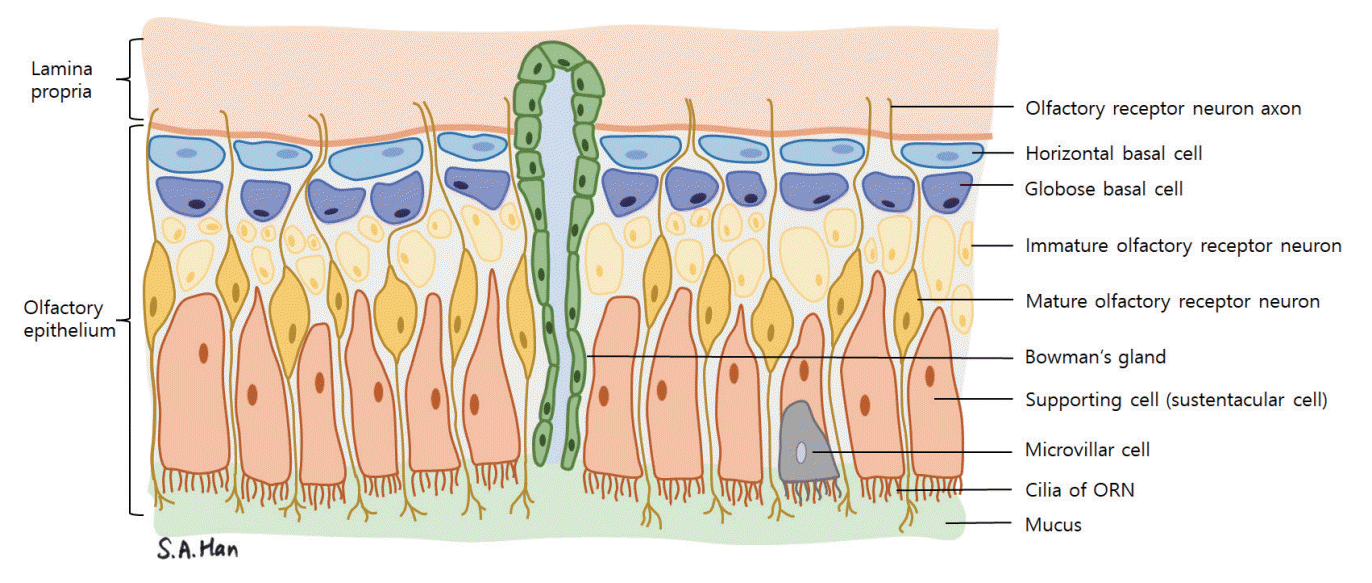
The structure of the olfactory mucosa can be differentiated from the respiratory mucosa that lines most of the nasal cavity [24]. It consists of the olfactory epithelium, basal lamina, and lamina propria. The olfactory epithelium is thicker than the respiratory mucosa and is made up of bipolar olfactory receptor neurons (also referred to as olfactory receptor cells or olfactory sensory neurons), basal cells, supporting cells (sustentacular cells) and other cells such as the microvillar cells (Fig. 2) [25]. Each olfactory receptor neuron expresses a gene coding for only one type of olfactory receptor and can only detect one type of odorant molecule [26]. Through axonal targeting, the axons of the olfactory neurons that express the same kind of receptors aggregate in the same area of the olfactory bulb in structures called the olfactory glomeruli [27]. The “one neuron, one receptor” rule and axonal targeting allow odor discrimination (Fig. 1). Signals from olfactory receptor neurons are delivered to the olfactory glomeruli in layer II, or the glomerulus layer of the olfactory bulb [28].
In olfactory receptor neurons, signals are relayed via the G-protein alpha subunit (Gαolf). Proteins such as Gαolf and adenylyl cyclase III (located downstream of the pathway), are mainly distributed in the cilia of olfactory receptor neurons [22]. Patients with mutations in genes related to the cilia of the olfactory receptor neuron, such as Bardet-Biedl syndrome, can exhibit anosmia [29,30]. Olfactory neurons have a unique ability to regenerate throughout life [31]. The basal cells of the olfactory epithelium can be categorized into horizontal basal cells (HBCs) and globose basal cells (GBCs). HBCs function as stem cells, while GBCs play a role in the regeneration of the olfactory receptor neurons [32,33]. In aging, the rate of GBC proliferation into olfactory receptor neurons slows, resulting in delayed replacement of damaged olfactory neurons. Therefore, olfactory dysfunction may follow if damaged olfactory neurons are not substituted promptly [34].
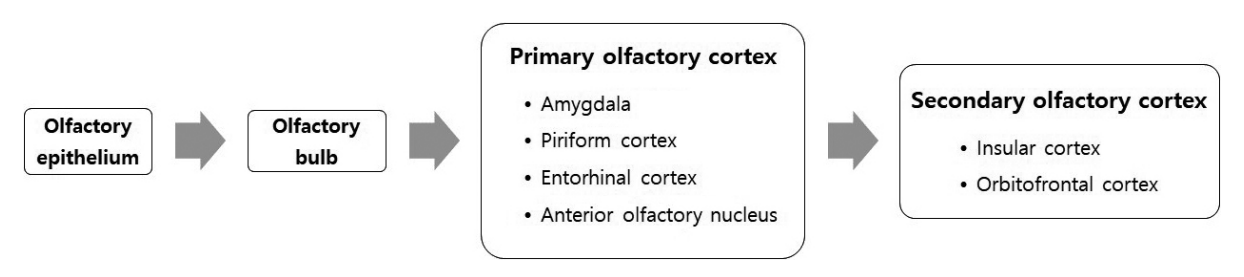
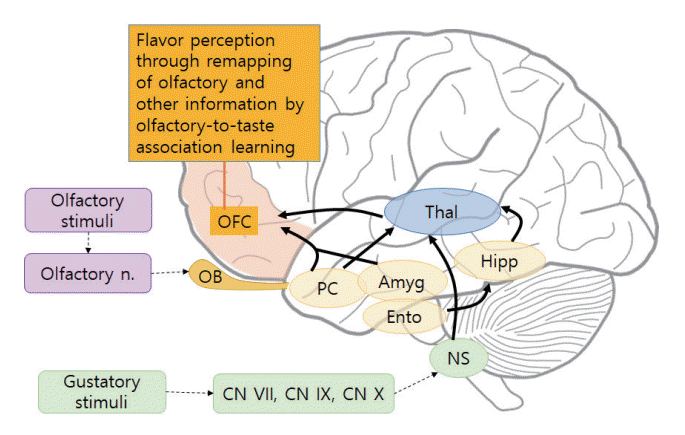
Olfactory receptor neurons form synapses with main olfactory bulb neurons, and mitral and tufted cells in the olfactory bulb project their axons to the primary olfactory cortex through the olfactory peduncle. From the olfactory peduncle, the axons then continue to the lateral olfactory tract, and thus the signals from the olfactory bulb are relayed to the primary olfactory cortex (anterior olfactory nucleus, piriform cortex, entorhinal cortex, and olfactory tubercle) (Fig. 3). The cortical networks that receive signals from the olfactory bulb commonly consist of multiple layers. Unlike the other senses, olfactory nerve fibers project to the primary olfactory cortex (amygdala, piriform cortex, anterior olfactory nucleus, and entorhinal cortex) without thalamic relay [35]. Thus, the olfactory system is a direct and rapidly responsive sense that quickly and subconsciously elicits emotions and/or memories. The orbitofrontal cortex, which consists of the secondary olfactory cortex, is responsible for combining taste and olfactory stimuli to form flavor, along with visual information [36].
The orbitofrontal cortex consists of the secondary olfactory and gustatory cortex, combining visual and tactile information to form flavor [37]. In this manner, olfaction, taste, vision, and touch combine to produce flavor. The response provoked in neurons by olfactory stimuli is also combined with taste, which allows training by associative learning. Odor perception occurs both through the binding of odorants to the olfactory receptor neurons and the remapping of olfactory information in the orbitofrontal cortex with other associated information to perceive it with appropriate meaning. For example, tasting wine involves a process of olfactory-to-taste association in which the aroma and the taste combine in the orbitofrontal cortex to form flavor (Fig. 4).
Gradual-onset olfactory dysfunction is a possible symptom of neurodegenerative diseases such as AD or PD [19]. Olfactory dysfunction is found in 90% of early-stage PD patients [38]. The olfactory bulb is one of the earliest affected regions in patients with PD. As PD progresses, pathology spreads from the olfactory bulb, anterior olfactory nucleus, and lower brainstem to other brain regions [39]. A hypothesis has suggested that environmental toxins that cannot move across the blood-brain barrier may induce inflammation in the olfactory mucosa and olfactory bulb, spreading to other brain regions [40]. The odor identification test in PD is considered one of the two-factor assessments for predicting the conversion risk of prodromal Parkinson’s patients [41].
In AD, neurofibrillary tangle formation and β-amyloid deposits have been observed in every layer of the olfactory bulb. The odor identification score of AD patients was significantly lower than that of normal controls, and this score worsened with the progression from minimal cognitive impairment to AD [42]. The odor identification test is also considered one of the 5-factor assessments to predict conversion from minimal cognitive impairment to AD within 3 years [41]. Thus, it has been suggested that declining olfactory function in the process of aging may be the result of early Alzheimer-type pathology in the olfactory system [43]. In addition, rapid olfactory decline during aging predicts dementia and gray matter volume loss in brain regions affected by AD [44]. Central nervous system demyelinating diseases, including multiple sclerosis, are also associated with olfactory loss [45], and patients with other neurological disorders, such as epilepsy, exhibit olfactory dysfunction [46]. Olfactory loss is noted as a common symptom of psychiatric conditions including schizophrenia, bipolar disorder, and major depressive disorder [47], and an increase in olfactory function is associated with a decline in depression severity [48]. Traumatic brain injury can also induce olfactory dysfunction and may indicate other neurologic complications [49]. In patients recovering from traumatic brain injury, neurogenesis and migration to the olfactory bulb from the subventricular region have been described previously [50]. As otorhinolaryngologists, we should consider the possibility of neurodegenerative or psychiatric diseases and collaborate with other specialists in this field when assessing patients with olfactory dysfunction.
The treatment options for olfactory dysfunction depend mainly on the etiology (e.g., trauma or sinonasal disease) and also involve correcting underlying metabolic or autoimmune conditions. The evidence for the use of local and systemic steroids, which are commonly prescribed for olfactory dysfunction, is limited. Intranasal sodium citrate may potentially improve olfactory outcomes in patients with PIOD [51]. Supplements such as zinc and α-lipoic acid have been studied, while in one randomized controlled trial, omega-3 fatty acids had a protective effect for patients undergoing endoscopic skull base surgery [52]. In a recent study, the use of platelet-rich plasma in the treatment has shown the potential to improve olfactory function in COVID-19–related smell loss [53]. Olfactory training is one of the few recommended treatment options for olfactory dysfunction, and combining other methods such as budesonide irrigation and omega-3 fatty acid supplementation may also be helpful [54,55].
The effect of olfactory training suggests the reversibility of olfactory dysfunction and plasticity of the olfactory system to some extent. After Hummel and colleagues reported the effects of olfactory training [56], many studies have investigated the possibility of olfactory training in improving olfactory function. Of note, the purity, concentration, and brand of odorants are not important, so patients can use anything with an odor to perform this training [57]. Olfactory training is one of the highly recommended treatment options in PIOD [20], and it has also been proven effective in a Korean cohort [58]. A systematic review and meta-analysis reported that olfactory training might be beneficial even for posttraumatic olfactory loss [59]. The mechanism of how olfactory training improves olfactory function has been investigated in several neuroimaging studies. Olfactory training increased the cortical thickness of several brain regions involved in olfactory processing, including the right inferior frontal gyrus, superior temporal gyrus, and entorhinal cortex [60]. Studies analyzing functional magnetic resonance imaging (MRI) in association with olfactory training have reported changes in connectivity within the visual cortex [61] and alterations in the connectivity networks [62], suggesting a reorganization of the neural associations. Studies have also demonstrated an increase in olfactory bulb volumes after olfactory training [63]. These studies suggest that improved olfaction consists of the reorganization of neural connectivity and possibly reflects structural changes in the olfactory system.
Several mechanisms for how SARS-CoV-2 infection induces olfactory dysfunction have been suggested. Radiological imaging analysis examining changes in olfactory structures has reported that COVID-19 patients with olfactory loss exhibited opacification of the olfactory cleft, suggesting a conductive type of olfactory dysfunction [64]. Others noted that SARS-CoV-2 infection was associated with damaged olfactory epithelium [65,66], as well as down-regulation of olfactory receptors and the signaling components [67]. Furthermore, structural changes were also reported, including olfactory bulb atrophy [68,69]. In a longitudinal study comparing MRI before and after SARS-CoV-2 infection, those with a history of infection showed (1) a greater reduction in gray matter thickness in the orbitofrontal cortex, (2) more remarkable changes in markers signifying tissue damage in regions functionally related to the primary olfactory cortex, and (3) more significant reduction in global brain size [70]. Furthermore, these subjects also showed a greater decline in cognitive abilities compared to the control group. In another longitudinal cohort study, severe COVID-19 (based on the American Thoracic Society guidelines for community-acquired pneumonia) was associated with an increase in risk of longitudinal cognitive decline [71]. A study examining postmortem samples of COVID-19 patients identified multifocal microvascular injury in brain and olfactory bulb through MRI and histopathological examinations, but with no sign of viral infection in these samples [72]. However, another study on deceased patient samples suggested that sustentacular cells were the main target cell type in SARS-CoV-2 infection, rather than the olfactory receptor neurons [73]. Olfactory dysfunction due to SARS-CoV-2 infection seems to involve either the central or the peripheral olfactory system, or both. Considering that olfactory receptor neurons regenerate every 30 to 90 days, the mechanism may be different in each case. In comparison, mechanisms such as inflammatory cell recruitment and secretion of cytokines causing the loss of olfactory receptor neurons have been suggested for PIOD [74]; however, no studies have examined in depth the effect of these viral infections on the central nervous system. A study comparing MRI findings in PIOD and COVID-19-related anosmia showed a higher rate of abnormal shapes and greater signal intensity of the olfactory bulbs in COVID-19 patients [75]. Patients with smell and taste disorder related to COVID-19 were younger and scored higher on olfactory function tests, and a lower percentage of them had anosmia or ageusia than patients with PIOD [76]. As for the prognosis, approximately one-third of PIOD patients recover after 1 year [77]. In a meta-analysis, 95.7% of COVID-19 patients recovered their sense of smell at 180 days, while it was estimated that 5.6% of patients may have persistent olfactory and taste dysfunction [78]. While COVID-19 is considered endemic in many countries as of 2023, the sequelae of long-haul COVID-19 remain to be investigated.
As olfactory dysfunction receives greater public attention as one of the major symptoms of SARS-CoV-2 infection, we have realized the importance of olfaction in our daily lives. Humans have an olfactory system with complex pathways that allow us to perform as well or even better than some other mammals. The “one neuron, one receptor” rule and axonal targeting allow us to discriminate tens of thousands of odors, if not more. Olfactory nerve fibers project to the primary olfactory cortex without thalamic relay, which elicits immediate emotional and memory recall.
The orbitofrontal cortex is responsible for combining taste and olfactory stimuli to form flavor by associative learning. In addition to being indicatory of disease in the nasal cavity, olfactory dysfunction may also suggest neurodegenerative or psychiatric disease. Olfactory training is now considered an important tool for improving smell by reorganizing olfactory processing and network connectivity. COVID-19 related olfactory dysfunction may involve not only the peripheral part of the olfactory system, but also the central olfactory regions. Otorhinolaryngologists must have a basic understanding of the olfactory system and the pathophysiological mechanism related to the peripheral and central olfactory pathways to help patients when diagnosing and managing olfactory dysfunction.
▪ Humans have complex olfactory pathways that allow us to perform as well as or even better than some other mammals.
▪ About one-fifth of the general population has olfactory dysfunction.
▪ Olfactory dysfunction is closely associated with neurodegenerative and psychiatric diseases.
▪ Sound localization performance improved after VR-based training in individuals with SSD and normal hearing.
▪ Olfactory training improves olfactory function, possibly through the reorganization of neural connectivity.
▪ Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may cause olfactory dysfunction by affecting peripheral and central parts of the olfactory system.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Allergy and Infectious disease (K08AI146220, 1R21AI168894-01) and Cystic Fibrosis Foundation K08 Boost Award (CHO20A0-KB) to DYC.
REFERENCES
1. Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci U S A. 2004; Feb. 101(8):2584–9.
2. McGann JP. Poor human olfaction is a 19th-century myth. Science. 2017; May. 356(6338):eaam7263.
3. Bushdid C, Magnasco MO, Vosshall LB, Keller A. Humans can discriminate more than 1 trillion olfactory stimuli. Science. 2014; Mar. 343(6177):1370–2.
4. Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008; Nov. 86(3):216–44.
5. Sarnat HB, Flores-Sarnat L, Wei XC. Olfactory development, part 1: function, from fetal perception to adult wine-tasting. J Child Neurol. 2017; May. 32(6):566–78.
6. Clark-Gambelunghe MB, Clark DA. Sensory development. Pediatr Clin North Am. 2015; Apr. 62(2):367–84.
7. Genter MB, Doty RL. Toxic exposures and the senses of taste and smell. Handb Clin Neurol. 2019; 164:389–408.
8. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014; 9(10):e107541.
9. Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2021; Feb. 164(2):244–54.
10. Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991; Apr. 65(1):175–87.
11. Desai M, Oppenheimer J. The importance of considering olfactory dysfunction during the COVID-19 pandemic and in clinical practice. J Allergy Clin Immunol Pract. 2021; Jan. 9(1):7–12.
12. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; Aug. 277(8):2251–61.
13. Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy. 2021; Mar. 35(2):195–205.
14. Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015; May. 125(5):1102–6.
15. Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016; Jun. 17(2):221–40.
16. Lee WH, Wee JH, Kim DK, Rhee CS, Lee CH, Ahn S, et al. Prevalence of subjective olfactory dysfunction and its risk factors: Korean national health and nutrition examination survey. PLoS One. 2013; 8(5):e62725.
17. Passali GC, Passali D, Cingi C, Ciprandi G. Smell impairment in patients with chronic rhinosinusitis: a real-life study. Eur Arch Otorhinolaryngol. 2022; Feb. 279(2):773–7.
18. Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000; Jul. 110(7):1071–7.
19. Doty RL, Hawkes CH. Chemosensory dysfunction in neurodegenerative diseases. Handb Clin Neurol. 2019; 164:325–60.
20. Hura N, Xie DX, Choby GW, Schlosser RJ, Orlov CP, Seal SM, et al. Treatment of post-viral olfactory dysfunction: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2020; Sep. 10(9):1065–86.
21. Burdach KJ, Doty RL. The effects of mouth movements, swallowing, and spitting on retronasal odor perception. Physiol Behav. 1987; 41(4):353–6.
22. Ronnett GV, Moon C. G proteins and olfactory signal transduction. Annu Rev Physiol. 2002; 64:189–222.
23. Cleland TA, Linster C. Central olfactory structures. Handb Clin Neurol. 2019; 164:79–96.
24. Smith TD, Bhatnagar KP. Anatomy of the olfactory system. Handb Clin Neurol. 2019; 164:17–28.
25. Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J Comp Neurol. 1990; Jul. 297(1):1–13.
26. Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994; Sep. 78(5):823–34.
27. Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998; Apr. 93(1):47–60.
28. Shepherd GM. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972; Oct. 52(4):864–917.
29. Feldmesser E, Bercovich D, Avidan N, Halbertal S, Haim L, Gross-Isseroff R, et al. Mutations in olfactory signal transduction genes are not a major cause of human congenital general anosmia. Chem Senses. 2007; Jan. 32(1):21–30.
30. Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004; Sep. 36(9):994–8.
31. Murrell W, Bushell GR, Livesey J, McGrath J, MacDonald KP, Bates PR, et al. Neurogenesis in adult human. Neuroreport. 1996; Apr. 7(6):1189–94.
32. Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995; Dec. 363(1):129–46.
33. Mackay-Sim A. Stem cells and their niche in the adult olfactory mucosa. Arch Ital Biol. 2010; Jun. 148(2):47–58.
34. London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008; Feb. 63(2):159–66.
35. Wilson DA, Xu W, Sadrian B, Courtiol E, Cohen Y, Barnes DC. Cortical odor processing in health and disease. Prog Brain Res. 2014; 208:275–305.
36. Rolls ET, Critchley HD, Mason R, Wakeman EA. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J Neurophysiol. 1996; May. 75(5):1970–81.
37. de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Tasteolfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003; Oct. 18(7):2059–68.
38. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012; May. 8(6):329–39.
39. Johnson ME, Bergkvist L, Mercado G, Stetzik L, Meyerdirk L, Wolfrum E, et al. Deficits in olfactory sensitivity in a mouse model of Parkinson’s disease revealed by plethysmography of odor-evoked sniffing. Sci Rep. 2020; Jun. 10(1):9242.
40. Niu H, Wang Q, Zhao W, Liu J, Wang D, Muhammad B, et al. IL-1β/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-Synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathol. 2020; Nov. 30(6):1102–18.
41. Dan X, Wechter N, Gray S, Mohanty JG, Croteau DL, Bohr VA. Olfactory dysfunction in aging and neurodegenerative diseases. Ageing Res Rev. 2021; Sep. 70:101416.
42. Quarmley M, Moberg PJ, Mechanic-Hamilton D, Kabadi S, Arnold SE, Wolk DA, et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J Alzheimers Dis. 2017; 55(4):1497–507.
43. Kovacs T, Cairns NJ, Lantos PL. Beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol. 1999; Dec. 25(6):481–91.
44. Pacyna RR, Han SD, Wroblewski KE, McClintock MK, Pinto JM. Rapid olfactory decline during aging predicts dementia and GMV loss in AD brain regions. Alzheimers Dement. 2023; Apr. 19(4):1479–90.
45. Joseph A, DeLuca GC. Back on the scent: the olfactory system in CNS demyelinating diseases. J Neurol Neurosurg Psychiatry. 2016; Oct. 87(10):1146–54.
46. Khurshid K, Crow AJ, Rupert PE, Minniti NL, Carswell MA, Mechanic-Hamilton DJ, et al. A quantitative meta-analysis of olfactory dysfunction in epilepsy. Neuropsychol Rev. 2019; Sep. 29(3):328–37.
47. Cothren TO, Evonko CJ, MacQueen DA. Olfactory dysfunction in schizophrenia: evaluating olfactory abilities across species. Curr Top Behav Neurosci. 2023; 63:363–92.
48. Sabiniewicz A, Hoffmann L, Haehner A, Hummel T. Symptoms of depression change with olfactory function. Sci Rep. 2022; Apr. 12(1):5656.
49. Schofield PW, Moore TM, Gardner A. Traumatic brain injury and olfaction: a systematic review. Front Neurol. 2014; 5:5.
50. Marin C, Langdon C, Alobid I, Mullol J. Olfactory dysfunction in traumatic brain injury: the role of neurogenesis. Curr Allergy Asthma Rep. 2020; Jul. 20(10):55.
51. Patel ZM, Holbrook EH, Turner JH, Adappa ND, Albers MW, Altundag A, et al. International consensus statement on allergy and rhinology: olfaction. Int Forum Allergy Rhinol. 2022; Apr. 12(4):327–680.
52. Yan CH, Rathor A, Krook K, Ma Y, Rotella MR, Dodd RL, et al. Effect of omega-3 supplementation in patients with smell dysfunction following endoscopic sellar and parasellar tumor resection: a multicenter prospective randomized controlled trial. Neurosurgery. 2020; Aug. 87(2):E91–8.
53. Yan CH, Jang SS, Lin HC, Ma Y, Khanwalkar AR, Thai A, et al. Use of platelet-rich plasma for COVID-19-related olfactory loss: a randomized controlled trial. Int Forum Allergy Rhinol. 2023; Jun. 13(6):989–97.
54. Hernandez AK, Woosch D, Haehner A, Hummel T. Omega-3 supplementation in postviral olfactory dysfunction: a pilot study. Rhinology. 2022; Apr. 60(2):139–44.
55. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018; Sep. 8(9):977–81.
56. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009; Mar. 119(3):496–9.
57. Patel ZM, Wise SK, DelGaudio JM. Randomized controlled trial demonstrating cost-effective method of olfactory training in clinical practice: essential oils at uncontrolled concentration. Laryngoscope Investig Otolaryngol. 2017; Apr. 2(2):53–6.
58. Choi BY, Jeong H, Noh H, Park JY, Cho JH, Kim JK. Effects of olfactory training in patients with postinfectious olfactory dysfunction. Clin Exp Otorhinolaryngol. 2021; Feb. 14(1):88–92.
59. Huang T, Wei Y, Wu D. Effects of olfactory training on posttraumatic olfactory dysfunction: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2021; Jul. 11(7):1102–12.
60. Al Ain S, Poupon D, Hetu S, Mercier N, Steffener J, Frasnelli J. Smell training improves olfactory function and alters brain structure. Neuroimage. 2019; Apr. 189:45–54.
61. Jiramongkolchai P, Jones MS, Peterson A, Lee JJ, Liebendorfer A, Klatt-Cromwell CN, et al. Association of olfactory training with neural connectivity in adults with postviral olfactory dysfunction. JAMA Otolaryngol Head Neck Surg. 2021; Jun. 147(6):502–9.
62. Kollndorfer K, Kowalczyk K, Hoche E, Mueller CA, Pollak M, Trattnig S, et al. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014; 2014:140419.
63. Negoias S, Pietsch K, Hummel T. Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging Behav. 2017; Aug. 11(4):998–1005.
64. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000; Mar. 100(6):693–702.
65. Reyna RA, Kishimoto-Urata M, Urata S, Makishima T, Paessler S, Maruyama J. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci Rep. 2022; Jan. 12(1):628.
66. Urata S, Maruyama J, Kishimoto-Urata M, Sattler RA, Cook R, Lin N, et al. Regeneration profiles of olfactory epithelium after SARSCoV-2 infection in golden Syrian hamsters. ACS Chem Neurosci. 2021; Feb. 12(4):589–95.
67. Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022; Mar. 185(6):1052–64.
68. Chiu A, Fischbein N, Wintermark M, Zaharchuk G, Yun PT, Zeineh M. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology. 2021; Jan. 63(1):147–8.
69. Liang YC, Tsai YS, Syue LS, Lee NY, Li CW. Olfactory bulb atrophy in a case of COVID-19 with hyposmia. Acad Radiol. 2020; Nov. 27(11):1649–50.
70. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022; Apr. 604(7907):697–707.
71. Liu YH, Chen Y, Wang QH, Wang LR, Jiang L, Yang Y, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022; May. 79(5):509–17.
72. Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2021; Feb. 384(5):481–3.
73. Khan M, Yoo SJ, Clijsters M, Backaert W, Vanstapel A, Speleman K, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021; Nov. 184(24):5932–49.
74. Addison AB, Wong B, Ahmed T, Macchi A, Konstantinidis I, Huart C, et al. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J Allergy Clin Immunol. 2021; May. 147(5):1704–19.
75. Yildirim D, Kandemirli SG, Tekcan Sanli DE, Akinci O, Altundag A. A comparative olfactory MRI, DTI and fMRI study of COVID-19 related anosmia and post viral olfactory dysfunction. Acad Radiol. 2022; Jan. 29(1):31–41.
76. Stankevice D, Fjaeldstad AW, Agergaard J, Ovesen T. Long-term COVID-19 smell and taste disorders differ significantly from other postinfectious cases. Laryngoscope. 2023; Jan. 133(1):169–74.
77. Damm M, Pikart LK, Reimann H, Burkert S, Goktas O, Haxel B, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014; Apr. 124(4):826–31.
78. Tan BK, Han R, Zhao JJ, Tan NK, Quah ES, Tan CJ, et al. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: meta-analysis with parametric cure modelling of recovery curves. BMJ. 2022; Jul. 378:e069503.
Fig. 1.
The path of olfactory perception from the olfactory epithelium to the olfactory bulb. An odorant molecule binds to an olfactory receptor neuron, generating an action potential that is relayed to the olfactory bulb and then to the olfactory cortex through the olfactory tract.
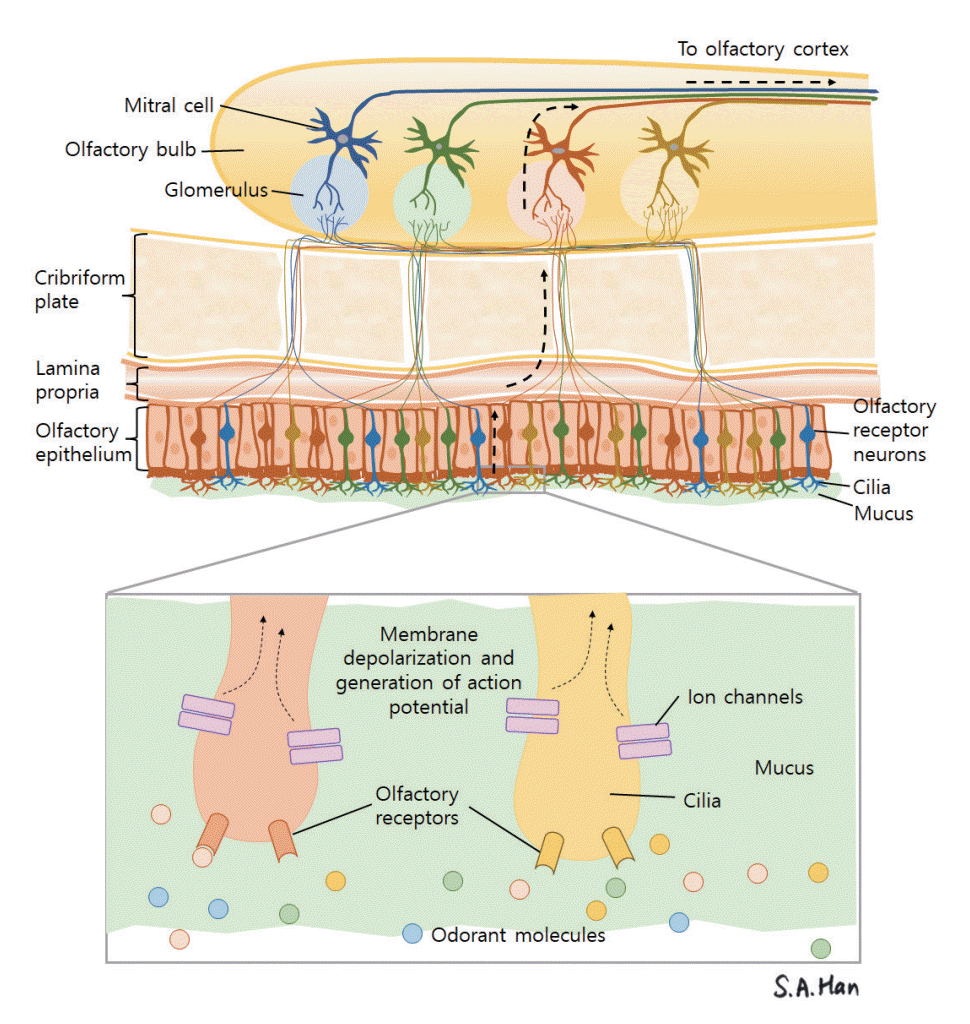
Fig. 2.
Schematic drawing of the olfactory mucosa. Olfactory receptor neurons (ORNs), globose and horizontal basal cells, supporting cells, and Bowman’s gland make up the olfactory epithelium.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download