TO THE EDITOR: Immunoglobulin-light-chain (AL) amyloidosis is a disease in which clonal plasma cells create excess monoclonal immunoglobulin light chains that aggregate into amyloid fibrils and accumulate in organs, ultimately leading to organ dysfunction. Current treatments for amyloidosis target clonal plasma cells to prevent the production of excess light chains and promote amyloid clearance through immunologic functions [1]. As such, anti-myeloma agents have been used to treat AL amyloidosis. The current standard treatment regimen comprises a combined regimen of daratumumab into bortezomib, cyclophosphamide, and dexamethasone [2]. Interestingly, daratumumab exerts immunomodulatory activity on immune cells, driving a depletion of regulatory T and B cells, as well as myeloid-derived suppressor cells, to facilitate amyloid clearance [3]. Likewise, lenalidomide, an immunomodulatory drug, is anticipated to have a therapeutic effect in amyloidosis. With this in mind, we planned a prospective single-arm study using lenalidomide and dexamethasone for relapsed/refractory AL amyloidosis patients with cardiac involvement.
Positron emission tomography (PET) has high sensitivity and specificity for amyloids, making it a useful tool to assess the myocardial amyloid burden [4]. In this study, we utilized 18F-florbetaben PET to quantify myocardial amyloid deposits and evaluate chemotherapy response in treated patients.
Due to competing studies, this study was terminated prematurely. However, we believe that it is nevertheless worthwhile to share the results obtained in the limited cases treated with lenalidomide and evaluated using amyloid PET.
Go to : 
This study was registered under the study protocol number NCT04298372. This trial investigated the efficacy of a combination therapy of oral lenalidomide (15–25 mg/day, days 1–21) and dexamethasone (40 mg/day, IV or PO, days 1–4) every 28 days. The starting dose of lenalidomide was 15 mg/day; when tolerated, the dose was increased to 20 mg/day for the next cycle, up to a maximum dose of 25 mg/day. If chemotherapy was well tolerated, treatment continued for up to 12 cycles. At the end of each cycle, hematological and organ responses were assessed. PET was performed before treatment and every 6 months after treatment initiation. The maximal uptake in the myocardium divided by the average liver uptake (myocardium-to-liver ratio, MLR) at a delayed time point (90 min after injection of 18F-florbetaben 300 MBq) was used to assess changes in amyloid deposits.
Go to : 
A 61-year-old Korean man presented with general weakness. Echocardiography revealed concentric left ventricular hypertrophy with mild pericardial effusion. Cardiac magnetic resonance imaging revealed diffuse fuzzy subendocardial enhancement of the global left ventricle. Endomyocardial biopsy was performed to confirm AL amyloidosis. Serum protein electrophoresis (PEP) and immunofixation electrophoresis (IFE) were normal; however, urine PEP/IFE revealed lambda-type Bence-Jones proteinuria with M-spike. The difference between the involved and uninvolved FLC (dFLC) was 109.7 mg/L. Bone marrow exam revealed hypercellular marrow (cellularity 51–60%) with Lambda-restricted plasma cells (4.4%), with no evidence of amyloid deposit in the bone marrow.
The patient was initially treated with oral melphalan (10 mg per square meter of body surface area, days 1–4) and high-dose dexamethasone (40 mg/day, days 1–4) (MelDex). After four cycles of MelDex, hematologic complete response (CR) and renal response were achieved (decrease in 24 h urinary protein from 1.8 g/day to 0.26 g/day). MelDex was discontinued at the request of the patient. After four years, he experienced relapse; urine PEP/IFE revealed lambda-type Bence-Jones proteinuria with M-spike. At this time, his dFLC level was 68.3 mg/L.
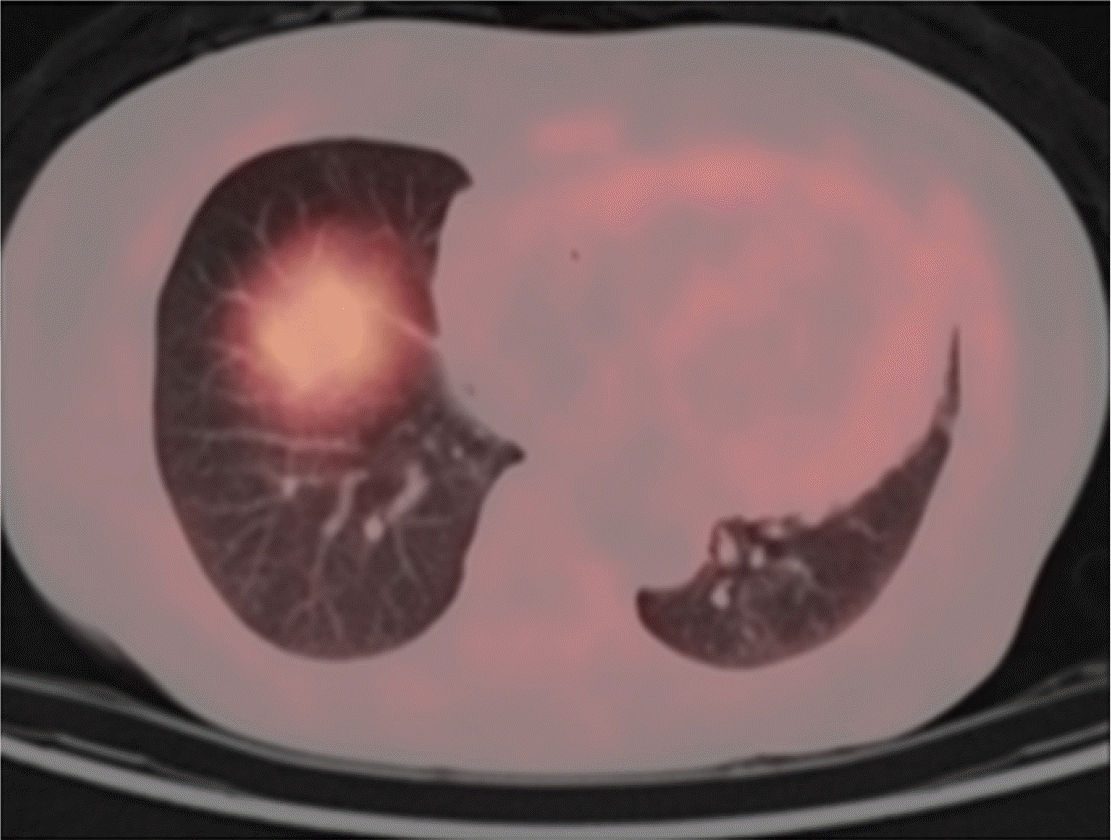
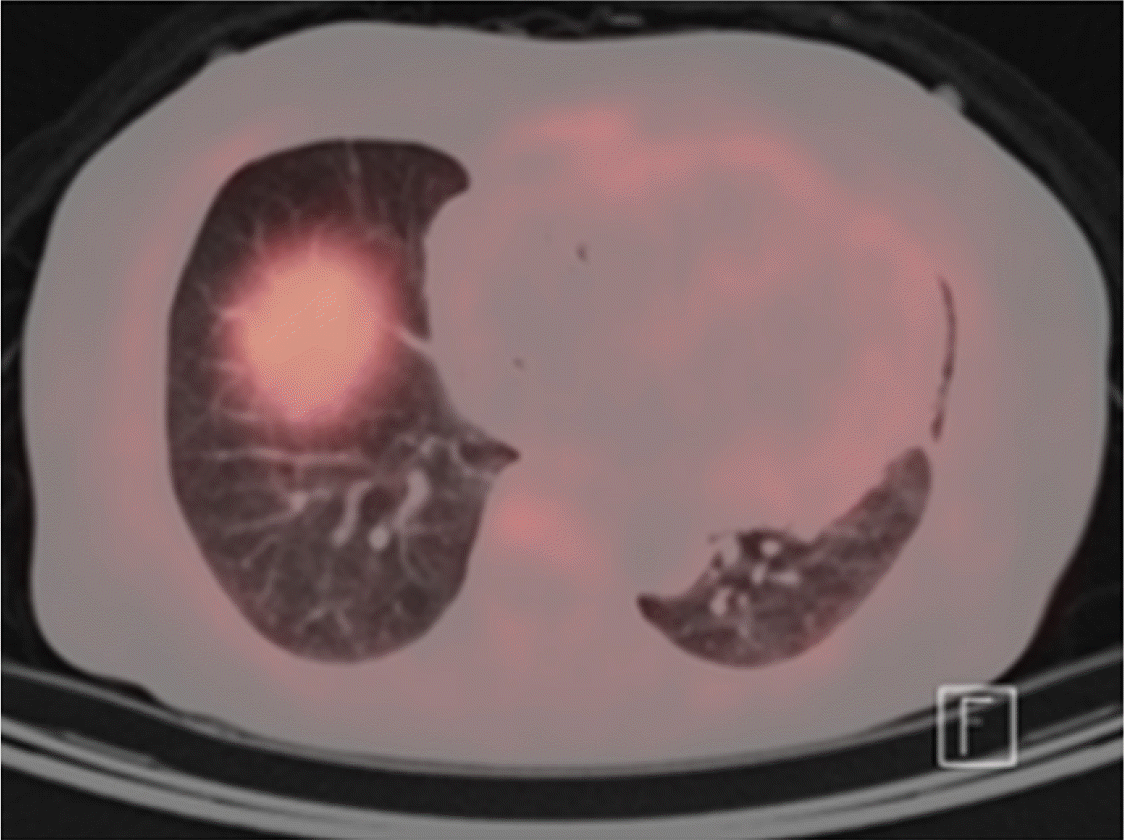
The patient was enrolled in the lenalidomide trial. After six cycles of lenalidomide plus dexamethasone, serum FLC levels normalized, the dFLC level reduced to 2 mg/L, and serum and urine immunofixation showed negative results. His 24 h urinary protein decreased more than 50% from 3.4 g/day to 0.29 g/day, while NT-proBNP levels decreased from 77,160 ng/L to 51,290 ng/L. PET showed a significant decrease in MLR from 2.28 to 1.51 (Table 1). After six cycles of chemotherapy, the patient was removed from the study because of lenalidomide-related adverse events, predominantly general weakness (NCI CTCAE Grade 3). After treatment, his organ function and fatigue improved, and at the time of the patient’s last follow-up (44 months since study initiation), the patient was still in hematological CR without organ dysfunction; the most recent NT-proBNP level was 33,100 ng/L, and echocardiography showed normal left ventricular (LV) systolic function (EF 58%) with no LV strain.
Go to : 
A 56-year-old Korean woman presented to our hospital with anorexia. She had experienced a sudden loss of consciousness after micturition and visited the emergency room. Echocardiography showed increased LV wall thickness with granular-sparkling appearance. Endomyocardial biopsy revealed amyloid deposits. Serum PEP/IFE revealed monoclonal gammopathy, lambda-type with M-spike. The dFLC level was 145.7 mg/L and the bone marrow exam showed normocellular marrow (cellularity 31–40%) with amyloid deposit (plasma cell 4.2%).
MelDex chemotherapy was initiated and after three cycles, hematologic partial response (PR) was achieved, with serum dFLC levels decreasing from 145.7 mg/L to 17.1 mg/L. Cardiac response was achieved as NT-proBNP levels decreased by more than 50% from 14,610 ng/L to 6,832 ng/L. The patient underwent high-dose melphalan conditioning followed by autologous peripheral blood stem cell transplantation.
After five years, the patient experienced relapse, with M-spikes reappearing in serum PEP. Consequently, she was enrolled in the lenalidomide trial. After six cycles of lenalidomide plus dexamethasone, serum dFLC decreased from 38.9 mg/L to 6.6 mg/L and serum immunofixation showed negative results. PET imaging showed low MLR at the initiation of treatment, which was maintained after six cycles (Table 2). After 10 cycles, hematologic CR was achieved and the treatment was terminated early at the patient’s discretion. At the time of the patient’s last follow-up (29 months since initiation of the study), the patient had CR. The NT-proBNP level did not increase at relapse (1,751 ng/L), and the most recent result remained low (1,150 ng/L).
Go to : 
Herein, we presented two cases of patients treated with lenalidomide-based chemotherapy achieving good hematologic responses. Although two patients were reviewed, it is notable that both were safely treated using this regimen, and the organ response was demonstrated using PET imaging.
The standard treatment for AL amyloidosis involves the use of antimyeloma agents that target clonal plasma cells. Cardiac dysfunction results not only from the interstitial infiltration of amyloid fibrils, but also from direct light chain toxicity [5]. Therefore, the goal of treatment in cardiac amyloidosis is a rapid reduction of amyloid light chain production and promotion of regression of pre-existing deposits to reverse organ dysfunction and improve survival [6].
The cardiac biomarker, NT-proBNP, is most frequently used to assess cardiac response [7]. However, there are limitations to the use of NT-proBNP, given that levels can fluctuate according to the patient’s hemodynamic status. Moreover, prior studies have reported the utility of amyloid PET, showing that the intensity of myocardial uptake reflected the degree of amyloid deposits in endomyocardial biopsy. Furthermore, the prognostic utility of PET has been studied in previous studies as well [8]. Accordingly, we used PET to quantify and visualize changes in amyloid deposits. In this study, PET achieved a significant decrease of MLR in the first case and a low MLR in the second case. Although cardiac response evaluated according to the consensus guideline [9] was not achieved in the second patient, both patients clinically improved after treatment with lenalidomide, with preserved cardiac function up until the most recent follow-up.
Fluid retention is a notorious adverse event of lenalidomide, which can hinder its use in patients [10]. However, in this study, we report that with adequate supportive care, this regimen can be applied safely in patients with cardiac amyloidosis. Lenalidomide has been previously tested for AL amyloidosis. In a single-center prospective trial of 50 patients treated with melphalan, lenalidomide, and dexamethasone (MLD) regimen as first-line treatment, hematological response rate was 68% (CR 18%) and organ response rate (including heart, kidney, liver, autonomic nervous system, and soft tissue) was 48% [11]. A multicenter prospective trial of 28 patients treated with cyclophosphamide, lenalidomide, and dexamethasone (CLD) regimen as first-line treatment showed a hematological response of 46% (CR 25%) and a cardiac response of 26% [12]. Both of these studies included high percentages of patients with Mayo stage III cardiac disease (Mayo 2004; 36% and 50%, respectively). Patients with advanced Mayo stage showed high early mortality in the MLD study, and were associated with a lower hematological response rate in the CLD study. Thus, patients with advanced Mayo stage were unlikely to benefit from and did not tolerate the regimen. Notably, both patients in our study, including second who had Mayo stage III disease, successfully received lenalidomide-based therapy over a long period. In fact, the lenalidomide dose in our regimen was 20 mg/day (increased up to 25 mg/day), which was higher than the dose in MLD and CLD studies. Patients were monitored for adverse events of lenalidomide, such as hematologic adverse events (cytopenia), rash, diarrhea, and edema. Both patients had CTCAE grade 1 peripheral edema which improved with diuretics, and the first patient had CTCAE grade 3 general weakness which improved after treatment cessation.
Our results were comparable to those of bortezomib-based treatments. Bortezomib-based treatments have consistently achieved a hematologic response rate of approximately 25% and cardiac response rate of 17% [13]. In one prior study, the combination of daratumumab and bortezomib achieved a hematologic CR rate of 53.3% and cardiac response rate of 41.5% [2].
Another consideration was the combination of lenalidomide and daratumumab. As daratumumab plus lenalidomide and dexamethasone (D-Rd) is a promising frontline regimen for elderly patients with myeloma [14], this study suggests the possibility of combining lenalidomide with daratumumab in AL amyloidosis patients.
Based on the results of this study and previous trials, lenalidomide is expected to be an important treatment option for relapsed/refractory AL amyloidosis patients, especially those with resistance to bortezomib. Further evaluation of the efficacy of lenalidomide in a larger cohort is warranted and the immunomodulatory effects of lenalidomide require further investigation.
Go to : 
Notes
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Go to : 
REFERENCES
1. Merlini G. 2017; AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology Am Soc Hematol Educ Program. 2017:1–12. DOI: 10.1182/asheducation-2017.1.1. PMID: 29222231. PMCID: PMC6142527.
2. Kastritis E, Palladini G, Minnema MC, et al. 2021; Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 385:46–58. DOI: 10.1056/NEJMoa2028631. PMID: 34192431.
3. Roccatello D, Fenoglio R, Sciascia S, et al. 2020; CD38 and anti-CD38 monoclonal antibodies in AL amyloidosis: targeting plasma cells and beyond. Int J Mol Sci. 21:4129. DOI: 10.3390/ijms21114129. PMID: 32531894. PMCID: PMC7312896. PMID: 41bf3818d5ba4b36b0615acd518994ea.
4. Lee SP, Suh HY, Park S, et al. 2020; Pittsburgh B compound positron emission tomography in patients with AL cardiac amyloidosis. J Am Coll Cardiol. 75:380–90. DOI: 10.1016/j.jacc.2019.11.037. PMID: 32000949.
5. Brenner DA, Jain M, Pimentel DR, et al. 2004; Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 94:1008–10. DOI: 10.1161/01.RES.0000126569.75419.74. PMID: 15044325.
6. Lachmann HJ, Gallimore R, Gillmore JD, et al. 2003; Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 122:78–84. DOI: 10.1046/j.1365-2141.2003.04433.x. PMID: 12823348.
7. Palladini G, Dispenzieri A, Gertz MA, et al. 2012; New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 30:4541–9. DOI: 10.1200/JCO.2011.37.7614. PMID: 23091105.
8. Choi YJ, Koh Y, Lee HJ, et al. 2022; Independent prognostic utility of 11C-pittsburgh compound B PET in patients with light-chain cardiac amyloidosis. J Nucl Med. 63:1064–9. DOI: 10.2967/jnumed.121.263033. PMID: 34916248. PMCID: PMC9258564.
9. Gertz MA, Comenzo R, Falk RH, et al. 2005; Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 79:319–28. DOI: 10.1002/ajh.20381. PMID: 16044444.
10. Dispenzieri A, Dingli D, Kumar SK, et al. 2010; Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 85:757–9. DOI: 10.1002/ajh.21822. PMID: 20872958. PMCID: PMC3691013.
11. Hegenbart U, Bochtler T, Benner A, et al. 2017; Lenalidomide/melphalan/dexamethasone in newly diagnosed patients with immunoglobulin light chain amyloidosis: results of a prospective phase 2 study with long-term follow up. Haematologica. 102:1424–31. DOI: 10.3324/haematol.2016.163246. PMID: 28522573. PMCID: PMC5541875.
12. Cibeira MT, Oriol A, Lahuerta JJ, et al. 2015; A phase II trial of lenalidomide, dexamethasone and cyclophosphamide for newly diagnosed patients with systemic immunoglobulin light chain amyloidosis. Br J Haematol. 170:804–13. DOI: 10.1111/bjh.13500. PMID: 25974382.
13. Palladini G, Sachchithanantham S, Milani P, et al. 2015; A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 126:612–5. DOI: 10.1182/blood-2015-01-620302. PMID: 25987656.
14. Dimopoulos MA, Oriol A, Nahi H, et al. 2016; Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 375:1319–31. DOI: 10.1056/NEJMoa1607751. PMID: 27705267.
Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download