Abstract
Background
Despite improved outcomes for pediatric patients with acute myeloid leukemia (AML), the prognosis for relapse remains poor. This study aimed to examine the clinical factors associated with prognosis in relapsed pediatric AML.
Methods
We conducted a chart review of pediatric patients with AML who experienced their first relapse and received treatment at our institution between 2008 and 2019. Risk stratification at diagnosis was performed according to the definition suggested by the ongoing AML 2012 study in Korea, and the clinical factors associated with prognosis were analyzed.
Results
A total of 27 pediatric patients with relapsed AML were identified. The 5-year overall survival (OS) and event-free survival (EFS) rates were 32.9% and 32.9%, respectively. A duration ≥12 months from diagnosis to relapse had a favorable impact on survival outcomes (5-yr OS, 64.0% vs. 15.7%; P=0.007). Patients who achieved complete remission (CR) after 1 course of chemotherapy following relapse (N=15) had a 5-year OS rate of 59.3%, while none of the other patients survived (P<0.0001). Additionally, the 5-year OS differed significantly based on the risk group at initial diagnosis (62.3% [favorable and intermediate prognosis groups, N=11] vs. 13.3% [poor prognosis group, N=15]; P=0.014).
Conclusion
Patients with a longer duration of CR before relapse, who achieved CR following 1 course of reinduction chemotherapy, and were in the favorable or intermediate prognosis group at diagnosis demonstrated better outcomes. These findings emphasize the importance of tailoring treatment strategies based on the expected prognosis at relapse in pediatric patients with AML.
The incidence of pediatric acute myeloid leukemia (AML) is estimated to be 6.6 to 8.4 per million children, making it the second most common leukemia [1, 2]. Compared to the early 1980s, when only 40% of children with AML survived, the current survival rate has increased to approximately 70% [3, 4]. However, even after treatment with chemotherapy and hematopoietic stem cell transplantation (HSCT), approximately 24% to 41% of pediatric patients with AML still experience relapse [5-10]. The probability of long-term survival for relapsed AML in pediatric patients is around 30% to 40% [11-13]. Poor outcomes in pediatric patients with relapsed AML have highlighted the need for extensive research. However, due to the relatively small size of this patient population, only a limited number of studies have focused on relapsed pediatric AML. We conducted a retrospective study to understand treatment outcomes and clinical factors associated with prognosis in relapsed pediatric AML.
We retrospectively reviewed the medical records of pediatric patients with a first relapse of AML who were treated at the Samsung Medical Center between 2008 and 2019. Patients with Down syndrome or acute promyelocytic leukemia were excluded from the study. To assess the prognosis after a first relapse of pediatric AML, we collected the following data from the patients’ medical records: sex, white blood cell (WBC) count at the time of initial AML diagnosis, age at the time of relapse, interval from initial diagnosis to first relapse, time point of achieving complete remission (CR) after relapse, prognosis group at initial diagnosis, site of relapse, and molecular abnormalities. The risk group at the time of initial diagnosis was classified as favorable, intermediate, or poor, with the prognosis based on the risk stratification of the AML 2012 protocol (Fig. 1), which is currently undergoing a prospective multicenter clinical trial in Korea. This study was reviewed and approved by the Institutional Review Board of the Samsung Medical Center (approval no. 2023-05-103).
An event was defined as relapse or death from any cause, whichever occurred first. Survival rates after relapse were estimated using the Kaplan-Meier method, and the significance of factors related to survival was analyzed using the log-rank test. A P-value of <0.05 was considered statistically significant. Multivariate analysis was conducted using the Cox regression analysis. All statistical analyses were performed using the SPSS software (IBM SPSS Statistics for Windows, Version 27.0, Armonk, NY, USA).
A total of 27 pediatric patients with relapsed AML were identified; their characteristics are presented in Table 1. There were 15 males and 12 females, the median WBC count at diagnosis was 20,400/μL (range, 1,650–265,800/μL), median age at relapse was 6.0 years (range, 1.0–18.0 yr), and the median time between diagnosis to relapse was 7 months (range, 2–33 mo). At initial diagnosis, a majority of patients (N=16) had AML, not otherwise specified (NOS), followed by AML with recurrent genetic abnormalities (N=6), AML with myelodysplasia-related changes (N=4), and myeloid sarcoma (N=1). Diagnoses were made according to the AML 2016 World Health Organization (WHO) classification. Based on the clinical risk factors at the time of initial diagnosis, the patients were stratified into 3 prognostic groups: 4 patients in the favorable group, 7 in the intermediate group, and 15 in the poor group. The one remaining patient could not be stratified due to a lack of information. Among the 27 patients, 21 experienced bone marrow relapse, 5 had isolated extramedullary relapse, and 1 had a combined relapse. Molecular abnormalities were identified in 7 patients: 4 with a FTL3/ITD mutation, 1 with a core-binding factor AML with a c-KIT mutation, and 2 with a CEBPA mutation. The remaining 20 patients had either no clinically relevant molecular abnormalities or did not undergo molecular testing.
Most patients (N=23, 85.2%) received the FLAG regimen (fludarabine, cytarabine, and granulocyte-macrophage stimulating factor) as reinduction therapy after the first relapse. Following relapse, 18 patients (66.7%) achieved a second CR, with 15 (55.6%) achieving it after 1 course of reinduction chemotherapy. HSCT was recommended for all patients, irrespective of their response to reinduction chemotherapy or history of prior transplantation. However, 5 patients did not undergo HSCT due to: death from infectious complications (N=3), parental refusal (N=1), and isolated central nervous system relapse with poor general condition (N=1).
The conditioning regimens for HSCT varied depending on the patients’ conditions, history of previous transplantation, and the graft source. Transplant recipients (N=11) received a myeloablative total body irradiation (TBI)-based regimen, 10 received combination busulfan and fludarabine, and one received low-dose TBI (3 Gy) in combination with busulfan and fludarabine.
The 5-year overall survival (OS) and event-free survival (EFS) rates were 32.9% and 32.9%, respectively (Fig. 2A). Two-thirds (N=18) of patients experienced an event; these were a second relapse before (N=1) or after (N=13) transplantation, death from infectious complications before transplantation (N=3), and a transplant-related mortality due to infection (N=1). Among the 5 patients who did not undergo HSCT, only 1 who had an isolated central nervous system relapse survived. As shown in Table 2, there were no statistically significant differences in survival rates according to sex (P=0.745), initial WBC count (P=0.989), or age at relapse (P=0.782). However, an interval time of >12 months from diagnosis to first relapse was identified as a favorable prognostic factor (P=0.007). The 5-year OS rate in this group was 64.0% (Fig. 2B). Among the 15 patients who achieved CR after 1 course of reinduction chemotherapy, the 2-year and 5-year OS rates were 80.0% and 59.3%, respectively. However, none of the patients who achieved CR after 2 or more courses or were refractory to chemotherapy survived (P<0.0001) (Fig. 2C). Among those patients who achieved CR after 1 course of reinduction chemotherapy, only one did not undergo HSCT but survived with chemotherapy after an isolated central nervous system relapse. Survival outcome was not affected by the relapse site (P=0.258). The 5-year OS rates for patients with FLT3/ITD mutations (N=4), c-KIT mutations in core-binding factor AML (N=1), CEBPA mutations (N=2), and the remaining 20 patients were 0%, 100%, 100%, and 30.9%, respectively. The 5-year OS rates for the favorable or intermediate prognosis groups (N=11) and poor prognosis group (N=15) were 62.3% and 13.3%, respectively (P=0.014) (Fig. 2D).
Among the prognostic factors with a P-value <0.1 by univariate analysis, CR after 1 course of reinduction chemotherapy was the only independent prognostic factor on multivariate analysis (Table 3).
Contemporary clinical trials involving relatively large numbers of pediatric patients with AML have shown OS and EFS rates between 60% to 75% and 50% to 65%, respectively [5-10]. Nevertheless, relapse remains the primary cause of initial treatment failure, occurring in 24% to 41% of patients. In our study, the 5-year OS rate was 32.9%, consistent with the results of previous reports.
Relapsed AML in pediatric patients poses several challenges to both patients and healthcare professionals. Limited treatment options are available, leading to poor outcomes for many patients. Chemotherapy resistance is a common issue among these patients and significantly limits the efficacy of standard treatments. Moreover, limited access to clinical trials can restrict experimental and personalized treatment options.
In a study by the Therapeutic Advances in Childhood Leukemia (TACL) Consortium on pediatric relapsed or refractory AML, the 5-year EFS and OS rates were <30% [11]. However, the disease-free survival rate was 43% in the 56% of patients who achieved CR after reinduction therapy, highlighting the importance of achieving CR through reinduction chemotherapy for these patients to survive. Consistent with this finding, our data demonstrated that only those patients who achieved CR after 1 course of reinduction therapy survived.
The optimal reinduction regimen for relapsed pediatric AML remains uncertain. A recent report by the AML- Berlin–Frankfurt–Munster (BFM) group indicated that 88% of children with relapsed AML received a fludarabine plus cytarabine-based combination, with or without an anthracycline and granulocyte-colony stimulating factor (G-CSF) [14]. They observed that 42% of late relapsers (defined as relapse >1 year from initial diagnosis) survived event-free, whereas only 14% of early relapsers (defined as relapse <1 year from initial diagnosis) survived. In our study, in which most patients received a fludarabine plus cytarabine-based reinduction chemotherapy, the survival difference between late and early relapsers was even more pronounced than in the AML-BFM study (14.0% vs. 68.6%, respectively).
Allogeneic HSCT is generally recommended for pediatric patients with relapsed AML. Additionally, reducing the disease burden as much as possible prior to HSCT is important for the treatment of malignant diseases. To achieve this, clinical trials exploring new combinations of therapy (e.g., clofarabine-based regimens) and various targeted agents are underway. Some studies have reported that clofarabine-based reinduction chemotherapy could lead to an approximately 50% OS rate in pediatric patients with relapsed or refractory AML, whereas non-responders exhibit much lower OS rates [15, 16]. However, these outcomes cannot be considered optimal, given the comparable ones in other studies, including ours.
Targeted therapies are gaining attention due to their improved response rates in pediatric AML. However, developing targeted therapies for pediatric AML faces challenges due to the relatively small population of pediatric patients with AML and the limited potential for financial return; these may discourage pharmaceutical companies from investing in development of such drugs. Despite these challenges, several targeted therapies for the treatment of pediatric AML—particularly for relapsed or refractory cases, are currently undergoing clinical trials. Gemtuzumab ozogamicin (GO), an antibody-drug conjugate against CD33, is the only targeted therapy currently approved for pediatric AML, while others are in early-phase clinical trials or preclinical studies [17]. A recent Children’s Oncology Group trial showed promising results when GO was added to chemotherapy, leading to improved EFS by reducing relapse rates in newly-diagnosed pediatric AML [8]. In a retrospective BFM study of pediatric relapsed or refractory AML, GO, with or without cytarabine, successfully bridged 49 of 76 patients to HSCT [18]. Similarly, in a French retrospective study that used a single dose of GO added to a fludarabine plus cytarabine plus anthracycline regimen in 26 patients with first relapse and 3 with refractory disease, 24 (83%) achieved CR, with an OS rate of 49% [19]. Notably, the OS rates for low-, intermediate-, and high-risk patients in that study were 56%, 51%, and 0%, respectively. These findings further highlight that the initial risk group is a highly significant prognostic factor in pediatric relapsed and refractory AML, similar to the findings in our study. Given the small number of patients in the favorable prognosis group (N=4) of our study, we compared the OS rates between the favorable and intermediate prognosis groups and the poor prognosis group. The higher OS rates in the favorable and intermediate prognosis groups and extremely poor outcomes in the poor prognosis group were consistent with the findings of other studies. They clearly underscore the predictive value of initial prognostic factors in determining clinical outcomes after relapse.
The small number of patients is a major limitation of our study. However, our study demonstrated that only patients who achieved CR after 1 course of reinduction therapy survived, and this was the only independent factor affecting survival outcomes. In line with our findings, several groups have reported that early achievement of CR is a strong prognostic factor [11, 20]. Given that rapid responders to therapy may have a higher chance of being salvaged, inducing early CR is a major primary endpoint for future clinical trials.
In conclusion, we showed that patients in the favorable or intermediate prognosis group at diagnosis, those with a longer duration of remission before relapse, and those who achieved CR after 1 course of reinduction chemotherapy demonstrated better outcomes. These findings support the need to tailor treatment strategies based on the expected prognosis at relapse in pediatric patients with relapsed AML. Future clinical trials using targeted therapies should be highly encouraged, especially in patients who are likely to be resistant to standard reinduction therapies.
REFERENCES
1. Park HJ, Moon EK, Yoon JY, et al. 2016; Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 48:869–82. DOI: 10.4143/crt.2015.290. PMID: 26790965. PMCID: PMC4946351.
2. Puumala SE, Ross JA, Aplenc R, Spector LG. 2013; Epidemiology of childhood acute myeloid leukemia. Pediatr Blood Cancer. 60:728–33. DOI: 10.1002/pbc.24464. PMID: 23303597. PMCID: PMC3664189.
3. Abrahamsson J, Forestier E, Heldrup J, et al. 2011; Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 29:310–5. DOI: 10.1200/JCO.2010.30.6829. PMID: 21149663.
4. Rubnitz JE, Inaba H, Dahl G, et al. 2010; Minimal residual disease- directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 11:543–52. DOI: 10.1016/S1470-2045(10)70090-5. PMID: 20451454.
5. Tsukimoto I, Tawa A, Horibe K, et al. 2009; Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 27:4007–13. DOI: 10.1200/JCO.2008.18.7948. PMID: 19620491.
6. Creutzig U, Zimmermann M, Bourquin JP, et al. 2013; Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 122:37–43. DOI: 10.1182/blood-2013-02-484097. PMID: 23704089.
7. Rubnitz JE, Lacayo NJ, Inaba H, et al. 2019; Clofarabine can replace anthracyclines and etoposide in remission induction therapy for childhood acute myeloid leukemia: the AML08 multicenter, randomized phase III trial. J Clin Oncol. 37:2072–81. DOI: 10.1200/JCO.19.00327. PMID: 31246522. PMCID: PMC7001777.
8. Gamis AS, Alonzo TA, Meshinchi S, et al. 2014; Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 32:3021–32. DOI: 10.1200/JCO.2014.55.3628. PMID: 25092781. PMCID: PMC4162498.
9. Pession A, Masetti R, Rizzari C, et al. 2013; Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 122:170–8. DOI: 10.1182/blood-2013-03-491621. PMID: 23673857.
10. Burnett AK, Hills RK, Milligan DW, et al. 2010; Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 28:586–95. DOI: 10.1200/JCO.2009.22.9088. PMID: 20038732.
11. Gorman MF, Ji L, Ko RH, et al. 2010; Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 55:421–9. DOI: 10.1002/pbc.22612. PMID: 20658611.
12. Creutzig U, Zimmermann M, Dworzak MN, et al. 2014; The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study relapsed AML 2001/01. Haematologica. 99:1472–8. DOI: 10.3324/haematol.2014.104182. PMID: 24763401. PMCID: PMC4562536.
13. Moritake H, Tanaka S, Miyamura T, et al. 2021; The outcomes of relapsed acute myeloid leukemia in children: results from the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05R study. Pediatr Blood Cancer. 68:e28736. DOI: 10.1002/pbc.28736. PMID: 32991072.
14. Rasche M, Steidel E, Zimmermann M, et al. 2021; Second relapse of pediatric patients with acute myeloid leukemia: a report on current treatment strategies and outcome of the AML-BFM Study Group. Cancers (Basel). 13:789. DOI: 10.3390/cancers13040789. PMID: 33672815. PMCID: PMC7918758. PMID: 1baebca711d84d67a51adec0cb9cf17a.
15. van Eijkelenburg NKA, Rasche M, Ghazaly E, et al. 2018; Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study. Haematologica. 103:1484–92. DOI: 10.3324/haematol.2017.187153. PMID: 29773602. PMCID: PMC6119144.
16. Ramaswamy K, Steinherz PG, Agrawal AK, et al. 2022; Clofarabine with topotecan, vinorelbine, and thiotepa reinduction regimen for children and young adults with relapsed AML. Blood Adv. 6:2688–94. DOI: 10.1182/bloodadvances.2021005753. PMID: 35008101. PMCID: PMC9043926.
17. Brivio E, Baruchel A, Beishuizen A, et al. 2022; Targeted inhibitors and antibody immunotherapies: novel therapies for paediatric leukaemia and lymphoma. Eur J Cancer. 164:1–17. DOI: 10.1016/j.ejca.2021.12.029. PMID: 35121370.
18. Niktoreh N, Lerius B, Zimmermann M, et al. 2019; Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. 104:120–7. DOI: 10.3324/haematol.2018.191841. PMID: 30093401. PMCID: PMC6312035.
19. Dhunputh C, Strullu M, Petit A, et al. 2022; Single-dose (4.5 mg/m2) gemtuzumab ozogamicin in combination with fludarabine, cytarabine and anthracycline as reinduction therapy in relapsed or refractory paediatric acute myeloid leukaemia. Br J Haematol. 198:373–81. DOI: 10.1111/bjh.18203. PMID: 35438187.
20. Kaspers GJ, Zimmermann M, Reinhardt D, et al. 2013; Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 31:599–607. DOI: 10.1200/JCO.2012.43.7384. PMID: 23319696.
Fig. 1
Determination of prognosis groups in AML 2012 trial. Both risk features and treatment response were taken into account in determining the prognostic group.
Abbreviations: AMKL, acute megakaryocytic leukemia; CBF, core-binding factor; CR, complete remission; NR, no response; PR, partial response.
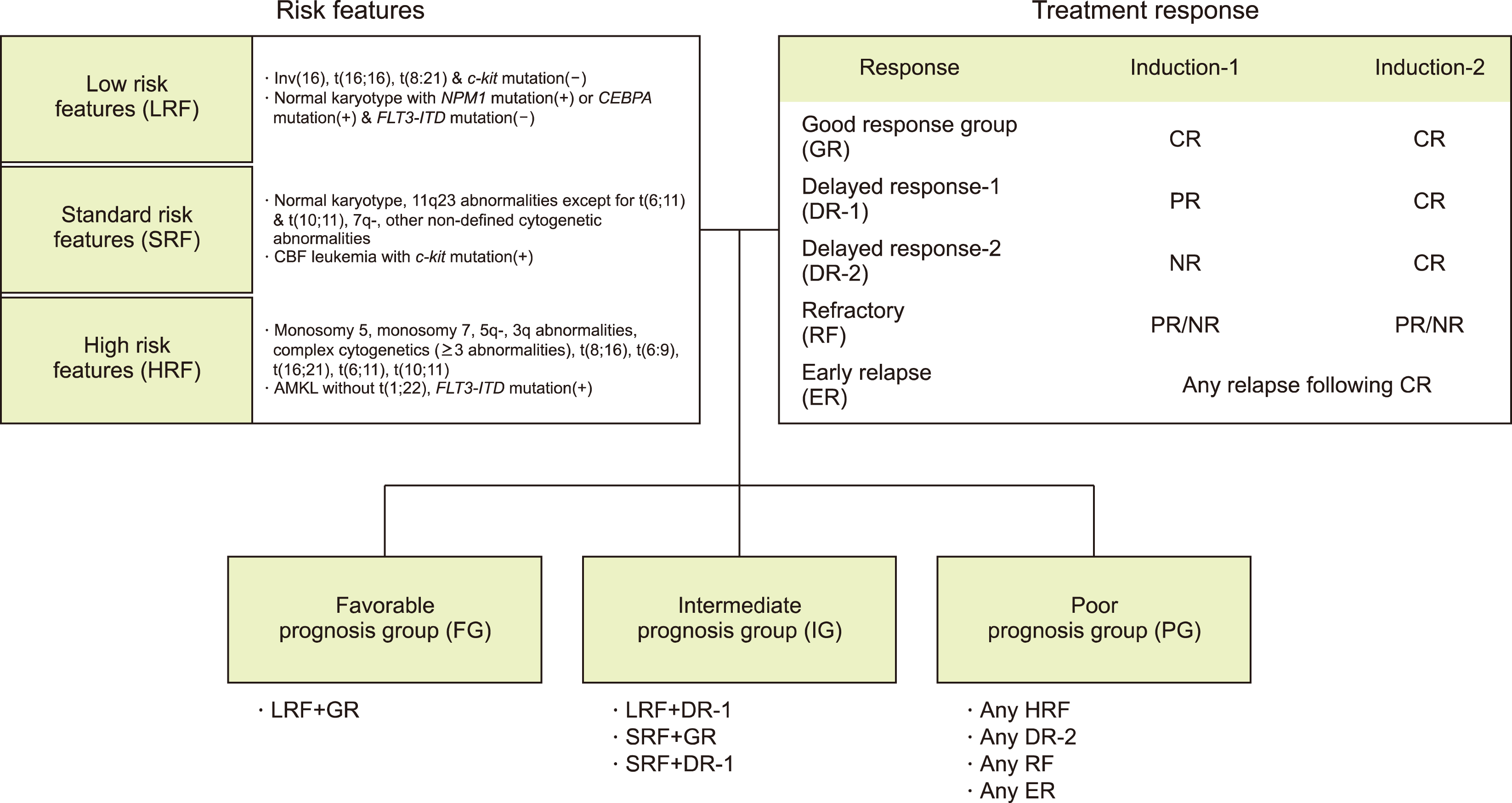
Fig. 2
Overall survival (OS) and event-free survival (EFS) rates. OS and EFS of all patients with first relapse of pediatric AML (A). Comparisons of OS rates between the early vs. late relapsers (B), those who achieved CR after 1 course of reinduction chemotherapy vs. others (C), and those in the favorable or intermediate prognosis groups (FG+IG) vs. poor prognosis group (PG) (D).
Table 1
Patient characteristics.
Table 2
Univariate analysis of prognostic factors.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download