Abstract
Background
Acromegaly leads to various skeletal complications, and fragility fractures are emerging as a new concern in patients with acromegaly. Therefore, this study investigated the risk of fractures in Korean patients with acromegaly.
Methods
We used the Korean nationwide claims database from 2009 to 2019. A total of 931 patients with acromegaly who had never used an osteoporosis drug before and were treated with surgery alone were selected as study participants, and a 1:29 ratio of 26,999 age- and sex-matched osteoporosis drug-naïve controls without acromegaly were randomly selected from the database.
Results
The mean age was 46.2 years, and 50.0% were male. During a median follow-up of 54.1 months, there was no difference in the risks of all, vertebral, and non-vertebral fractures between the acromegaly and control groups. However, hip fracture risk was significantly higher (hazard ratio [HR], 2.73; 95% confidence interval [CI], 1.32 to 5.65), and non-hip and non-vertebral fractures risk was significantly lower (HR, 0.40; 95% CI, 0.17 to 0.98) in patients with acromegaly than in controls; these results remained robust even after adjustment for socioeconomic status and baseline comorbidities. Age, type 2 diabetes mellitus, cardio-cerebrovascular disease, fracture history, recent use of acid-suppressant medication, psychotropic medication, and opioids were risk factors for all fractures in patients with acromegaly (all P<0.05).
Growth hormone (GH) and insulin-like growth factor-1 (IGF-1) play critical roles in linear bone growth, the acquisition of peak bone mass, and bone homeostasis [1,2]. Both GH deficiency and pathologically high GH impair skeletal health, causing secondary osteoporosis, and acromegaly, a chronic disease most often caused by a GH-secreting pituitary tumor, is traditionally regarded as one of the causes of secondary osteoporosis [3]. GH hypersecretion and the resultant overproduction of IGF-1 lead to numerous musculoskeletal complications of acromegaly, including acral enlargement, carpal tunnel syndrome, osteoarthritis, and vertebral fractures [4,5], highlighting the clinical importance of bone health in patients with acromegaly.
Under physiological conditions, GH and IGF-1 increase bone formation by directly or indirectly stimulating cells of the osteoblast lineage and modulating the process of bone remodeling, coupled with bone formation and bone resorption [6]. Recently, researchers have examined the effect of excess GH on bone metabolism and revealed increased bone turnover in patients with acromegaly in proportion to the disease activity [7,8]. Bone resorption markers are increased compared with bone formation markers in patients with acromegaly, explaining the increased bone loss and damaged bone structure in GH excess [9-11]. Thus, abnormalities in bone microarchitecture and deterioration in skeletal strength increase the risk of fragility fractures, especially vertebral fractures, in patients with acromegaly [8,12]. This increased risk of fractures is an emerging concern in patients with acromegaly [13]. However, owing to the rarity of the disease, there is an unmet need for large-scale longitudinal studies on acromegalic osteopathy, especially in Asia.
Therefore, this study aimed to assess the risk of fractures in patients with acromegaly using a nationwide cohort in Korea. The current study aimed to investigate the incidence and risk of all fractures but also, in particular, site-specific fractures in patients with acromegaly. We also determined the risk factors for site-specific fractures in patients with acromegaly.
Korea has a unique medical system: the National Health Insurance Service (NHIS), which provides medical insurance to approximately 97% of the Korean population [14,15]. Since 1989, it has been mandatory for all Korean citizens to subscribe to the NHIS. All medical service providers submit data on hospital admissions and outpatient visits to the NHIS for reimbursement, including sociodemographic data, diagnostic, prescription, and procedure codes [14,15]. Therefore, the NHIS captures all medical utilization data in Korea and stores and integrates it into a single database called the National Health Information Database (NHID) [14,15]. The NHID provides anonymized medical information for the entire Korean population for research purposes. This includes the International Classification of Disease, 10th Revision (ICD-10) codes, pharmaceutical claim records, procedure codes, hospital admissions, and outpatient visits [14,15]. The current study was conducted using NHID data from 2009 to 2019.
The study protocol was approved by the Institutional Review Board of Yonsei University College of Medicine, Seoul, Republic of Korea (approval no: 4-2020-0733), and the need for informed consent was waived because of the anonymity of the data.
We retrospectively analyzed the data of patients with acromegaly who underwent surgery alone between January 2009 and December 2019 in South Korea. A patient was defined as having acromegaly when both of the following criteria were satisfied: (1) at least one claim with the ICD-10 code of acromegaly (E22.0) and (2) at least one claim of acromegaly-related treatment (surgery, medication, or radiotherapy) within 2 years of the date of the first diagnosis of acromegaly [16,17]. Patients who had claim data with ICD-10 code E22.0 from January 2002 to December 2008 were excluded for the washout period (n=1,993) for the analysis of incident cases of acromegaly. Among all patients with acromegaly, 1,519 patients who underwent acromegaly-related surgery within 2 years from the date of the first diagnosis were extracted. Of the 1,519 patients who underwent acromegaly-related surgery, 588 were excluded based on the following exclusion criteria: (1) patients who had received acromegaly-related treatment other than surgery alone (n=550) and (2) patients who used osteoporosis medication within 2 years before the diagnosis of acromegaly (n=38). In the current study, osteoporosis medication was defined as bisphosphonates or a combination of bisphosphonates and vitamin D. A total of 931 osteoporosis drug-naïve patients with acromegaly who were treated with surgery alone were enrolled in the final analyses, and 1:29 age- and sex-matched osteoporosis drug-naïve controls without ICD-10 code E22.0 were randomly selected from the database (n=26,999). The research design is illustrated in Fig. 1. The first claim date of ICD-10 code E22.0 was defined as the index date.
Baseline comorbidity was considered present if there were claims for at least one inpatient or two outpatient ICD-10 codes or a combination of an ICD-10 code with a prescription of medication for the comorbidity within 2 years of the index date (Supplemental Table S1). Baseline comorbidities included type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, heart failure, stroke, previous myocardial infarction, dementia, depression, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis (RA), chronic liver disease, Parkinson’s disease, chronic kidney disease (CKD), including end-stage renal disease (ESRD), hyperthyroidism, previous fracture history, osteoporosis, inflammatory bowel disease, and hypogonadism. Recent use of corticosteroids, acid-suppressant medications, psychotropic medications, opioids, and warfarin, which can negatively impact skeletal health, was identified in the database. Recent use of medication was defined as >90 days of prescription from 1 year before to 2 months after the index date.
The primary outcomes of the study were all fracture and sitespecific (vertebrae, hip, proximal humerus, and distal radius) fracture risk in patients with acromegaly treated with surgery alone. The operational definition of site-specific fractures was based on the ICD-10, procedure, or radiologic imaging codes (Supplemental Table S2). The secondary outcome was the identification of risk factors for fractures in patients with acromegaly. Incident fracture cases were analyzed from the date after the index date to the day of death or until December 2019.
Continuous data are presented as mean±standard deviation for normally distributed variables and median and interquartile range for non-normally distributed variables. Categorical data are presented as numbers and percentages. A conditional logistic regression model was used to compare the baseline characteristics between the acromegaly and matched control groups. The incidence of fractures is presented as the rate per 1,000 person-years by dividing the number of events by the total person-years. We performed Kaplan-Meier survival analysis and the log-rank test to compare the cumulative incidence between the two groups. Stratified Cox proportional hazards regression models (by age and sex) were used to evaluate the risk of fracture, and the results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). We estimated adjusted HRs by including socioeconomic status (SES) and baseline comorbidities as covariates in the model. The sub-distribution HR (sHR) was obtained using the Fine-Gray model, which considers death to be a competing risk. In these models, patients who died before experiencing a fracture were not censored and remained in the risk set until the end of the follow-up. To identify the risk factors affecting the fracture event, we performed univariate analysis for each variable and then estimated the multivariable sHR by including age, sex, and variables with significant univariate test results in the model. All reported P values were two-sided, and statistical significance was set at P<0.05. All statistical analyses were performed using the SAS Enterprise Guide version 7.1 (SAS Institute Inc., Gary, NC, USA).
The baseline characteristics of the 931 surgically treated patients with acromegaly and 26,999 age- and sex-matched controls are summarized in Table 1. The mean age of patients with acromegaly was 46.2 years, and neither men nor women showed a predominance in sex distribution. The acromegaly group had a significantly higher rate of baseline comorbidities than the control group, including T2DM, hypertension, dyslipidemia, heart failure, stroke, depression, COPD, RA, malignancy, hyperthyroidism, and the Charlson Comorbidity Index (all P<0.05). Nevertheless, previous fracture history and osteoporosis showed no significant differences between the two groups. In addition, the recent use of corticosteroids, acid-suppressant medication, psychotropic medication, and opioids was significantly higher in the acromegaly group than in the control group (all P<0.05).
The risk of fractures in patients with acromegaly and age- and sex-matched controls is shown in Table 2. Compared with the control group, the acromegaly group did not show a higher risk of all fractures, including those of the vertebrae, hip, proximal humerus, and distal radius (Table 2, Fig. 2A). Additionally, a site-specific fracture risk analysis was performed. Although vertebral and non-vertebral fracture risks showed no differences between the acromegaly and the control groups (Table 2, Fig. 2B, C), hip fracture showed a significantly higher risk in patients with acromegaly compared with the controls (HR, 2.73; 95% CI, 1.32 to 5.65, model 1). The Kaplan-Meier curve demonstrated a significantly higher cumulative incidence of hip fractures in the acromegaly group than in the control group (Fig. 2D). Similar results were observed for all, vertebral, non-vertebral, and hip fractures, even after adjusting for SES and baseline comorbidities, including T2DM, cardio-cerebrovascular disease, heart failure, COPD, RA, chronic liver disease, CKD (including ESRD), and previous fracture history (model 2). The hip fracture risk remained more prominent in patients with acromegaly than in controls in the sub-distribution hazard model (sHR, 2.58; 95% CI, 1.19 to 5.59, model 3). The increased risk of hip fracture in patients with acromegaly remained robust after further adjustment with underlying malignancy as a covariate in model 2 (Supplemental Table S3, model 4). The risk of non-hip and non-vertebral fractures (NHNVF) was significantly lower in patients with acromegaly than in controls (Table 2).
The risk factors for site-specific fractures in all study participants were analyzed using the Cox proportional hazards model and are presented in Supplemental Table S4. High SES reduced the risk of all fractures (multivariable sHR, 0.84; 95% CI, 0.71 to 0.99), whereas cardio-cerebrovascular disease and previous fracture history increased the risk of all fractures (multivariable sHR 1.45, 95% CI 1.08 to 1.94; multivariable sHR 4.89, 95% CI 3.59 to 6.67, respectively). Similarly, cardio-cerebrovascular disease and previous fracture history also increased the risk of vertebral fracture (multivariable sHR, 1.69 [95% CI, 1.13 to 2.51], multivariable sHR, 5.49 [95% CI, 3.68 to 8.19], respectively). CKD, including ESRD and previous history of fracture, were common risk factors for non-vertebral and hip fractures, and notably, acromegaly was a risk factor for hip fracture (multivariable sHR, 2.58; 95% CI, 1.19 to 5.59). In contrast, acromegaly was a protective factor in NHNVF, and as in other types of fractures, previous fracture history was a risk factor for NHNVF.
The risk factors for all types of fractures in patients with acromegaly are listed in Table 3. In the univariate analysis, age, T2DM, cardio-cerebrovascular disease, previous fracture history, recent use of acid-suppressant medication, psychotropic medication, and opioids were risk factors for all types of fractures in patients with acromegaly. Age and previous fracture history remained risk factors for all types of fractures in the multivariable analysis. Likewise, age, cardio-cerebrovascular disease, previous fracture history, and recent use of corticosteroids, acidsuppressant medication, psychotropic medication, and opioids were risk factors for vertebral fractures in the univariate model (Supplemental Table S5). Only age, cardio-cerebrovascular disease, previous fracture history, and recent use of corticosteroids and acid-suppressant medications were significantly associated with vertebral fractures in the multivariable analysis. Previous fracture history was the only risk factor for non-vertebral fractures in the univariate and multivariable models (Supplemental Table S6), and for hip fractures, hypogonadism significantly increased the risk in both the univariate and multivariable models (Supplemental Table S7). The risk factors for NHNVF in patients with acromegaly are presented in Supplemental Table S8.
The current study analyzed the risk of fractures in patients with acromegaly and demonstrated an increased risk of hip fractures in patients with acromegaly who were treated with surgery alone, compared to the age- and sex-matched controls. Age, cardiocerebrovascular disease, hypogonadism, previous fracture history, and recent use of corticosteroids and acid-suppressant medication were risk factors for fractures in the multivariable analysis in patients with acromegaly, as in the general population [18].
The most striking result of this study was the higher risk of hip fracture in surgically treated patients with acromegaly, than in age- and sex-matched controls during the follow-up period. A previous study addressed the association between supraphysiological GH and altered bone geometry, demonstrating reduced trabecular bone volume and trabecular thickness with increased trabecular separation, while cortical thickness increased but porosity and pore volume also increased [19]. Concerning the fact that the hip bone is composed of both cortical and trabecular bone (femoral neck: 25% trabecular bone and 75% cortical bone; greater trochanteric area of the femur: 50% trabecular bone and 50% cortical bone) [20], this may suggest that hip bone structure is impaired in patients with acromegaly. Notably, structural abnormalities of the hip bone in patients with acromegaly have been suggested in an earlier study using dual-energy X-ray absorptiometry-based three-dimensional analysis [21]. The study showed higher total hip and femoral neck cortical thickness and lower lumbar areal bone mineral density (BMD), trabecular bone score, and total hip trabecular volumetric BMD in patients with acromegaly than in healthy controls [21]. Another study showed an increase in femoral cortical bone thickness and an enlargement in bone diameter in patients with active acromegaly due to the prominent effect of periosteal bone formation [22]. Then, after 1 year of pituitary surgery, the femoral cortical bone thickness decreased with unchanged bone dimension, and this unbalanced geometry may consequently lead to inferior mechanical properties of the hip in patients with acromegaly [22]. Additionally, after GH-lowering treatments, endocortical bone resorption becomes more prominent than periosteal bone formation, similar to normal aging, leading to decreased cortical bone thickness [22]. Altogether, during the active phase of acromegaly, the trabecular bone is mainly damaged, and the cortical bone compensates for it [21,22]. However, endocortical bone resorption increases following GH-lowering treatment, resulting in decreased cortical thickness and the disappearance of cortical bone compensation in treated patients with acromegaly, which may explain the increased risk of hip fractures [21,22]. These studies support our finding of an increased hip fracture risk in patients with acromegaly who were treated with surgery alone.
Extensive studies have been conducted to investigate factors that affect bone metabolism. Age, sex, fracture history, T2DM, renal disease, cardiovascular disease, dementia, hypogonadism, chronic liver disease, inflammatory disease, and certain medications are widely accepted risk factors for osteoporosis [23,24]. This study analyzed the risk factors for fractures in patients with acromegaly, and the results were consistent with those of previous studies [23,24]. Old age and previous fracture history were risk factors for all fractures in patients with acromegaly in the multivariable models. Risk factors for site-specific fractures in the multivariable model showed similar results to those of previous studies [23-27], with age, cardio-cerebrovascular disease, previous fracture history, and recent use of corticosteroids and acid-suppressant medication being the risk factors for vertebral fractures and hypogonadism being the risk factor for hip fractures. The present study suggests that previously accepted risk factors for fractures are also applicable to surgically treated patients with acromegaly, and we recommend careful attention and regular examination of patients with acromegaly with these risk factors during long-term follow-up.
The high prevalence of vertebral fractures in patients with acromegaly has been previously reported. Interestingly, vertebral fracture risk persists, and fractures even progress despite biochemical control of the disease [12,28,29]. However, contrary to expectations, there was no significant increase in the risk of vertebral fractures in patients with acromegaly compared with in controls. The incidence of vertebral fractures was numerically higher in the acromegaly group than in the control group; however, the difference was not statistically significant. Approximately 60% of vertebral fractures are asymptomatic, making it difficult to detect all osteoporotic vertebral fractures unless all individuals with acromegaly undergo spinal radiography as a screening [30]. Since we defined vertebral fractures based on the ICD-10 diagnosis, procedure, or imaging codes, there might be a limitation in the detection of asymptomatic vertebral fractures patients that do not visit the hospital. Thus, our results did not necessarily contradict other prior studies showing more prevalent vertebral fractures in patients with acromegaly, and our data need to be interpreted with caution [12,31,32]. Furthermore, point estimation of vertebral fractures showed an increasing trend in the acromegaly group compared with the control group, which could be statistically significant with a sufficient sample size. Further large-scale studies using imaging modalities are required to accurately estimate the risk of vertebral fractures in patients with acromegaly.
The major strength of the present study was that it was a nationwide population-based cohort study that included a large number of patients with acromegaly and the follow-up period was relatively long (up to 10 years). A washout period of 2 to 3 years might not be sufficient owing to the nature of fracture healing rate; thus, to reduce the influence of previous fractures on new fractures, we set the longest possible washout period from 2002, the year NHID became available, to 2008, before the initiation of the benefit extension policy. Moreover, by analyzing only patients with acromegaly treated with surgery alone, we were able to determine the incidence of fractures in patients with acromegaly, which was assumed to be under biochemical control. A recent study demonstrated that the mortality of patients treated with surgery alone did not differ from that of the general population, whereas patients who received postoperative adjuvant medication or radiotherapy had higher mortality than the controls [33]. The prognosis of patients differed depending on the treatment modality; therefore, we conducted the study with a homogenous group of patients who underwent only surgery with a similar prognosis. Lastly, to the best of our knowledge, this is the first study in Asia to demonstrate an increased risk of hip fractures in patients with acromegaly who were treated with surgery alone.
However, our study has several limitations. Fractures were operationally defined based on the ICD-10 diagnosis, procedure, or imaging codes; therefore, asymptomatic fracture patients who did not visit a hospital might have been missed, resulting in possible underestimation of fracture incidence, especially vertebral fractures. The risk of NHNVF was significantly lower in patients with acromegaly compared with the age- and sex-matched controls; however, this result should be interpreted with caution owing to the insufficient number of events. A future large-scale study is required to assess the risk of NHNVF in patients with acromegaly. The present study is based on a claims database primarily used for reimbursement, possibly resulting in a lack of clinical granularity. We could not obtain information on potential confounding factors, such as blood test results for GH, IGF-1, and bone turnover markers; imaging data, including BMD and brain magnetic resonance imaging; or treatment compliance. Accordingly, there is a need for additional studies involving the integration of hospital data. Moreover, owing to this limitation in the nature of claims database, we could only assume that patients who underwent pituitary surgery alone, without adjuvant therapy, achieved biochemical control after surgery. In addition, SES and baseline comorbidities were the only covariates included in the regression model; thus, other potential confounding factors might have been overlooked. It would be valuable to include body mass index, alcohol consumption, smoking, regular exercise, and laboratory test results as covariates using the national health checkup program; however, in addition to the limited number of patients with acromegaly owing to its rarity, the number of included patients will be reduced by about 50% if only patients with health checkup data are enrolled. Thus, to maintain the number of study participants, only variables obtainable from the NHID were selected as covariates in this study. Future studies using NHID and hospital data including these covariates are necessary for better understanding of potential confounding factors for fracture risk in patients with acromegaly. We aimed to investigate fracture risk in patients with acromegaly while excluding the influence of osteoporosis medications; therefore, among 969 patients who underwent surgery only, we excluded 38 patients with a history of osteoporosis medication use. The percentage of these patients was relatively low (3.9%); thus, excluding them from the study population would not significantly compromise the representation of patients with acromegaly. However, obtaining a substantial number of osteoporosis drug-naïve patients through a longer study period and multinational approach could enhance the reliability of future studies. This was a retrospective study; however, we matched for age and sex and adjusted for SES and baseline comorbidities to minimize the effects of confounding factors.
The current study demonstrated an increased risk of hip fractures in patients with surgically treated acromegaly compared to age- and sex-matched controls. Additionally, by analyzing surgically treated patients with acromegaly, we suggest that the risk of hip fracture might persist even after successful GH-lowering treatment. The risk factors for fracture in patients with acromegaly were consistent with widely accepted risk factors, including age, T2DM, cardio-cerebrovascular disease, hypogonadism, previous fracture history, and recent use of medications such as acid-suppressant medication, psychotropic medication, and opioids. Thus, we recommend regular examinations for fragility fractures, not only vertebral fractures but also hip fractures, in patients with acromegaly, and careful attention is needed for patients with these risk factors.
Supplementary Material
Supplemental Table S1.
Operational Definition of Baseline Comorbidities
Supplemental Table S2.
Operational Definition of Site-Specific Fracture
Supplemental Table S3.
Risk of Fractures in Surgically Treated Acromegaly Patients Compared with Controls
Supplemental Table S4.
Risk Factors for All Fractures and Site-Specific Fractures in the Study Participants
Supplemental Table S5.
Risk Factors of Vertebral Fracture in Patients with Acromegaly
Supplemental Table S6.
Risk Factors of Non-Vertebral Fracture in Patients with Acromegaly
Supplemental Table S7.
Risk Factors of Hip Fracture in Patients with Acromegaly
Supplemental Table S8.
Risk Factors of Non-Hip and Non-Vertebral Fracture in Patients with Acromegaly
Notes
ACKNOWLEDGMENTS
We would like to thank Ipsen, “Team Science Award” of the Yonsei University College of Medicine, Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea, Committee of Rare Endocrine Disease of the Korean Endocrine Society, and the participants of the Korean Health Insurance Cohort study
REFERENCES
1. Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008; 29:535–59.

2. Courtland HW, Sun H, Beth-On M, Wu Y, Elis S, Rosen CJ, et al. Growth hormone mediates pubertal skeletal development independent of hepatic IGF-1 production. J Bone Miner Res. 2011; 26:761–8.

3. Mirza F, Canalis E. Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015; 173:R131–51.
4. Colao A, Grasso LF, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Primers. 2019; 5:20.

5. Hong JW, Ku CR, Kim SH, Lee EJ. Characteristics of acromegaly in Korea with a literature review. Endocrinol Metab (Seoul). 2013; 28:164–8.

6. Mazziotti G, Maffezzoni F, Frara S, Giustina A. Acromegalic osteopathy. Pituitary. 2017; 20:63–9.

7. Mazziotti G, Chiavistelli S, Giustina A. Pituitary diseases and bone. Endocrinol Metab Clin North Am. 2015; 44:171–80.

8. Bonadonna S, Mazziotti G, Nuzzo M, Bianchi A, Fusco A, De Marinis L, et al. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J Bone Miner Res. 2005; 20:1837–44.

9. Stepan J, Marek J, Havranek T, Dolezal V, Pacovsky V. Bone isoenzyme of serum alkaline phosphatase in acromegaly. Clin Chim Acta. 1979; 93:355–63.
10. Scillitani A, Chiodini I, Carnevale V, Giannatempo GM, Frusciante V, Villella M, et al. Skeletal involvement in female acromegalic subjects: the effects of growth hormone excess in amenorrheal and menstruating patients. J Bone Miner Res. 1997; 12:1729–36.
11. Ueland T, Bollerslev J, Godang K, Muller F, Froland SS, Aukrust P. Increased serum osteoprotegerin in disorders characterized by persistent immune activation or glucocorticoid excess: possible role in bone homeostasis. Eur J Endocrinol. 2001; 145:685–90.
12. Mazziotti G, Bianchi A, Porcelli T, Mormando M, Maffezzoni F, Cristiano A, et al. Vertebral fractures in patients with acromegaly: a 3-year prospective study. J Clin Endocrinol Metab. 2013; 98:3402–10.
13. Mazziotti G, Lania AGA, Canalis E. Management of endocrine disease: bone disorders associated with acromegaly: mechanisms and treatment. Eur J Endocrinol. 2019; 181:R45–56.
14. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance system. Diabetes Metab J. 2014; 38:395–403.
15. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15.
16. Park KH, Lee EJ, Seo GH, Ku CR. Risk for acromegaly-related comorbidities by sex in Korean acromegaly. J Clin Endocrinol Metab. 2020; 105:dgz317.
17. Cho SW, Kim JH, Choi HS, Ahn HY, Kim MK, Rhee EJ. Big data research in the field of endocrine diseases using the Korean National Health Information Database. Endocrinol Metab (Seoul). 2023; 38:10–24.
18. Panday K, Gona A, Humphrey MB. Medication-induced osteoporosis: screening and treatment strategies. Ther Adv Musculoskelet Dis. 2014; 6:185–202.
19. Dalle Carbonare L, Micheletti V, Cosaro E, Valenti MT, Mottes M, Francia G, et al. Bone histomorphometry in acromegaly patients with fragility vertebral fractures. Pituitary. 2018; 21:56–64.
20. Cassidy JT, Laxer RM, Petty RE, Lindsley B. Textbook of pediatric rheumatology. 6th ed. Philadelphia: Saunders;2011.
21. Kuzma M, Vanuga P, Sagova I, Pavai D, Jackuliak P, Killinger Z, et al. Non-invasive DXA-derived bone structure assessment of acromegaly patients: a cross-sectional study. Eur J Endocrinol. 2019; 180:201–11.
22. Godang K, Lekva T, Normann KR, Olarescu NC, Oystese KA, Kolnes A, et al. Hip structure analyses in acromegaly: decrease of cortical bone thickness after treatment: a longitudinal cohort study. JBMR Plus. 2019; 3:e10240.
23. Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int. 2005; 16:581–9.
24. Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018; 14:2029–49.
25. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296:2947–53.
26. Ramnemark A, Nyberg L, Borssen B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int. 1998; 8:92–5.
27. den Uyl D, Nurmohamed MT, van Tuyl LH, Raterman HG, Lems WF. (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther. 2011; 13:R5.
28. Claessen KM, Kroon HM, Pereira AM, Appelman-Dijkstra NM, Verstegen MJ, Kloppenburg M, et al. Progression of vertebral fractures despite long-term biochemical control of acromegaly: a prospective follow-up study. J Clin Endocrinol Metab. 2013; 98:4808–15.
29. Pelsma IC, Biermasz NR, Pereira AM, van Furth WR, Appelman-Dijkstra NM, Kloppenburg M, et al. Progression of vertebral fractures in long-term controlled acromegaly: a 9-year follow-up study. Eur J Endocrinol. 2020; 183:427–37.
30. Ross PD. Clinical consequences of vertebral fractures. Am J Med. 1997; 103(2A):30S–43S.
31. Mazziotti G, Bianchi A, Bonadonna S, Cimino V, Patelli I, Fusco A, et al. Prevalence of vertebral fractures in men with acromegaly. J Clin Endocrinol Metab. 2008; 93:4649–55.
32. Mazziotti G, Biagioli E, Maffezzoni F, Spinello M, Serra V, Maroldi R, et al. Bone turnover, bone mineral density, and fracture risk in acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2015; 100:384–94.
33. Kim J, Hong N, Choi J, Moon JH, Kim EH, Hong JW, et al. Sex differences in mortality in patients with acromegaly: a nationwide cohort study in Korea. Eur J Endocrinol. 2023; 189:225–34.
Fig. 1.
Flow chart of the study design. ICD-10, International Classification of Disease, 10th Revision.
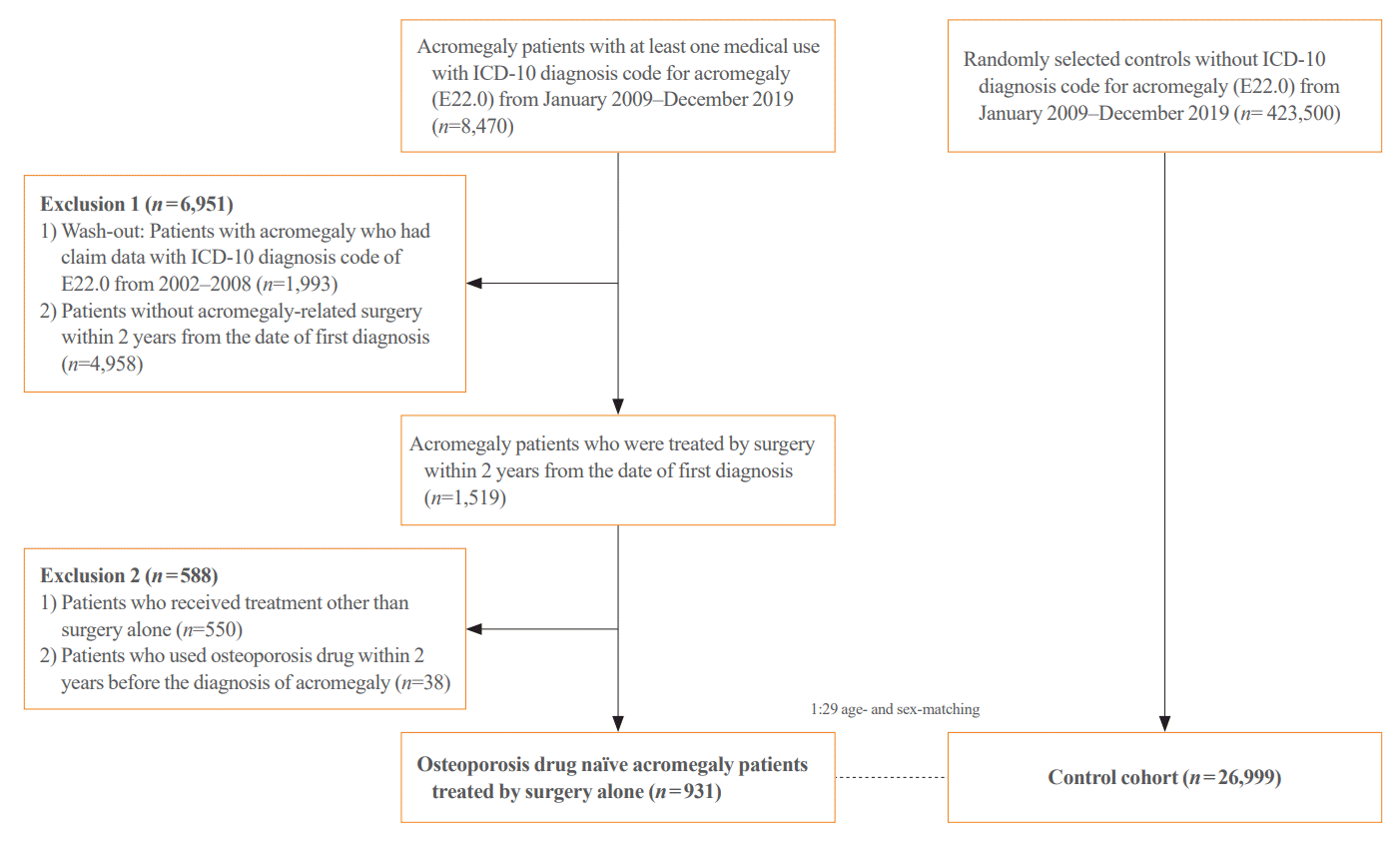
Fig. 2.
The Kaplan-Meier curve of site-specific fractures. (A) All fracture. (B) Vertebral fracture. (C) Non-vertebral fracture. (D) Hip fracture.
Table 1.
Baseline Clinical Characteristics of Study Participants
| Characteristic | Surgically treated acromegaly patients (n=931) | Controls (n=26,999) | P value |
|---|---|---|---|
| Age, yr | 46.2±12.6 | 46.2±12.6 | - |
| Sex | - | ||
| Male | 465 (50.0) | 13,485 (50.0) | |
| Female | 466 (50.1) | 13,514 (50.1) | |
| Socioeconomic status | 0.333 | ||
| Low | 216 (23.2) | 6,605 (24.5) | |
| Middle | 309 (33.2) | 9,265 (34.3) | |
| High | 406 (43.6) | 11,129 (41.2) | |
| Baseline comorbidities | |||
| Type 2 diabetes mellitus | 223 (24.0) | 1,647 (6.1) | <0.001a |
| Hypertension | 146 (15.7) | 1,491 (5.5) | <0.001a |
| Dyslipidemia | 176 (18.9) | 3,848 (14.3) | <0.001a |
| Heart failure | 20 (2.2) | 208 (0.8) | <0.001a |
| Cardio-cerebrovascular disease | 37 (4.0) | 704 (2.6) | 0.010a |
| Stroke | 33 (3.5) | 595 (2.2) | 0.006a |
| Previous myocardial infarction | 4 (0.4) | 119 (0.4) | 0.960 |
| Dementia | 1 (0.1) | 71 (0.3) | 0.368 |
| Depression | 58 (6.2) | 1,244 (4.6) | 0.020a |
| COPD | 59 (6.3) | 1,263 (4.7) | 0.019a |
| Rheumatoid arthritis | 44 (4.7) | 472 (1.8) | <0.001a |
| Chronic liver disease | 45 (4.8) | 1,342 (5.0) | 0.849 |
| Malignancy | 74 (7.9) | 707 (2.6) | <0.001a |
| Parkinson disease | 0 | 28 (0.1) | 0.948 |
| CKD/ESRD | 6 (0.6) | 124 (0.5) | 0.415 |
| Hyperthyroidism | 31 (3.3) | 312 (1.2) | <0.001a |
| Previous fracture history (overall) | 9 (1.0) | 246 (0.9) | 0.860 |
| Hip | 0 | 19 (0.1) | - |
| Vertebral | 5 (0.5) | 81 (0.3) | 0.205 |
| Radius | 3 (0.3) | 126 (0.5) | 0.524 |
| Humerus | 1 (0.1) | 25 (0.1) | 0.884 |
| Osteoporosis | 4 (0.4) | 86 (0.3) | 0.555 |
| Inflammatory bowel disease | 1 (0.1) | 39 (0.1) | 0.770 |
| Hypogonadism | 16 (1.7) | 400 (1.5) | 0.551 |
| Charlson comorbidity index | 3 (1–4) | 2 (0–3) | <0.001a |
| Recent medication use | |||
| Corticosteroids | 34 (3.7) | 268 (1.0) | <0.001a |
| Acid-suppressant medication | 99 (10.6) | 1,559 (5.8) | <0.001a |
| Psychotropic medication | 73 (7.8) | 1,421 (5.3) | 0.001a |
| Opioid | 41 (4.4) | 582 (2.2) | <0.001a |
| Warfarin | 1 (0.1) | 38 (0.1) | 0.789 |
Table 2.
Risk of Fractures in Surgically Treated Acromegaly Patients Compared with Controls
|
No. of event (IR) |
Hazard ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Acromegaly | Control | Model 1 | P value | Model 2 | P value | Model 3 | P value | |
| All fracture | 25 (5.12) | 729 (5.15) | 0.99 (0.67–1.47) | 0.959 | 0.98 (0.66–1.47) | 0.925 | 0.99 (0.66–1.47) | 0.940 |
| Vertebral fracture | 12 (2.44) | 316 (2.21) | 1.10 (0.62–1.92) | 0.751 | 1.16 (0.66–2.05) | 0.608 | 1.16 (0.66–2.05) | 0.612 |
| Non-vertebral fracturesb | 13 (2.65) | 438 (3.07) | 0.86 (0.49–1.49) | 0.587 | 0.81 (0.46–1.43) | 0.475 | 0.82 (0.47–1.45) | 0.499 |
| Hip fracture | 8 (1.63) | 85 (0.59) | 2.73 (1.32–5.65) | 0.007a | 2.53 (1.16–5.50) | 0.019a | 2.58 (1.19–5.59) | 0.017a |
| Non-hip and non-vertebral fracturesc | 5 (1.01) | 357 (2.50) | 0.40 (0.17–0.98) | 0.045a | 0.39 (0.16–0.96) | 0.040a | 0.40 (0.16–0.96) | 0.041a |
Model 1: matched by age and sex; Model 2: model 1+adjusted for socioeconomic status and comorbidities (type 2 diabetes mellitus, cardio-cerebrovascular disease, heart failure, chronic obstructive pulmonary disease, rheumatoid arthritis, chronic liver disease, chronic kidney disease/end-stage renal disease, and previous fracture history); Model 3: sub-distribution hazard model for model 2.
IR, incidence rate (/1,000 person-years); CI, confidence interval.
Table 3.
Risk Factors for All Fractures in Patients with Acromegaly
| Variable |
All fractures |
Univariate sHR (95% CI) | P value | Multivariable sHR (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Yes (n=25) | No (n=906) | ||||||
| Age, yr | 55.0±13.1 | 46.0±12.5 | 1.07 (1.03–1.12) | 0.002a | 1.05 (1.01–1.10) | 0.021a | |
| Sex | |||||||
| Male | 11 (44.0) | 454 (50.1) | 1 (Ref.) | 1 (Ref.) | |||
| Female | 14 (56.0) | 452 (49.9) | 1.28 (0.58–2.80) | 0.544 | 0.82 (0.37–1.81) | 0.620 | |
| Socioeconomic status | 0.110 | ||||||
| Low | 9 (36.0) | 207 (22.8) | 1.39 (0.56–3.42) | 0.476 | |||
| Middle | 10 (40.0) | 299 (33.0) | 1 (Ref.) | ||||
| High | 6 (24.0) | 400 (44.2) | 0.47 (0.17–1.28) | 0.139 | |||
| Baseline comorbidities | |||||||
| Type 2 diabetes mellitus | 10 (40.0) | 213 (23.5) | 2.30 (1.03–5.10) | 0.042a | 1.31 (0.59–2.90) | 0.501 | |
| Hypertension | 7 (28.0) | 139 (15.3) | 2.31 (0.97–5.49) | 0.058 | |||
| Cardio-cerebrovascular disease | 4 (16.0) | 33 (3.6) | 4.40 (1.50–12.89) | 0.007a | 2.85 (0.91–8.91) | 0.072 | |
| COPD | 1 (4.0) | 58 (6.4) | 0.59 (0.08–4.30) | 0.604 | |||
| Rheumatoid arthritis | 1 (4.0) | 43 (4.7) | 0.71 (0.10–4.97) | 0.729 | |||
| Chronic liver disease | 2 (8.0) | 43 (4.7) | 1.73 (0.40–7.52) | 0.462 | |||
| Previous fracture history | 2 (8.0) | 7 (0.8) | 15.85 (3.38–74.37) | 0.001a | 12.50 (1.86–84.20) | 0.010a | |
| Hypogonadism | 1 (4.0) | 15 (1.7) | 3.16 (0.42–24.00) | 0.266 | |||
| Recent medication use | |||||||
| Corticosteroids | 2 (8.0) | 32 (3.5) | 2.44 (0.57–10.51) | 0.231 | |||
| Acid-suppressant medication | 7 (28.0) | 92 (10.2) | 3.87 (1.59–9.42) | 0.003a | 1.79 (0.54–5.90) | 0.338 | |
| Psychotropic medication | 5 (20.0) | 68 (7.5) | 2.91 (1.09–7.75) | 0.033a | 1.53 (0.52–4.44) | 0.438 | |
| Opioid | 4 (16.0) | 37 (4.1) | 4.74 (1.64–13.68) | 0.004a | 1.76 (0.48–6.39) | 0.392 | |
| Warfarin | 0 | 1 (0.1) | - | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download