INTRODUCTION
METHODS
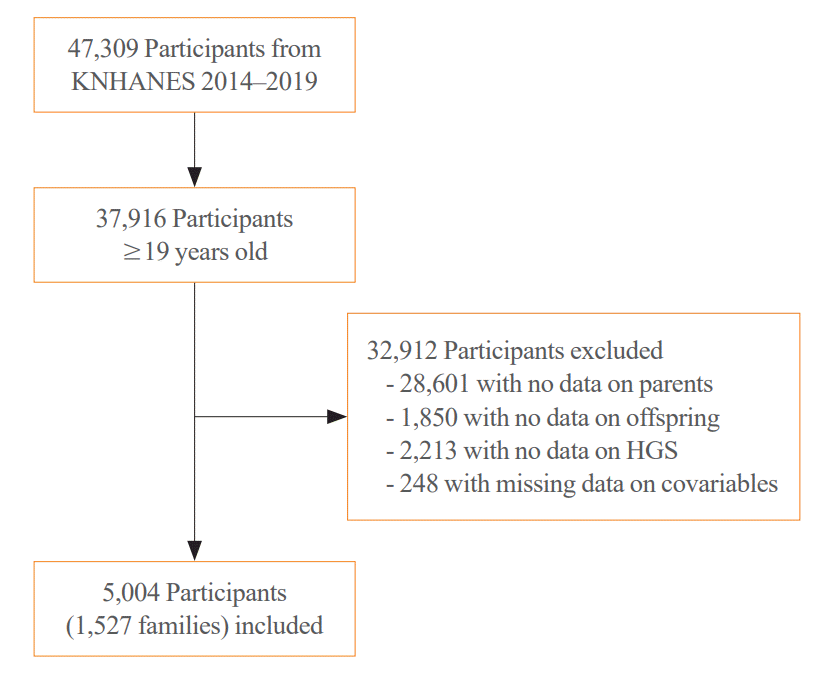
Study participants
Anthropometric, lifestyle, and dietary parameters
Measurement of HGS
Statistical analysis
RESULTS
Baseline characteristics of the study participants
Table 1.
| Variable |
Parents |
Offspring |
|||||
|---|---|---|---|---|---|---|---|
| Father (n=1,527) | Mother (n=1,527) | P valuea | Son (n=1,017) | Daughter (n=933) | P valuea | ||
| Age, yr | 58.4±7.9 | 55.1±7.6 | <0.001 | 27.8±7.6 | 26.8±6.6 | 0.004 | |
| BMI, kg/m2 | 24.4±2.9 | 23.9±3.4 | <0.001 | 24.3±4.1 | 21.7±3.8 | <0.001 | |
| HGS, kg | 39.3±7.5 | 24.4±5.0 | <0.001 | 41.8±8.0 | 24.0±4.8 | <0.001 | |
| Weak HGS | 121 (7.9) | 105 (6.9) | 0.269 | 53 (5.2) | 58 (6.2) | 0.339 | |
| Smoking | 1,187 (79.1) | 57 (3.8) | <0.001 | 533 (53.1) | 111 (12.0) | <0.001 | |
| Drinking | 326 (21.7) | 45 (3.0) | <0.001 | 159 (16.0) | 68 (7.3) | <0.001 | |
| Exercise | 429 (29.7) | 220 (14.8) | <0.001 | 366 (37.4) | 174 (19.0) | <0.001 | |
| Protein intake, g/day | 80.9 (68.4) | 58.9 (27.2) | <0.001 | 95.3 (58.9) | 67.2 (38.2) | <0.001 | |
| Comorbidities | |||||||
| Diabetes | 288 (19.8) | 151 (10.2) | <0.001 | 16 (1.7) | 11 (1.2) | 0.441 | |
| Hypertension | 626 (41.9) | 421 (27.9) | <0.001 | 94 (9.5) | 27 (2.9) | <0.001 | |
| Arthritis | 65 (4.5) | 212 (14.2) | <0.001 | 8 (0.8) | 5 (0.6) | 0.472 | |
| Thyroid disease | 21 (1.4) | 53 (3.6) | <0.001 | 3 (0.3) | 7 (0.8) | 0.170 | |
| Cancers | 70 (4.8) | 106 (7.1) | 0.009 | 3 (0.3) | 11 (1.2) | 0.023 | |
| Stroke | 18 (1.2) | 4 (0.3) | 0.002 | 2 (0.2) | 0 | 0.171 | |
| Coronary artery disease | 60 (4.1) | 17 (1.1) | <0.001 | 2 (0.2) | 0 | 0.171 | |
| Chronic kidney disease | 8 (0.6) | 2 (0.1) | 0.052 | 1 (0.1) | 2 (0.2) | 0.526 | |
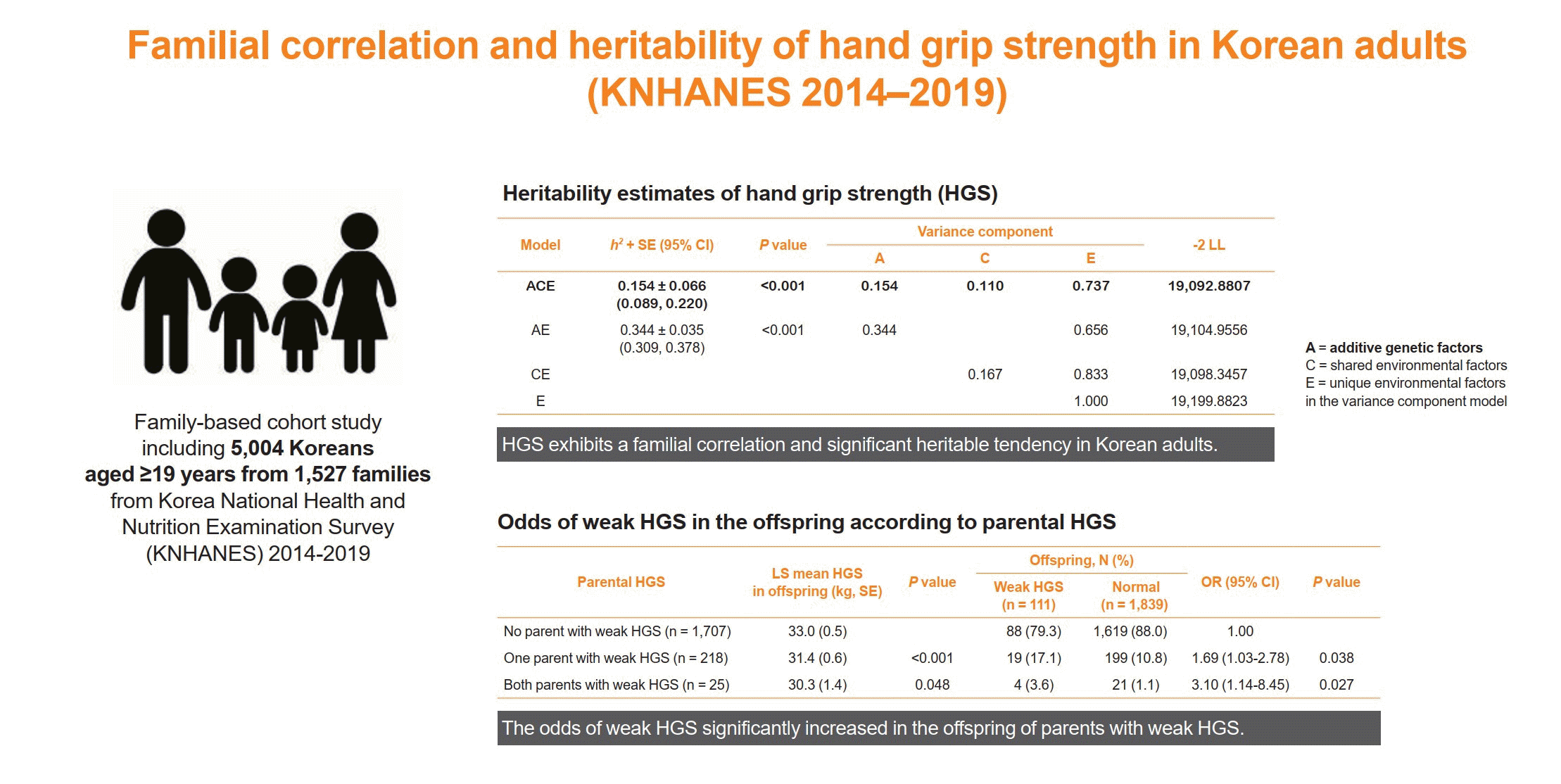
Familial correlation of HGS
Table 2.
ICC for hand grip strength were estimated to evaluate familial correlations between specific family pairs within the pedigrees, after adjustments for important confounding variables, such as sex, age, body mass index, protein intake, smoking status, alcohol consumption, exercise habits, and comorbidities.
ICC, intraclass correlation coefficient; CI, confidence interval.
Heritability of HGS
Table 3.
| Modela |
Crude model |
Adjusted model |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| h2±SE (95% CI) | P value | –2 LLb | h2±SE (95% CI) | P value |
Variance componentc |
–2 LLd | |||
| A | C | E | |||||||
| ACEe | 0.215±0.038 (0.177–0.253) | <0.001 | 28,363.2036 | 0.154±0.066 (0.089–0.220)e | <0.001e | 0.154e | 0.110e | 0.737e | 19,092.8807e |
| AE | 0.215±0.038 (0.177–0.253) | <0.001 | 28,363.2036 | 0.344±0.035 (0.309–0.378) | <0.001 | 0.344 | 0.656 | 19,104.9556 | |
| CE | 28,395.8128 | 0.167 | 0.833 | 19,098.3457 | |||||
| E | 28,395.8128 | 1.000 | 19,199.8823 | ||||||
Heritability (h2) was estimated as the proportion of the total phenotypic variance explained by additive genetic effects using variance component analysis.
Adjusted model included sex, age, body mass index, protein intake, smoking status, alcohol consumption, exercise habits, and comorbidities as confounders.
SE, standard error; CI, confidence interval; LL, log-likelihood.
a ACE=additive genetic factors and shared and unique environmental factors included in the variance component model, AE=additive genetic factors and unique environmental factors included in the variance component model, CE=shared and unique environmental factors included in the variance component model, and E=unique environmental factors included in the variance component model;
Odds of weak HGS in offspring according to parental muscle strength
Table 4.
| Parental HGS | LS mean HGS in offspring, kg | P valuea |
Offspring |
OR (95% CI) | P valueb | |
|---|---|---|---|---|---|---|
| Weak HGS (n=111) | Normal (n=1,839) | |||||
| No parent with weak HGS (n=1,707) | 33.0±0.5 | - | 88 (79.3) | 1,619 (88.0) | 1.00 | - |
| One parent with weak HGS (n=218) | 31.4±0.6 | <0.001 | 19 (17.1) | 199 (10.8) | 1.69 (1.03–2.78) | 0.038 |
| Both parents with weak HGS (n=25) | 30.3±1.4 | 0.048 | 4 (3.6) | 21 (1.1) | 3.10 (1.14–8.45) | 0.027 |
Values are expressed as mean±standard error or number (%) unless otherwise indicated. The cutoff values for weak HGS were defined as <28.9 and <16.8 kg in men and women, respectively.
HGS, hand grip strength; LS, least-square; OR, odds ratio; CI, confidence interval.
Table 5.
| Parental HGS | LS mean HGS in offspring, kg | P valuea |
Offspring |
OR (95% CI) | P valueb | ||
|---|---|---|---|---|---|---|---|
| Weak HGS | Normal | ||||||
| Son | n=53 | n=964 | |||||
| No parent with weak HGS (n=891) | 40.8±1.2 | - | 44 (83.0) | 847 (87.9) | 1.00 | - | |
| One parent with weak HGS (n=109) | 39.2±1.4 | 0.063 | 7 (13.2) | 102 (10.6) | 1.30 (0.59–2.89) | 0.519 | |
| Both parents with weak HGS (n=17) | 37.5±2.2 | 0.095 | 2 (3.8) | 15 (1.6) | 2.38 (0.58–9.83) | 0.230 | |
| Daughter | n=58 | n=875 | |||||
| No parent with weak HGS (n=816) | 24.4±0.4 | - | 44 (75.9) | 772 (88.2) | 1.00 | - | |
| One parent with weak HGS (n=109) | 22.5±0.6 | <0.001 | 12 (20.7) | 97 (11.1) | 2.04 (1.08–3.87) | 0.029 | |
| Both parents with HGS (n=8) | 22.5±1.8 | 0.283 | 2 (3.5) | 6 (0.7) | 4.64 (1.12–19.13) | 0.034 | |
Values are expressed as mean±standard error or number (%) unless otherwise indicated. The cutoff values for weak HGS were defined as <28.9 and <16.8 kg in men and women, respectively.
HGS, hand grip strength; LS, least-square; OR, odds ratio; CI, confidence interval.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download